Published online May 16, 2020. doi: 10.4253/wjge.v12.i5.159
Peer-review started: February 18, 2020
First decision: March 28, 2020
Revised: April 12, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: May 16, 2020
Longstanding ulcerative colitis (UC) is associated with an increased risk of colonic neoplasia. Various endoscopic modalities, such as chromoendoscopy (CE), narrow band imaging (NBI) and random biopsy have been introduced for surveillance, however, there exists a paucity of direct comparisons between them. We aimed to conduct a network meta-analysis of randomized controlled trials (RCTs) performed for surveillance of neoplasia in UC.
To provide a comparative evaluation of the efficacy of the above-mentioned various modalities.
We searched MEDLINE/PubMed, Web of Science, Embase, Google Scholar and Cochrane Central Registry through May 2016 for RCTs evaluating the efficacy of endoscopic modalities for surveillance of neoplasia in UC. The primary outcomes of interest were dysplasia (low- or high-grade) detection rates per biopsy and per patient, and dysplasia numbers per patient. Studies were simultaneously analyzed using a random-effects network meta-analysis under the Bayesian framework to identify the modality with the highest dysplasia detection rate. The best ranking probability for the dysplasia detection rate was analyzed by surface under the cumulative ranking (SUCRA) technique.
Six prospective RCTs of a total 1038 patients were identified. We identified 4 different modalities; white light (WL) high definition (HD) or standard definition (SD), CE HD, and NBI HD. For dysplasia per biopsy, direct meta-analysis showed superiority of NBI HD over WL HD and CE HD over WL SD. Network meta-analysis demonstrated the rank order of best modality as NBI HD, CE HD, WL HD and WL SD with close SUCRA scores of the first two. For dysplasia per patient, direct meta-analyses showed equivocal results between each modality. Network meta-analysis demonstrated the rank order of best modality as WL HD, NBI HD, CE HD and WL SD with small differences of the SUCRA score among the first two. For dysplasia numbers per patient, direct meta-analysis showed superiority of CE HD over WL SD. Network meta-analysis demonstrated the rank order of best modality as WL HD, NBI HD, CE HD, and WL SD with small differences of the SUCRA score among the first three.
We demonstrated that there were small differences among WL HD, NBI HD, and CE HD, while WL SD was inferior, in detecting dysplasia in UC.
Core tip: Ulcerative colitis (UC) is associated with increased neoplasia risk. Chromoendoscopy (CE), narrow band imaging (NBI) and random biopsy are used for surveillance, but data is limited to conclude best surveillance rank order. We did a network meta-analysis of UC surveillance randomized controlled trials. We identified 4 modalities; white light (WL) high definition (HD) or standard definition (SD), CE HD, and NBI HD. Results showed no differences among WL HD, NBI HD, and CE HD, while WLSD was inferior. The use of HD colonoscopes with or without image enhancement may provide improved detection of dysplasia in UC surveillance.
- Citation: Gondal B, Haider H, Komaki Y, Komaki F, Micic D, Rubin DT, Sakuraba A. Efficacy of various endoscopic modalities in detecting dysplasia in ulcerative colitis: A systematic review and network meta-analysis. World J Gastrointest Endosc 2020; 12(5): 159-171
- URL: https://www.wjgnet.com/1948-5190/full/v12/i5/159.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i5.159
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that causes inflammation of the colon and rectum. It may involve the rectum only, but many will experience progression and extension of disease to the proximal colon[1-3]. Due to the longstanding inflammation, patients with UC are at risk for colorectal cancer and dysplasia[4,5]. The risk is higher among those with pancolonic inflammation and with longer disease duration[5-7]. Current guidelines recommend endoscopic surveillance of CRC in patients with UC after 8-10 years of diagnosis[3,8-14].
Detection of dysplasia is often difficult as they can be subtle and difficult to discriminate between surrounding mucosa. For a long time, recommendations included random 4 quadrant non-targeted biopsies every 10 cm, resulting in a minimum of 32 biopsies per colonoscopy[15-18]. The random biopsy method is laborious and actually only a small area of the entire mucosal surface is biopsied. More recently, advanced modalities such as chromoendoscopy (CE) and narrow-band imaging (NBI) have been introduced to clinical practice[19]. They have shown improved detection of neoplasia in screening colonoscopies and have been applied to surveillance for dysplasia in UC[20-22]. Although retrospective and few prospective studies on dysplasia detection in UC have been performed, there exists a paucity of head-to-head comparisons between different modalities. Furthermore, the number of prospective randomized controlled trial (RCTs) is limited and the study population and treatment modality reported vary among studies.
Network meta-analysis is a type of meta-analysis in which multiple treatments are compared using direct comparisons of treatments, here in our case within RCTs, and indirect comparisons across RCTs based on a common comparator[23,24]. In order to provide a comparative evaluation among different endoscopic modalities and to rank the modalities based on their detection rate, we conducted a network meta-analysis comparing RCTs of surveillance for dysplasia in UC.
We searched MEDLINE (1993-July 2016), Google Scholar (1993-July 2016), Scopus (1993-July 2016), EMBASE (1993-July 2016), Web of Science (1980-July 2016) and Cochrane Central Register of Controlled Trials (through July 2016) for studies on UC dysplasia surveillance. For Google Scholar, only the first one thousand articles were reviewed as it does not provide results beyond it. We also searched abstracts from scientific meetings (American Gastroenterology Association, American College of Gastroenterology, United European Gastroenterology, 2001-2016), and bibliographies of identified articles for additional references.
To be eligible for inclusion, we only considered prospective RCTs evaluating the dysplasia detection in UC that compared outcomes between two or more different endoscopic modalities. We imposed no restrictions regarding age, sex, and duration of study but we tried to include studies with similar age, sex and duration ranges. We imposed no geographic or language restrictions and articles in languages other than English were translated if necessary. Two authors (Gondal B and Komaki Y) independently screened each of the potential titles and abstracts in the primary search to exclude studies that did not address the research question of interest. The full text of the remaining articles was examined to determine whether it contained relevant information and were eligible for inclusion. Areas of disagreement or uncertainty were resolved by consensus between the two authors. The corresponding authors of studies were contacted to provide additional information on trials if required. Studies were searched with a combination of terms including “UC”, “dysplasia”, “surveillance”, “cancer”, “chromoendoscopy”, “NBI”, “narrow band imaging” (both as medical subject headings and free text terms). These were combined by using the set operator and with studies identified with the terms.
All data were independently abstracted in duplicate by two authors (Gondal B and Komaki Y) by using a data abstraction form. Data on the study characteristics, such as author name, year of publication, study design, sample size, mean age of patients, type of scope and endoscopic modality used, total number of procedures, number of dysplastic lesions per random and targeted biopsy, and procedure withdrawal time, were collected. Studies that included patients with known neoplasia, colorectal cancer, poor preparation, severe inflammation, or no histology and fewer patients (less than 10) were excluded from the analysis. The Jadad score, a scale that assesses the methodological quality of a clinical trial, and Cochrane Risk of Bias Assessment Instrument were used to evaluate the methodological quality of the RCTs[25,26].
The primary outcome measures of interests were dysplasia detection rate per biopsy, dysplasia detection rate per patient, and numbers of dysplastic lesions detected per patients among different modalities. We also compared average procedure withdrawal times. Dysplastic lesions were defined as at least low-grade dysplasia per each study’s criteria. Results of the direct meta-analysis between modalities, where applicable, were also shown for reference in supplementary figures.
We followed the PRISMA for Network Meta-Analyses in the report of this meta-analysis[27]. The protocol of this meta-analysis has not been published or registered to any databases. A random-effects network meta-analysis using the Bayesian Markov chain Monte Carlo method was performed with ADDIS 1.16.1 and further details can be found at drugis.org[28]. Inconsistency and node-splitting models were applied to assess the consistency of the RCTs included in the network. Loop inconsistency models appeal directly to the intuition about how inconsistencies in networks of evidence might arise[29]. When loops of therapies were available, inconsistency factors were calculated to assess the strength of the data. The results were considered to show no significant inconsistency when 95% confidence intervals (95%CI) of inconsistency factors included zero or when a large probability P > 0.05 for the comparison between direct and indirect effects in the node splitting analysis was shown. The ranking probability for each modality, i.e., the most efficacious, the second-best, the third-best, and so on, was calculated and the overall ranks were interpreted by surface under the cumulative ranking (SUCRA) technique[30]. The larger SUCRAs denote better endoscopic modality. The effect sizes related to the studied outcomes and their corresponding 95%CI were also reported.
Direct meta-analysis was also performed with ADDIS 1.16.1 for reference. Odds ratio (OR) of detecting dysplasia were compared between the two groups. We evaluated the presence of heterogeneity across trials of each therapy by using the I2 statistic. An I2 < 25% indicates low heterogeneity, 25%-75% moderate heterogeneity, and > 75% high heterogeneity, respectively[31]. We followed the Cochrane Handbook for Systematic Reviews of Interventions in the report of this meta-analysis[25].
We identified 2927 citations through literature search, excluded 2862 titles and abstracts after initial screening, and assessed 65 articles for eligibility (Figure 1). Upon detailed review, we further excluded 59 more articles, and ultimately included 6 prospective RCTs which looked at dysplasia detection rates in WL HD, WL SD, CE HD, and NBI HD. All the studies were parallel studies. A total of 1038 patients were included in the analysis for this network meta-analysis. The characteristics and outcomes of the included studies are summarized in Table 1[32-37]. The quality of the studies assessed by the Jadad score showed a median of 3 (range 1-4). The majority of trials were rated to be of good methodological quality, despite most of the trials not mentioning the procedures for allocation, concealment and blinding of outcome assessment (Table 2).
| Study | Ref. | Design | Reference | Comparator | Disease Type, Age | Reference | Comparator | ||||
| Bx with dysplasia/total bx (n) | Patients with dysplasia/total patients (n) | Bx with dysplasia/total patients (n) | Bx with Dysplasia/total Bx (n) | Patients with dysplasia/total patients (n) | Bx with dysplasia/total patients (n) | ||||||
| NBI HD vs WL HD | Dekker et al[32], 2007 | PRCT | NBI HD | WL HD | UC, Adults | 9/52 | 8/42 | 9/42 | 21/1550 | 7/42 | 21/42 |
| Van den Broek, et al[33], 2011 | PRCT | NBI HD | WL HD | UC, Adults | 13/105 | 8/48 | 13/48 | 14/1657 | 12/48 | 14/48 | |
| Ignjatovic et al[34], 2012 | PRCT | NBI HD | WL HD | UC, Adults | 6/1380 | 5/56 | 6/56 | 7/1375 | 5/56 | 7/56 | |
| Leifeld et al[35], 2015 | PRCT | NBI HD | WL HD | UC, Adults | 31/1883 | 22/159 | 31/159 | 30/7565 | 24/159 | 30/159 | |
| CE HD vs WL SD | Kiesslich et al[36], 2003 | PRCT | CE HD | WL SD | UC, Adults | 35/3545 | 13/84 | 35/84 | 10/3094 | 6/81 | 10/81 |
| CE HD vs NBI HD | Watanabe et al[37], 2016 | PRCT | CE HD | NBI HD | UC, Adults | 19/181 | 11/130 | 19/130 | 27/183 | 17/133 | 27/133 |
| Ref. | Was the study described as randomized? | Was the randomization scheme described and appropriate? | Was the study described as doubleblind? | Was the method of double blinding appropriate? | Was there a description of dropouts and withdrawals? | Total Jadad score | Se-quence generation (for arm randomization) | Allocation con-cealed | Blinding of outcomes | Incomplete outcome data addressed | ITT | Sample size calculation |
| Dekker et al[32], 2006 | o | NA | o | o | o | 4 | x | x | o | o | o | o |
| Van den Broek et al[33], 2011 | o | NA | x | NA | x | 1 | ? | ? | x | ? | o | x |
| Ignjatovic et al[34], 2012 | o | NA | o | NA | o | 3 | ? | ? | ? | o | o | o |
| Leifeld et al[35], 2015 | o | NA | x | NA | x | 1 | o | o | x | ? | ? | x |
| Kiesslich et al[36], 2003 | o | o | o | NA | o | 4 | o | o | o | o | o | o |
| Watanabe et al[37], 2016 | o | o | o | NA | o | 4 | o | o | o | o | o | o |
As shown in Supplementary Figure 1A, there were 4 studies that compared the effectiveness of WL HD vs NBI HD in detecting dysplasia per biopsy, and the pooled results showed superiority of NBI HD (OR: 5.71, 95%CI: 1.87-17.47). Significant heterogeneity was seen as shown by an I2 value of 88.2%, which appeared due to variable outcomes between studies. A single study showed superiority of CE HD over WL SD and another study was in slight favor of NBI HD over CE HD (Supplementary Figure 1B and C). It was difficult to draw any conclusion from the direct meta-analyses as quantitative assessment of the effectiveness between the 4 modalities in detecting dysplasia per biopsy was not possible.
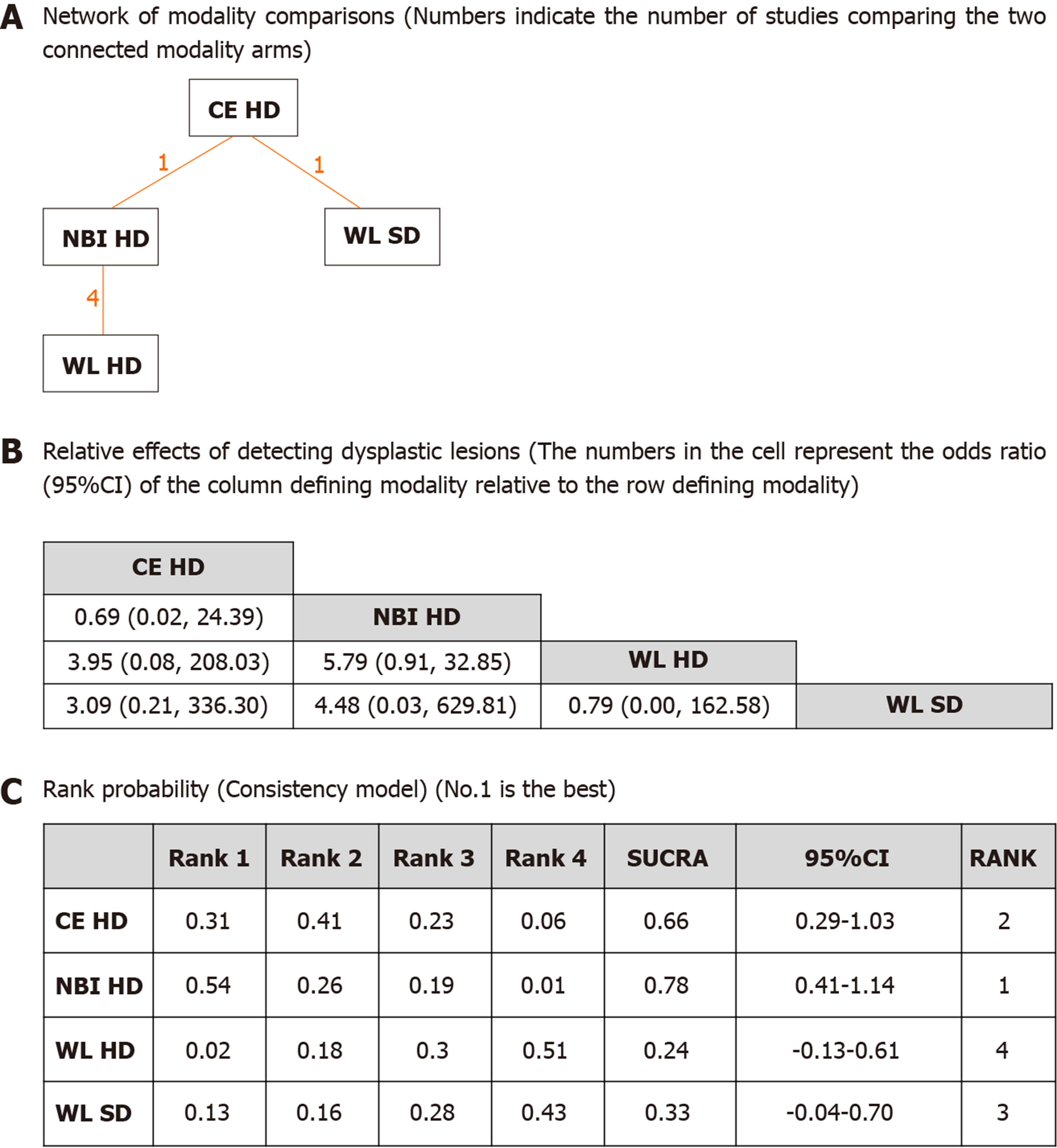
The pictorial network of all modality comparisons analyzed for best modality in detecting dysplasia per biopsy is shown in Figure 2A. Figure 2B shows the OR of detecting dysplastic lesions per all biopsies analyzed by the network meta-analysis. CE HD and NBI HD were numerically superior to WL HD or WL SD modalities, but the differences were not statistically significant. Ranking probabilities for each modality in detecting dysplasia per biopsy are shown in Figure 2C. The rank probability of dysplasia detection interpreted by SUCRA was as follows (in ascending order of rank), NBI HD (0.78, 95%CI: 0.41-1.14), CE HD (0.66, 95%CI: 0.29-1.03), WL SD (0.33, 95%CI: -0.04-0.70) and WL HD (0.24, 95%CI: -0.13-0.61). The difference between NBI HD and CE HD was small, but their differences between the WL modalities were moderate suggesting that CE or NBI are more efficient in detecting dysplasia per biopsy than WL. Inconsistency model analysis and node-splitting analysis were not possible as no loop was created among the modalities (Figure 2A).
Direct meta-analysis of 4 studies showed no difference between NBI HD vs WL HD (Supplementary Figure 2A) with low heterogeneity. Single studies were in slight favor of NBI HD over CE HD and CE HD over WL SD, respectively, in detecting dysplasia on a per patient basis (Supplementary Figure 2B and C). It was difficult to draw any conclusion from the direct meta-analyses as quantitative assessment of the effectiveness between the 4 modalities in detecting dysplasia per patient was not possible.
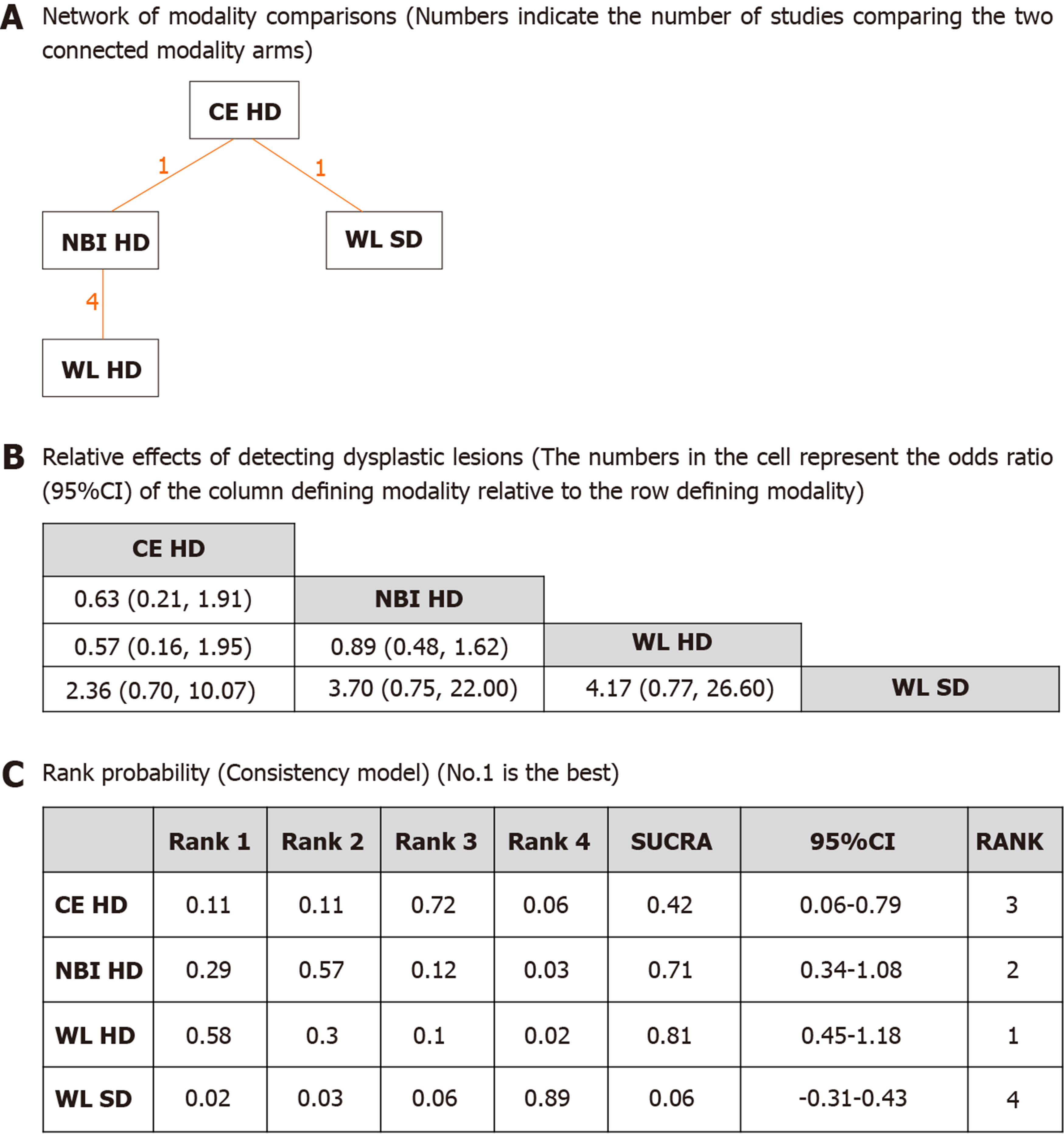
The network of all modality comparisons analyzed for detecting dysplasia per patient is shown in Figure 3A. Figure 3B shows the OR of efficacy (number of patients with dysplasia) analyzed by the network meta-analysis. As shown in Figure 3C, the rank probability of efficacy interpreted by SUCRA was as follows (in ascending order of rank); WL HD (0.81, 95%CI: 0.45-1.18), NBI HD (0.71, 95%CI: 0.34-1.08), CE HD (0.42, 95%CI: 0.06-0.79) and WL SD (0.06, 95%CI: -0.31-0.43). The difference between the first two modalities was small, but the differences between WL SD and the rest of the three were large suggesting that WL SD may be inferior to the other modalities. Inconsistency model analysis and node-splitting analysis were not possible as no loop was created among the modalities (Figure 3A).
Direct meta-analysis of 4 studies showed no difference between NBI HD vs WL HD (Supplementary Figure 3A) with moderate heterogeneity. A single study showed superiority of CE HD over WL SD and another study was in slight favor of NBI HD over CE HD (Supplementary Figure 3B , C). It was difficult to draw any conclusion from the direct meta-analyses as quantitative assessment of the effectiveness between the 4 modalities in detected numbers of dysplasia per patient was not possible.
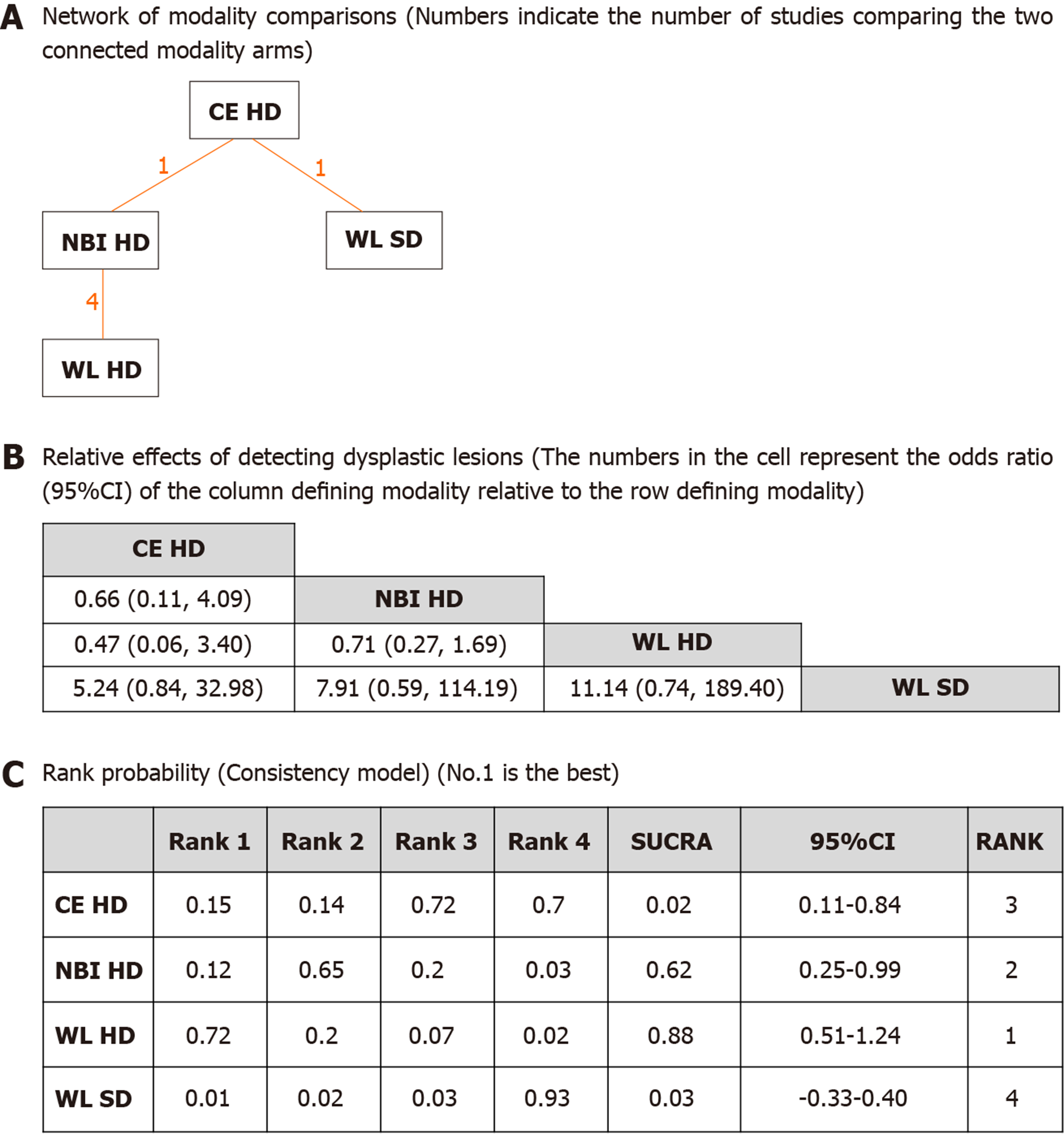
The network of all modality comparisons analyzed for detected numbers of dysplasia per patient is shown in Figure 4A. Figure 4B shows the OR of efficacy (detected numbers of dysplasia per patient) analyzed by the network meta-analysis. As shown in Figure 4C, the rank probability of efficacy interpreted by SUCRA was as follows (in ascending order of rank); WL HD (0.88, 95%CI: 0.51-1.24), NBI HD (0.62, 95%CI: 0.25-0.99), CE HD (0.48, 95%CI: 0.11-0.84) and WL SD (0.03, 95%CI: -0.33-0.40). The differences between the first three modalities were moderate, and the differences between WL SD and the rest were large without overlap of 95%CIs. It was suggested that WL SD appeared to be inferior to the other modalities.
Inconsistency model analysis and node-splitting analysis were not possible as no loop was created among the modalities (Figure 4A).
Our comprehensive network meta-analysis simultaneously compared various endoscopic modalities for dysplasia surveillance in UC. A few direct meta-analyses comparing two modalities have been previously reported, however, the rationale of our study was to add evidence through indirect comparisons by network meta-analysis, where direct head-to-head comparisons were lacking. On a per biopsy basis, NBI HD or CE HD appeared to be more effective compared to WL modalities. For detecting dysplasia on a per patient basis, though, WL HD and NBI HD, ranked relatively close, and over other modalities. Another finding is that WL SD consistently ranked worse compared to the other three modalities suggesting that its use cannot be rationalized for dysplasia surveillance in UC. Overall, the results of our study suggest that the use of a HD colonoscope may be more important rather than the use of image enhancing in detecting dysplasia in patients with UC.
The rank order of efficacy in the present network meta-analysis is somewhat different from various international societal recommendations where CE is recommended over other modalities. CE has been increasingly used in surveillance of dysplasia in UC since Kiesslich et al[36] reported its effectiveness over the traditional random biopsy method. Since then, many cohort studies have demonstrated the utility of CE which has led to its inclusion and recommendation in guidelines. However, the number of RCT showing the effectiveness of CE over random biopsy is small and the evidence supporting its use may not be robust. NBI system is one of the most commonly used image enhanced technology which has been shown to be effective in detecting colonic neoplasia in colon cancer screening. NBI with target biopsy has not been shown to be consistently more effective than WL exam, however, a recent study by Watanabe et al[37] showed its non-inferiority over CE. It was thus difficult to draw any conclusion from the existing RCT whether WL, CE or NBI were superior to or similar to each other by means of dysplasia detection in UC surveillance. Furthermore, some modalities lacked direct comparisons by RCT.
The fundamental rationales of our network meta-analysis were to overcome the lack of direct comparison of modalities and to perform indirect comparisons to objectively assess their comparative efficacy in detecting dysplasia. Network meta-analysis is now widely recognized and utilized in clinical questions where direct comparisons among treatments are not possible[38,39]. It can simultaneously compare multiple treatments by using direct comparisons of treatments within RCTs and indirect comparisons across RCTs based on a common comparator[23]. This analytical approach is justified when its key assumptions are satisfied, and we acknowledged the methodological challenge while calculating and interpreting statistics carefully.
We analyzed four different endoscopic modalities assessed in 7 head to head RCTs; WL SD, WL HD, CE HD (methylene blue or indigo carmine) and NBI HD. We found that among the 4 endoscopic modalities, NBI HD was the best modality for dysplasia detection per biopsy, but WL HD ranked slightly higher in terms of dysplasia per patient or numbers of dysplasia per patient. We acknowledge that our result was not solid enough to conclude that any one modality was the best and further investigation including larger RCTs is warranted. However, it does appear that WL SD appears to be inferior to the other modalities and its use cannot be justified based on the results of our network meta-analysis. Until further evidence is available, the use of NBI HD, WL HD and CE HD can all be utilized for dysplasia surveillance in UC. This is supported by observational studies demonstrating low yield of random biopsies compared to target biopsies[40].
One of the shortcomings of this network meta-analysis is that the number of included studies was relatively small with only one study comparing outcomes between CE HD vs NBI HD or CE HD vs WL SD. There were no studies comparing CE HD to WL HD or WL SD to WL HD, which resulted in a lack of loop of comparisons. Thus, we were unable to perform any inconsistency model analysis and node-splitting analysis in the subgroup analysis due to the lack of a loop among the comparison of included studies. Some studies included in our meta-analysis had potential risks of bias related to allocation concealment and blinding of outcome assessment, however, the median Jadad score was 3 and the majority of trials were rated to be of good methodological quality.
In conclusion, this systematic review and network meta-analysis comparing multiple modalities used in UC dysplasia surveillance suggests that the three modalities, WL HD, NBI HD and CE HD, showed overall similar effectiveness in detecting dysplasia. WL SD consistently ranked worse compared to the other modalities suggesting the importance of using a HD colonoscope.
Patients with ulcerative colitis (UC), which is a chronic inflammation of the colon and rectum, are at high risk of developing colorectal cancer (CRC), mainly if the inflammation involves the whole colon and/or lasts for a long duration. Currently, it is recommended to perform endoscopic surveillance looking for colon cancer and dysplasia in those patients after 8-10 years from the diagnosis of UC. Our study compares the best modality to use in those surveillance colonoscopies.
The main modalities used in CRC surveillance in UC are white light high definition (WL HD), WL standard definition (WL SD), chromoendoscopy (CE) HD, and narrow-band imaging (NBI) HD. There is a paucity of head-to-head comparison among these modalities to help physician in deciding what is the best option to use for early detection of dysplasia. This study is constructed to assist the clinician to choose the best yielding modality.
The main objective is to demonstrate the best modality to use in terms of detecting dysplasia and targeted biopsies. We realized that not all the modalities are equal in efficacy nor in yielding results. These objectives would be of major impact in future research and clinical practice.
The research methods (e.g., experiments, data analysis, surveys, and clinical trials) that were adopted to realize the objectives, as well as the characteristics and novelty of these research methods, should be described in detail. The methods used to reach these objectives were literature search of studies on UC dysplasia surveillance on MEDLINE, Google Scholars, Scopus, Embase, Web of Science, and Cochrane Central Register of Controlled Trials (CRC). Only prospective randomized controlled trials (RCTs) evaluating the dysplasia detection in UC that compared outcomes between two or more different endoscopic modalities were included. Data were extracted independently using a data abstraction form. Jadad score, a scale that assesses the methodological quality of a clinical trial, and Cochrane Risk of Bias Assessment Instrument were used to evaluate the methodological quality of the RCTs.
We found that for dysplasia per biopsy basis, the best modalities were NBI HD and CE HD, while on dysplasia per patient basis, WL HD and NBI HD were the highest ranked. For dysplasia numbers per patient, the three HD modalities were superior to WL SD. The striking finding was that regardless of the image enhancing modality used, HD was the most important option in detecting dysplasia. These finding could help the clinician in choosing the best yielding modality to use for CRC/dysplasia surveillance in patients with UC.
Regardless of the image enhancing modality used, HD was the most important option in detecting dysplasia in patients with UC. When HD colonoscopes are used, image enhancing modality may not be required in detecting dysplasia in patients with UC. HD was the most important option in detecting dysplasia in patients with UC. The best modality to use in surveillance colonoscopies of UC was unclear. Based on the current guidelines for colorectal cancer surveillance in patients with ulcerative colitis, we found that HD is the best option in detecting dysplasia, while white light standard definition is the most inferior option. The increased use of HD would yield the best results in both dysplasia detection rate and targeted biopsies.
Not all imaging or endoscopic modalities are equal in detecting dysplasia in UC. More data and research required to decide what is the single best modality to use in CRC/dysplasia surveillance in patients with UC. RCTs simultaneously comparing multiple modalities or a follow-up network meta-analysis when more studies become available.
An abstract of this manuscript was presented as a poster at Digestive Disease Week (DDW) 2017.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu C, Tsou YK S-Editor: Wang J L-Editor: A E-Editor: Liu JH
| 1. | Ekbom A, Helmick CG, Zack M, Holmberg L, Adami HO. Survival and causes of death in patients with inflammatory bowel disease: a population-based study. Gastroenterology. 1992;103:954-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 188] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 271] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-4; quiz e12-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1955] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 5. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1294] [Cited by in F6Publishing: 1162] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 6. | Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 334] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 308] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-23; quiz 524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 899] [Cited by in F6Publishing: 891] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 9. | Ullman T, Odze R, Farraye FA. Diagnosis and management of dysplasia in patients with ulcerative colitis and Crohn's disease of the colon. Inflamm Bowel Dis. 2009;15:630-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Iacucci M, Uraoka T, Fort Gasia M, Yahagi N. Novel diagnostic and therapeutic techniques for surveillance of dysplasia in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2014;28:361-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Itzkowitz SH, Present DH; Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 368] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 12. | Eaden JA, Mayberry JF; British Society for Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51 Suppl 5:V10-V12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 819] [Cited by in F6Publishing: 769] [Article Influence: 54.9] [Reference Citation Analysis (2)] |
| 14. | Huguet JM, Suárez P, Ferrer-Barceló L, Ruiz L, Monzó A, Durá AB, Sempere J. Endoscopic recommendations for colorectal cancer screening and surveillance in patients with inflammatory bowel disease: Review of general recommendations. World J Gastrointest Endosc. 2017;9:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, Munkholm P, Sandborn WJ. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Loftus EV. Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35:517-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | East JE, Toyonaga T, Suzuki N. Endoscopic management of nonpolypoid colorectal lesions in colonic IBD. Gastrointest Endosc Clin N Am. 2014;24:435-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV, Faigel DO, Gan SI, Hirota WK, Lichtenstein D, Qureshi WA, Rajan E, Zuckerman MJ, VanGuilder T, Fanelli RD; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639-651.e28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 20. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, Ordás I, Repici A, Rosa B, Sebastian S, Kucharzik T, Eliakim R; European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 540] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 21. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 22. | Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 341] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 23. | Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 527] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 24. | Komaki Y, Komaki F, Micic D, Yamada A, Suzuki Y, Sakuraba A. Pharmacologic therapies for severe steroid refractory hospitalized ulcerative colitis: A network meta-analysis. J Gastroenterol Hepatol. 2017;32:1143-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. England: Wiley 2008; 672. [Cited in This Article: ] |
| 26. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12275] [Cited by in F6Publishing: 12453] [Article Influence: 444.8] [Reference Citation Analysis (0)] |
| 27. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4165] [Cited by in F6Publishing: 4192] [Article Influence: 465.8] [Reference Citation Analysis (0)] |
| 28. | Gertvan Valkenhoef T, TijsZwinkels, Bertde Brock, HansHillege. ADDIS: A decision support system for evidence-based medicine. Decision Support Systems. 2013;55:459-475. [DOI] [Cited in This Article: ] |
| 29. | Jackson D, Boddington P, White IR. The design-by-treatment interaction model: a unifying framework for modelling loop inconsistency in network meta-analysis. Res Synth Methods. 2016;7:329-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1832] [Cited by in F6Publishing: 2362] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39087] [Cited by in F6Publishing: 41947] [Article Influence: 1997.5] [Reference Citation Analysis (1)] |
| 32. | Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Ignjatovic A, East JE, Subramanian V, Suzuki N, Guenther T, Palmer N, Bassett P, Ragunath K, Saunders BP. Narrow band imaging for detection of dysplasia in colitis: a randomized controlled trial. Am J Gastroenterol. 2012;107:885-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Leifeld L, Rogler G, Stallmach A, Schmidt C, Zuber-Jerger I, Hartmann F, Plauth M, Drabik A, Hofstädter F, Dienes HP, Kruis W; Detect Dysplasia Study Group. White-Light or Narrow-Band Imaging Colonoscopy in Surveillance of Ulcerative Colitis: A Prospective Multicenter Study. Clin Gastroenterol Hepatol. 2015;13:1776-1781.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 538] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 37. | Watanabe K, Nishishita M, Shimamoto F, Fukuchi T, Esaki M, Okamoto Y, Maehata Y, Oka S, Nishiyama S, Fujii S. 722 Comparison Between Newly-Developed Narrow Band Imaging and Panchromoendoscopy for Surveillance Colonoscopy in Patients With Longstanding Ulcerative Colitis: A Prospective Multicenter Randomized Controlled Trial, Navigator Study. Gastrointestinal Endoscopy. 2016;83:AB172. [DOI] [Cited in This Article: ] |
| 38. | Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, Danese S. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385-1397.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 39. | Vickers AD, Ainsworth C, Mody R, Bergman A, Ling CS, Medjedovic J, Smyth M. Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis. PLoS One. 2016;11:e0165435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 307] [Article Influence: 21.9] [Reference Citation Analysis (0)] |