Published online Aug 16, 2022. doi: 10.4253/wjge.v14.i8.495
Peer-review started: February 18, 2022
First decision: April 12, 2022
Revised: April 23, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 16, 2022
The endocytoscope with ultra-high magnification (x 520) allows us to observe the cellular structure of the colon epithelium during colonoscopy, known as virtual histopathology. We hypothesized that the endocytoscope could directly observe colorectal histopathological specimens and store them as endocyto-pathological images by the endoscopists without a microscope, potentially saving the burden on histopathologists.
To assess the feasibility of endocyto-pathological images taken by an endoscopist as adequate materials for histopathological diagnosis.
Three gastrointestinal pathologists were invited and asked to diagnose 40 cases of endocyto-pathological images of colorectal specimens. Each case contained seven endocyto-pathological images taken by an endoscopist, consisting of one loupe image, three low-magnification images, and three ultra-high magnification images. The participants chose hyperplastic polyp or low-grade adenoma for 20 cases of endocyto-pathological images (10 hyperplastic polyps, and 10 Low-grade adenomas in conventional histopathology) in study 1 and high-grade adenoma/ shallow invasive cancer or deep invasive cancer for 20 cases [10 tumor in situ/T1a and 10 T1b] in study 2. We investigated the agreement between the histopathological diagnosis using the endocyto-pathological images and conventional histopathological diagnosis.
Agreement between the endocyto-pathological and conventional histopathological diagnosis by the three gastrointestinal pathologists was 100% (95%CI: 94.0%–100%) in studies 1 and 2. The interobserver agreement among the three gastrointestinal pathologists was 100%, and the κ coefficient was 1.00 in both studies.
Endocyto-pathological images were adequate and reliable materials for histopathological diagnosis.
Core Tip: The endocytoscope allows us to observe the histological structure of the colon epithelium, but it is a virtual histopathology. We directly observed pathological specimens by the endocytoscope and evaluated the practical usefulness of endocyto-pathology in this pilot study.
- Citation: Inoue F, Hirata D, Iwatate M, Hattori S, Fujita M, Sano W, Sugai T, Kawachi H, Ichikawa K, Sano Y. New application of endocytoscope for histopathological diagnosis of colorectal lesions. World J Gastrointest Endosc 2022; 14(8): 495-501
- URL: https://www.wjgnet.com/1948-5190/full/v14/i8/495.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i8.495
The endocytoscope, which was launched in early 2018 by Olympus Medical Systems Corporation (Tokyo, Japan), can provide ultra-high magnification (x 520) images in real time during colonoscopy. The endocytoscopy allows us to observe the cellular structure of the colorectal lesions, known as virtual histopathology and has provided high diagnostic performance in estimating their histopathology[1-5]. There is growing evidence that the diagnostic accuracy of endocytoscopy with computer-aided diagnosis (CAD) was greater than that of non-expert and comparable to expert endoscopists[6-12].
Based on the background of the shortage of histopathologists, we have explored a new application of endocytoscope for histopathological diagnosis of colorectal lesions[13]. We hypothesized that the endocytoscope could directly observe colorectal histopathological specimens and store them as endocyto-pathological images by the endoscopists themselves without a microscope. The endocyto-pathological images taken by endoscopists can be stored in the same system as the endoscopic images so that both images can be obtained as needed, making it possible to hold clinicopathological conferences efficiently even in countries with a few pathologists. Furthermore, a combination of endocyto-pathological images and the CAD system may lead to saving the burden of histopathologists in the future.
This pilot study aimed to assess the feasibility of endocyto-pathological images taken by an endoscopist as adequate materials for histopathological diagnosis.
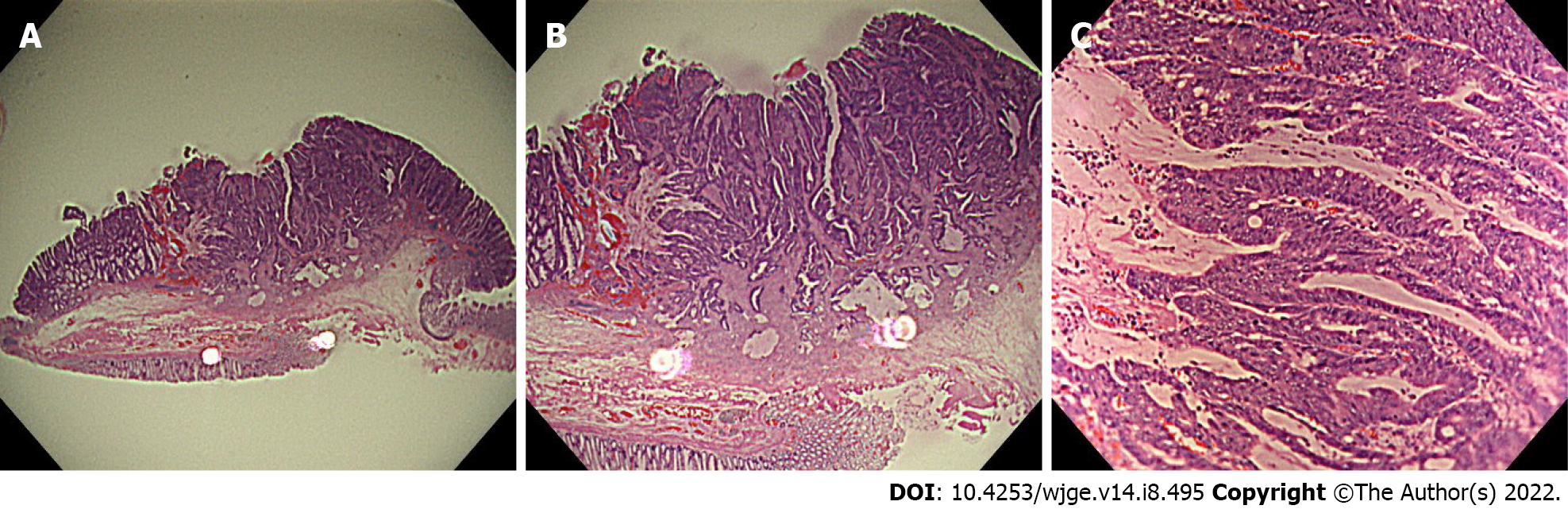
First, each specimen was placed horizontally in a white container filled with water to control the diffuse reflection of the scope light. An endoscopist (FI) took the ultra-magnifying images of the specimens (endocyto-pathological images) with the right hand firmly fixed by touching the edge of the container and holding the tip of the scope using a penhold grip (Figure 1). This method helps bring high-quality endocyto-pathological images into focus. Seven endocyto-pathological images were obtained for each case (one loupe image, three low-magnification images, and three ultra-high magnification images) (Figures 2 and 3).
Candidate colorectal specimens were selected from histopathologically-known material obtained by endoscopic or surgical resection at Sano Hospital between January 2017 and January 2021. Candidates samples with poor preservation, incomplete resection of the lesion, or other candidates deemed inappropriate by the investigators were excluded. Among these candidates samples, 10 specimens for each of the following categories hyperplastic polyps, low-grade adenoma, high-grade adenoma/ shallow invasive cancer (10 tumor in situ (Tis)/T1a), and deep invasive cancer (T1b) were randomly selected. The number of specimens in each category was masked to the participants.
Three gastrointestinal pathologists (TS, HK, KI) were invited and asked to read the endocyto-pathological images for 40 cases (7 images for each case) of colorectal specimens from May to July 2021. The participants were asked to choose hyperplastic polyp or low-grade adenoma for 20 cases of endocyto-pathological images (10 hyperplastic polyps and 10 Low-grade adenomas diagnosed by the conventional method) in study 1 and high-grade adenoma/shallow invasive cancer (Tis/T1a) or deep invasive cancer (T1b) for 20 cases (10 Tis/T1a and 10 Tib cancer) in study 2.
The study protocol was reviewed and approved by the Institutional Review Board at Sano Hospital (202106-02). This study was registered with Japan Registry of Clinical Trials (jRCT1050210046).
The primary outcome measure was the agreement between the histopathological diagnosis using the endocyto-pathological images and conventional histopathological diagnosis.
The secondary outcome measure was the interobserver agreement rate and Fleiss’s Kappa statistics among three pathologists.
This study was conducted as an exploratory research investigation without calculating sample size due to the lack of data in previous studies.
Tables 1 and 2 show the agreement between the histopathological diagnosis by three gastrointestinal pathologists using the endocyto-pathological images and conventional histopathological diagnosis in differentiating low-grade adenoma from hyperplastic polyp (study 1) and T1b from Tis/T1a cancer (study 2). The agreement between the endocyto-pathological and conventional histopathological diagnosis was 100% (95%CI: 94.0%-100%) in study 1 and 100% (94.0%-100%) in study 2. The interobserver agreement among the three gastrointestinal pathologists was 100%, and the κ coefficient was 1.00 in both studies.
| Conventional pathological diagnosis | ||
| Low-grade adenoma (n = 30) | Hyperplastic polyp (n = 30) | |
| Endocyto-pathological diagnosis | ||
| Low-grade adenoma | 30 | 0 |
| Hyperplastic polyp | 0 | 30 |
| Conventional pathological diagnosis | ||
| T1b cancer (n = 30) | Tis/T1a cancer (n = 30) | |
| Endocyto-pathological diagnosis | ||
| T1b cancer | 30 | 0 |
| Tis/T1a cancer | 0 | 30 |
To our knowledge, this is the first report of a new clinical application of the endocytoscope for histopathological specimens. The quality of endocyto-pathological images taken by an endoscopist was sufficiently high to make a histopathological diagnosis. We attempted to take pathological images of histopathological specimens by conventional magnifying endoscopy (x 85 maximum optical magnification with approximately 2mm of a minimum depth of observation); however, cytological findings could not be evaluated owing to a lack of resolution power and focus depth. In contrast, the endocytoscope easily enables the evaluation of cytological findings by taking ultra-high power magnification images with contact on the histological slides. For better quality, the specimens were placed horizontally in a white container filled with water to control the diffuse reflection of the diffuse reflection of the scope light.
Linking endoscopic and histopathological images is a clinically essential step for endoscopists to improve endoscopic diagnosis for estimating the histopathology of gastrointestinal lesions. In situations where pathologists are scarce, it would be better to have endoscopists obtain histopathological images using a microscope. However, most endoscopists do not have microscopes in their institutions or are generally unfamiliar with using them. In this context, we considered it meaningful to have endoscopists obtain histopathological images using endocytoscopes. Additionally, our endocyto-pathological images have the advantage of being stored with endoscopic images in the same endoscopic system, which is helpful when holding clinicopathological conferences. We believe the endocyto-pathological diagnosis will reduce the growing burden on histopathologists, including their time and cost, when especially made with the CAD system. Further studies will be required to prove the hypothesis.
This study has limitations. First, knowledge of histopathology is required for endoscopists to take diagnosable ultra-high magnification images, especially for cancer depth diagnosis. Taking inadequate images would lead to the wrong endocyto-pathological diagnosis. Second, endocytoscopes have not yet been disseminated worldwide. However, the results of this study may encourage the spread of the endocytoscopes, especially in countries with a few pathologists.
In conclusion, endocyto-pathological images of colorectal lesions were adequate and reliable materials for histopathological diagnosis. Endocytoscopes will be disseminated in the future and have the potential for endocyto-pathology worldwide.
Based on the background of the shortage of histopathologists, we explore the new application of endocytoscope for directly observing histopathological specimens of colorectal lesions and storing them as endocyto-pathological images with their endoscopic images.
Endocyto-pathological images taken by endoscopists potentially reduce the burden of histopathologists and facilitate holding clinicopathological conferences more simply.
To assess the feasibility of endocyto-pathological images taken by an endoscopist as adequate materials for histopathological diagnosis.
This was a single-center prospective pilot study. Three gastrointestinal pathologists were asked to diagnose 40 cases of endocyto-pathological images of colorectal specimens (Each case contained seven images: one loupe image, three low-magnification images, and three ultra-high magnification images). The participants chose hyperplastic polyp or low-grade adenoma for 20 cases of endocyto-pathological images (10 hyperplastic polyps, and 10 Low-grade adenomas in conventional histopathology) in study 1 and high-grade adenoma/shallow invasive cancer or deep invasive cancer for 20 cases [10 tumor in situ (Tis)/T1a and 10 T1b] in study 2.
Agreement between the endocyto-pathological and conventional histopathological diagnosis by the three gastrointestinal pathologists was 100% (95%CI: 94.0%–100%) in studies 1 and 2. The interobserver agreement among the three gastrointestinal pathologists was 100%, and the κ coefficient was 1.00 in both studies.
Endocyto-pathological images were adequate and reliable materials for histopathological diagnosis.
Endocyto-pathological images taken by endoscopists will reduce the growing burden on histopathologists, including their time and cost, when especially used with the computer-aided diagnosis system.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq; Tousidonis M, Spain S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Takamaru H, Wu SYS, Saito Y. Endocytoscopy: technology and clinical application in the lower GI tract. Transl Gastroenterol Hepatol. 2020;5:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Rotondano G, Bianco MA, Salerno R, Meucci C, Prisco A, Garofano ML, Sansone S, Cipolletta L. Endocytoscopic classification of preneoplastic lesions in the colorectum. Int J Colorectal Dis. 2010;25:1111-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Mori Y, Kudo SE, Ogawa Y, Wakamura K, Kudo T, Misawa M, Hayashi T, Katagiri A, Miyachi H, Inoue H, Oka S, Matsuda T. Diagnosis of sessile serrated adenomas/polyps using endocytoscopy (with videos). Dig Endosc. 2016;28 Suppl 1:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sugihara Y, Kudo SE, Miyachi H, Wakamura K, Mori Y, Misawa M, Hisayuki T, Kudo T, Hayashi T, Hamatani S, Okoshi S, Okada H. In vivo detection of desmoplastic reaction using endocytoscopy: A new diagnostic marker of submucosal or more extensive invasion in colorectal carcinoma. Mol Clin Oncol. 2017;6:291-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kudo T, Kudo SE, Mori Y, Wakamura K, Misawa M, Hayashi T, Miyachi H, Katagiri A, Ishida F, Inoue H. Classification of nuclear morphology in endocytoscopy of colorectal neoplasms. Gastrointest Endosc. 2017;85:628-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Mori Y, Kudo SE, Wakamura K, Misawa M, Ogawa Y, Kutsukawa M, Kudo T, Hayashi T, Miyachi H, Ishida F, Inoue H. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc. 2015;81:621-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Mori Y, Kudo SE, Chiu PW, Singh R, Misawa M, Wakamura K, Kudo T, Hayashi T, Katagiri A, Miyachi H, Ishida F, Maeda Y, Inoue H, Nimura Y, Oda M, Mori K. Impact of an automated system for endocytoscopic diagnosis of small colorectal lesions: an international web-based study. Endoscopy. 2016;48:1110-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 300] [Article Influence: 50.0] [Reference Citation Analysis (1)] |
| 9. | Takeda K, Kudo SE, Mori Y, Misawa M, Kudo T, Wakamura K, Katagiri A, Baba T, Hidaka E, Ishida F, Inoue H, Oda M, Mori K. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy. 2017;49:798-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Misawa M, Kudo SE, Mori Y, Takeda K, Maeda Y, Kataoka S, Nakamura H, Kudo T, Wakamura K, Hayashi T, Katagiri A, Baba T, Ishida F, Inoue H, Nimura Y, Oda M, Mori K. Accuracy of computer-aided diagnosis based on narrow-band imaging endocytoscopy for diagnosing colorectal lesions: comparison with experts. Int J Comput Assist Radiol Surg. 2017;12:757-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Misawa M, Kudo SE, Mori Y, Nakamura H, Kataoka S, Maeda Y, Kudo T, Hayashi T, Wakamura K, Miyachi H, Katagiri A, Baba T, Ishida F, Inoue H, Nimura Y, Mori K. Characterization of Colorectal Lesions Using a Computer-Aided Diagnostic System for Narrow-Band Imaging Endocytoscopy. Gastroenterology. 2016;150:1531-1532.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Mori Y, Kudo SE, Misawa M, Hotta K, Kazuo O, Saito S, Ikematsu H, Saito Y, Matsuda T, Kenichi T, Kudo T, Nemoto T, Itoh H, Mori K. Artificial intelligence-assisted colonic endocytoscopy for cancer recognition: a multicenter study. Endosc Int Open. 2021;9:E1004-E1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Provenzano E, Driskell OJ, O'Connor DJ, Rodriguez-Justo M, McDermott J, Wong N, Kendall T, Zhang YZ, Robinson M, Kurian KM, Pell R, Shaaban AM. The important role of the histopathologist in clinical trials: challenges and approaches to tackle them. Histopathology. 2020;76:942-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |