Published online Sep 16, 2018. doi: 10.4253/wjge.v10.i9.145
Peer-review started: March 14, 2018
First decision: April 23, 2018
Revised: April 28, 2018
Accepted: June 8, 2018
Article in press: June 9, 2018
Published online: September 16, 2018
Solid pseudopapillary neoplasm (SPN) is a rare tumor with malignant potential which is generally located in the tail of pancreas. The prevalence of SPN has increased with widespread use of cross sectional imaging. SPN is often misdiagnosed due to nonspecific clinical presentation and accurate diagnosis is essential for optimal management. Endoscopic ultrasound-FNA with immunohistochemistry can help in preoperative diagnosis. Surgery is the treatment of choice and a successful R0 resection is curative. Overall, SPN has a good prognosis. This review article focuses on pathogenesis, diagnosis and management of SPN.
Core tip: Solid pseudopapillary neoplasm (SPN) is a rare pancreatic tumor that predominantly affects young females. SPNs are usually indolent but they do have malignant potential. The pathogenesis of SPN is not entirely clear. Accurate diagnosis is essential in the management of SPN. Endoscopic ultrasound guided fine needle aspiration with immunohistochemistry can help distinguish SPNs from other aggressive pancreatic tumors preoperatively. Surgical resection with clear margins is curative and should be offered whenever feasible. The prognosis of SPN is good even in the presence of metastasis.
- Citation: Lanke G, Ali FS, Lee JH. Clinical update on the management of pseudopapillary tumor of pancreas. World J Gastrointest Endosc 2018; 10(9): 145-155
- URL: https://www.wjgnet.com/1948-5190/full/v10/i9/145.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i9.145
Pancreatic cysts are diagnosed more frequently because of increasing use of computed tomography (CT) and magnetic resonance imaging (MRI). They are found incidentally in more than 2 percent of CT and MRI for unrelated reasons[1,2]. According to World Health Organization (WHO) classification, pancreatic cysts are classified based on histology into serous, mucinous cystic neoplasm, intraductal papillary mucinous neoplasm (IPMN) and solid pseudopapillary neoplasm (SPN)[3]. SPN had various names in the past including Frantz’s tumor, solid and papillary tumor, solid-cystic tumor, papillary cystic tumor as well as solid and papillary epithelial neoplasm[4]. SPN accounts for about 3% of resected pancreatic cystic tumors[5]. SPNs are more common in females who are in their second or third decade of life. Less than 10% of SPNs are diagnosed in men. They are commonly found in the tail of pancreas[7,8].
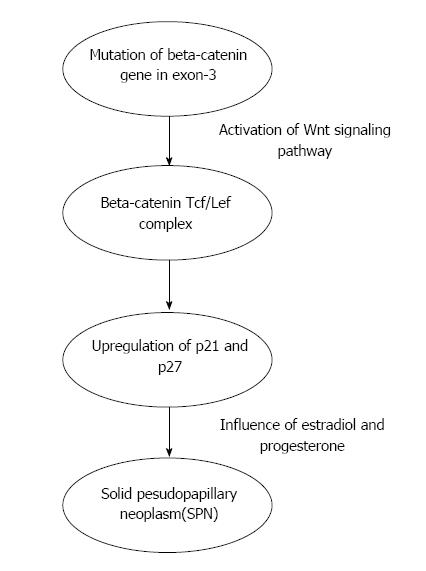
The cellular origin of SPN is unclear. During normal pancreas development, beta-catenin signaling within the beta-catenin/Wnt pathway is necessary and in the adult organ this pathway is usually downregulated[9]. The majority (85%-90%) of SPN have exon-3 mutations and in 10%-15% mutations are present in other exons[10]. The aberrant protein expression in SPN is strongly correlated with mutations in beta-catenin gene[10]. Mutations in beta-catenin gene exon-3 lead to Wnt signaling activation which plays an important role in the development of SPN[11]. Cell cycle-associated proteins like cyclin D1 and cyclin D3 are overexpressed in SPN because of deregulation of cell cycle[12,13]. The role of genetic aberration of EWS/FLI-1 in SPN was studied. FLI-1 is identified in endothelial and mesodermal tissues[14] and it is the earliest marker of blood vessels during embryogenesis[15]. Although FLI-1 was expressed in SPN, it was not accompanied by CD 34 positivity or EWS/FLI-1 translocation[16]. Hence, the diagnosis of SPN is less likely if EWS/FLI-1 translocation is positive. Chromosome 11 is vulnerable to specific genetic changes as it harbors several genes for proteins (cyclin D1, FLI-1, progesterone receptor and CD56) which are overexpressed in SPN[17]. The low tumor growth rate in SPN is explained by the role of cyclin-dependent kinase inhibitors p21 and p27 in controlling the activated Wnt/beta-catenin signaling pathway[17]. SPNs are considered hormone sensitive because they express progesterone receptor[18]. P21, P27 and cyclin D1 expression is influenced by estradiol and progesterone[19,20]. BCL9 and BCL9L play an important role in enhancing Wnt signaling by increasing beta-catenin transcriptional activity and tumorigenesis[21]. BCL9L is differentially expressed among various pancreatic neoplasms [overexpressed in pancreatic ductal adenocarcinoma (PDAC) but decreased in SPN] but BCL9 did not show any difference[22] (Figure 1).
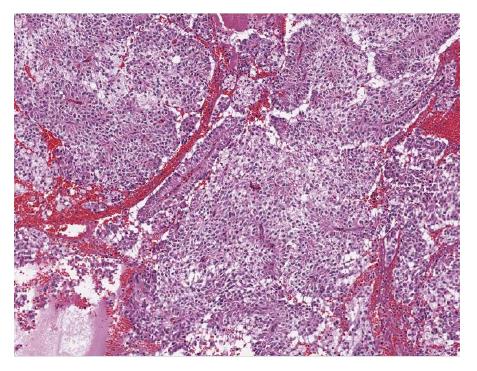
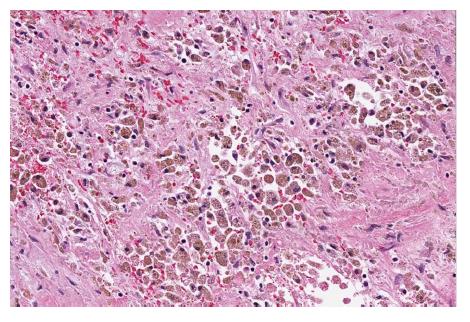
Grossly, SPN appear as solid and cystic components with areas of hemorrhage, calcifications, with a rim of fibrous capsule[23]. They are well-demarcated and invasion of adjacent organs like spleen and duodenum is rare[4]. Cut sections of SPN show alternate solid and yellow areas with cystic, necrotic and hemorrhagic zones. The solid portions of the tumor are usually composed of uniform, polygonal epithelioid cells with vascularized stroma which are not cohesive in arrangement[24]. Hyaline globules are periodic acid-shiff (PAS) intracytoplasmic inclusions that are immunoreactive for alpha1. Hyaline globules are classically associated with SPN. However, hyaline globules are positive in about 5% of pancreatic neuroendocrine tumors (PanNETs)[25]. Insidious pattern of invasion, clear cells and nuclear groove can help distinguish SPN from PanNETs[25]. Electron microscopy shows abundant mitochondria in polygonal cells and sparse endoplasmic reticulum. Tumor cells have round to oval nuclei with occasional indentation of eccentrically located nucleolus and peripheral clumping of chromatin[26]. Tumor cells contain membrane covered corpuscles are similar to zymogen granules about 8-1.2 micro meter in diameter[27].
Histological variants of SPN include clear cell, pleomorphic and oncocytic[24]. Clear cell variant contains multiple cytoplasmic vacuoles with solid and diffuse growth pattern. Pleomorphic variant contains polymorphism (variation in nuclear size, shape, hyperchromasia and multinucleate) in more than 20% of total tumor area. Oncocytic variant contains mostly oncocytic cells. Histological features suggestive of aggressive behavior of SPN include extensive necrosis, nuclear atypia, high mitotic rate and sarcomatoid areas[24]. Angioinvasion, perineural invasion and deep invasion of adjacent surrounding pancreatic parenchyma are considered potentially malignant features[4] (Figures 2 and 3).
In general, the immunostaining of SPN is positive for beta-catenin (nuclear and cytoplasmic), vimentin, synaptophysin, progesterone receptor (nuclear), CD56, NSE (neuron-specific enolase), CD10, and E-cadherin (loss of membrane and nuclear)[10]. Nuclear betacatenin expression and membranous E-cadherin loss are important immunoprofiles useful in distinguishing SPN from other pancreatic neoplasms[28]. E-cadherin is a transmembrane protein that mediates cell adhesion through interactions with catenins and it is linked to the actin skeleton. The exact mechanism for the loss of E-cadherin expression is not clear. Tang et al[29] proposed that loss of E-cadherin is a result of promoter silencing and overexpression of transcription repressors such as Snail. Pseudopapillary pattern of SPN is explained by loss of cell cohesiveness by loss of E-cadherin[30]. Enzyme histochemistry for trypsin, alpha-1 antitrypsin, chymotrypsin, amylase and lipase give inconsistent results and they are not useful in differentiating SPN from other pancreatic neoplasms. CD 10 is usually positive (80%) in the majority of SPN[10]. CD 10 is an endopeptidase which reduces the local concentration of biologic modulators by catabolizing them[31]. It is hypothesized that cell proliferation in SPN is from increased biologic modulators because of decreased CD10 expression[10].
CD 56 and CD10 are also positive in PanNET, acinar cell carcinoma (ACC), renal cell carcinoma (RCC) and malignant melanoma (MM). Progesterone receptor is positive in PanNET, ACC and MM. Synaptophysin is positive in PanNET and ACC. Yang et al[32] showed in their systematic study that Ki-67 index ≥ 4% was significantly associated with decreased recurrence free survival (RFS) and disease specific survival (DSS) in SPN. Ki-67 is a cell-proliferation marker used for evaluation of proliferative activity in tumors. Ki-67 is expressed in all phases of the cell cycle (G1, S, G2 and M)[33]. They concluded that Ki-67 should be routinely included in immunohistochemical staining of SPN.
Kim et al[34] identified androgen receptor (AR), lymphoid enhancer-binding factor 1 (LEF-1) and transcription factor for immunoglobulin heavy-chain enhancer 3 (TFE3) as putative diagnostic markers of SPN in addition to beta-catenin. This study showed that the sensitivity and specificity of beta-catenin in SPN were 98.9% and 97% respectively. when beta-catenin, LEF-1 and TFE3 were combined, the sensitivity and specificity of SPN diagnosis increased to 100% and 91%, respectively[34]. They concluded that when these markers are incorporated in to immunohistochemical panel, they can help differentiate SPN from pancreatic adenocarcinoma and neuroendocrine tumor. LEF-1 is a member of the lymphoid enhancer binding factor 1/T-cell factor (LEF1/TCF) complex and it acts as a regulator of the Wnt/CTNNB1 signaling pathway. When LEF-1 interacts with CTNNB1, it leads to upregulation of LEF-1 in SPN. CTNNB1 is primarily located in the cytoplasmic plasma cell membrane and it plays a key role in the Wnt signal transduction pathway. Singhi et al[35] analyzed the immunohistochemical staining for LEF-1 and CTNNB1 in pancreatic tumors. They concluded that abnormal CTNNB1 accumulation with nuclear LEF-1 expression was found in both SPN and pancreatoblastoma but with diffuse nuclear LEF-1 expression in SPN[35].
TFE3 is a member of the microphthalmia (MiT) family of transcription factors. MiT transcription factors regulate cellular proliferation, survival, motility, metabolism, melanocyte development by binding to target promoters[36]. These are deregulated during oncogenic process. TFE3 is expressed in 74.7% of SPN[34]. Park et al[37] showed activation of androgen receptor signaling pathway in SPN and they demonstrated increased AR expression at transcriptional and translational levels. This study confirmed high level of nuclear androgen receptor expression in all SPN (14/14). Kim et al[34] showed AR expression in 81.3% of SPN.
SOX-11 is a member of the SOX (SRY-related HMG-box) family of transcription factors. They play an important role in cell differentiation, sex determination, development of the central nervous system, hematopoietic, and other organ systems by regulating lineage and tissue-specific gene expression[38]. SOX proteins have been shown to be key modulators of Wnt/beta-catenin signaling pathway. However, the interaction between SOX-11 and Wnt/beta-catenin signaling is not reported so far. Harrison et al[39] showed a sensitivity and specificity of 100% and 84%, respectively in expression of SOX-11 in SPN. They concluded that immunohistochemistry with beta-catenin, SOX-11 and TFE3 should be combined to achieve optimal sensitivity and in diagnosing SPN. Foo et al[40] evaluated the nuclear reactivity of SOX-11 in SPN and they showed that the sensitivity and specificity was 100%, respectively in EUS-FNA specimens.
Symptoms are nonspecific. SPNs can present with palpable abdominal mass, indigestion, abdominal discomfort, epigastric pain, nausea, vomiting, asthenia, itching, weight loss, back pain, early satiety, bloating, jaundice and pancreatitis[23,27,41,42]. SPN outside the pancreas can occur in the retro-peritoneum , liver, stomach, mesentery, duodenum, omentum, ovary or lung[43,44]. The most common manifestation of malignant acting SPN is peripheral parenchymal infiltration[45]. SPN can also be found in regional lymph nodes, portal vein, colon, spleen and blood vessels[24,46,47].
Differential diagnosis of SPN include cystic tumors like cyst adenoma, cystadenocarcinoma, microcystic adenoma, PanNET, lymphangioma, sarcoma, cystic islet cell tumors, acinar cell cystadenocarcinoma, discogenic cysts, pseudocysts and hydatid cysts[48].
Ultrasonographic (US) examination of SPN shows homogeneous, hypoechoic mass with hyperechoic rim and contrast enhanced ultrasound (CEUS) shows hyperenhancement of the rim in the arterial phase[49]. CEUS can identify the cystic areas of the tumor and the peripheral rim of SPN better than US which improves the diagnosis of SPN[49]. Contrast CT and MRI are superior to US in identifying capsule and intramural hemorrhage which are more specific characteristics for diagnosing SPN[50].
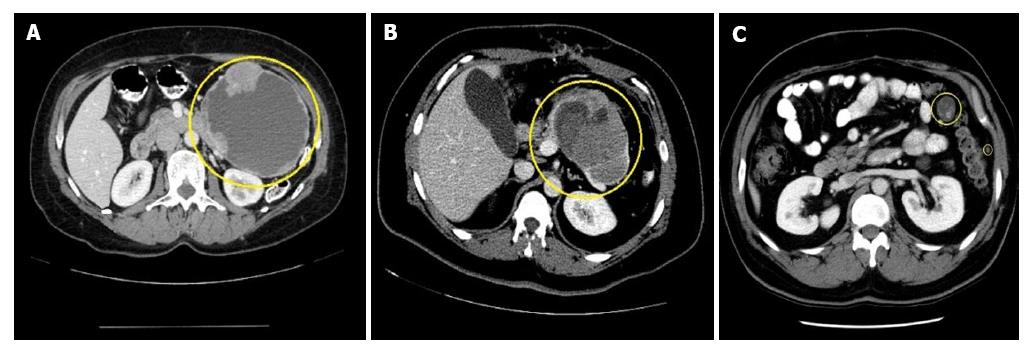
CT features of SPN include encapsulated mass with varying solid and cystic components secondary to hemorrhagic degeneration (Figure 4). At the periphery of the mass, calcification and solid areas can be identified[51]. On multiphasic CT scan appear as a solid pancreatic mass with sharp borders[52]. During CT pancreatic phase, there is weak enhancement when compared to the surrounding pancreatic parenchyma and in hepatic venous phase there is gradual increase in enhancement of small SPNs[52]. Typical SPN on CT has surrounding capsule with demarcation between solid and cystic components and hypoattenuation during pancreatic phase[50,51]. Atypical SPNs on CT have no surrounding capsule, solid or cystic component with hyper attenuation during pancreatic phase and dense internal calcification with no defined margin[52]. Park et al[53] studied CT imaging features of SPNs in males and females. The results showed that lobulated shape is more common among males and oval shape in females[53].
MRI is considered superior to CT in terms of correlation of clinicopathological and radiological findings of SPN[54]. It has advantage over CT especially when the patient has contrast allergy or renal insufficiency. SPN is identified on MRI as an encapsulated lesion with both solid and cystic component as well as hemorrhage without septation[54]. Yu et al[54] proposed three MRI features (Type 1, Type 2 and Type 3) that correlated with clinicopathological features. Type1 image in SPN is completely solid, homogeneously hypointense on T1W1 image and slightly hyperintense when compared to surrounding pancreatic parenchyma on T2W1 image. Type 2 image in SPN has both solid mass and hemorrhage, hypointense with surrounding heterogeneously hyperintense area on T1W1 image and hyperintense areas on T1W1 appear more hyperintense on T2W1 image. Type 3 image in SPN has massive hemorrhage, mainly hyperintense with intermediate and hypointense area on T1W1 image and hyperintense areas on T1W1 appear more hyperintense on T2W1 image.
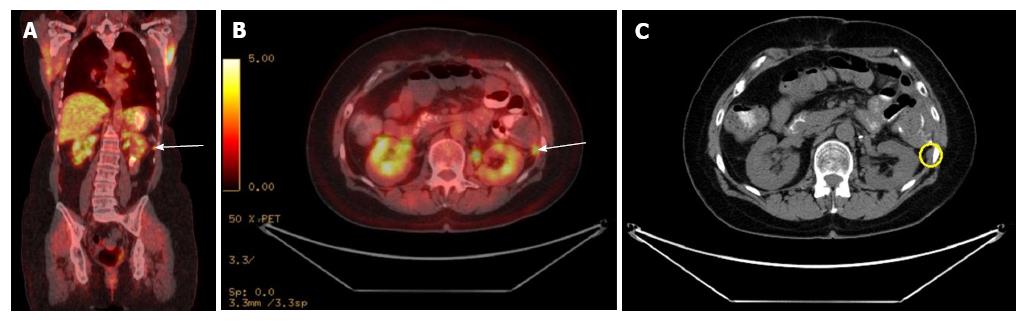
The F-18 fluorodeoxy glucose (FDG) uptake of SPN on positron emission tomography (PET) is not well studied. Dong et al[55] showed that FDG uptake of SPN on PET is related to tumor cellularity, proliferative index or histological malignancy. SPN with a greater proportion of solid component has more FDG uptake than with cystic or hemorrhagic component (Figure 5). Small SPN has more FDG uptake because they have less cystic component. Hence, increased FDG uptake does not necessarily correlate with malignancy. Sato et al[56] suggested that high FDG uptake should be appropriately correlated by use of clinical, lab and radiologic findings. Nakamoto et al[57] evaluated the usefulness of delayed FDG-PET scanning in distinguishing between benign and malignant pancreatic lesions. False positives were seen in patients with acute pancreatitis and autoimmune pancreatitis, but clinical information helped distinguish benign from malignant. They concluded that delayed FDG-PET scanning at 2 h post injection can help differentiate benign from malignant pancreatic lesions when interpreted carefully[57].
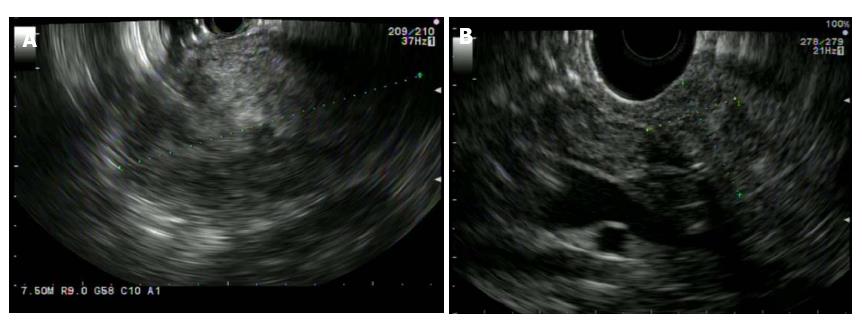
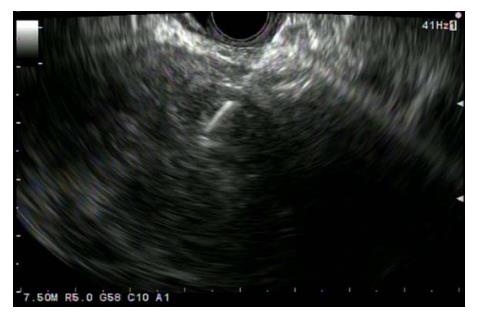
SPN is characterized on EUS as a well circumscribed, hypoechoic, heterogeneous solid mass with cystic, hemorrhagic and calcified components found predominantly on the body and tail of pancreas (Figure 6). EUS-Fine needle aspiration (EUS-FNA) can help in more definitive diagnosis of SPN (Figure 7). It is safe, reliable and can provide cytological specimens preoperatively which can guide targeted surgical approach[58]. Stoita et al[58] showed that a preoperative diagnosis of SPN was achieved from EUS-FNA cytology in 83% (5/6, 1/6 had insufficient sample) of cases. Jani et al[59] conducted a multicenter study on the role of EUS-FNA in preoperative diagnosis of SPN. Results showed that EUS-FNA had 75% (21/28) accuracy. Two/28 had insufficient EUS-FNA sample or acellular for accurate diagnosis. Five/28 were preoperatively misdiagnosed as panNET because of immunostaining results. This study recommends immunohistochemical staining with vimentin, CD10 and beta-catenin for more definitive diagnosis of SPN. EUS-FNA sample with immunohistochemical staining can differentiate SPN, panNET and acinar cell carcinoma[60]. SPN is positive for beta-catenin, vimentin, CD10 and CD56. PanNET is positive for chromogranin [50%-70% of PanNET (functioning and non-functioning)] and synaptophysin. Acinar cell carcinoma is positive for trypsin and chymotrypsin.
EUS-Fine needle biopsy (EUS-FNB) has advantage of providing adequate tissue sample for more accurate diagnosis of SPN. Maimone et al[61] showed that preoperative diagnosis of SPN was achieved in all 5/5 patients. This study concluded that EUS-FNB with ProCore needle (biopsy needle with side fenestrations 19G or 22G) provides good quality and quantity of sample which can increase the yield of preoperative diagnosis of SPN. EUS-FNA can lead to tumor seeding along the needle tract especially in pancreatic adenocarcinoma[62]. Micames et al[63] evaluated the frequency of peritoneal carcinomatosis in non-metastatic pancreatic cancer patients diagnosed with percutaneous FNA vs EUS-FNA. EUS-FNA has lower likelihood of tract seeding compared to percutaneous FNA because of the shorter needle tract. They concluded that EUS-FNA should be the preferred method to obtain tissue sample for potentially resectable localized pancreatic cancer[63]. However, no case reports of tumor seeding with EUS-FNA of SPN were published so far.
Hirooka et al[64] described a case report of peritoneal dissemination after EUS-FNA of IPMN located in pancreatic body. They concluded that pancreatic lesions located in the body/tail have more risk because of needle passage through lesser sac. Kita et al[65] described another case report of needle tract seeding (NTS) in posterior wall of stomach after EUS-FNA of pancreatic cancer located in pancreatic body. They concluded that NTS is rare in pancreatic head lesions as the needle passage is through duodenum and usually they undergo pancreaticoduodenectomy which includes needle tract (duodenal bulb). However, pancreatic lesions located in body/tail have higher risk of NTS as the needle passage is through transgastric (lesser sac) and they usually undergo distal pancreatectomy which doesn’t include needle tract (lesser sac)[65]. Hence, there is more risk of peritoneal dissemination or NTS with transgastric than transduodenal approach.
Sakamoto et al[66] described a case report of NTS after EUS-FNA of pancreatic adenocarcinoma located in pancreatic tail. Also, application of low suction during EUS-FNA decreases the blood contamination and increases the diagnostic accuracy[67]. The technique involves the stylet where it is slowly withdrawn from the needle and in and out motion is performed with in the target lesion. During needle strokes, if the needle is pulled out too far, and/or because of difficult recognition of the sidehole on EUS images, the tissue collected can exit out of the needle hole and there is potential for NTS. Hence, they concluded that slow-pull technique with side-hole needle should be avoided in cases scheduled for resection of pancreatic body/tail cancer to prevent NTS[66].
Surgical resection is the treatment of choice for SPN and organ preservation is advocated if feasible[68]. Distal pancreatectomy with spleen preservation is recommended for SPN located in corpus and tail of the pancreas[69]. Central pancreatectomy with distal pancreatojejunostomy or pancreaticogastrostomy is preferred for SPN located in the neck of the pancreas[70,71]. However, central pancreatectomy is associated with pancreaticoenteric anastomotic leak because of the oversewn proximal pancreatic remnant and distal pancreaticoenteric anastomosis site yielding two potential sources of pancreatic leakage and hence it should be performed in only experienced centers[72]. Pylorus preserving pancreaticoduodenectomy (PPD) is recommended for SPN located in the head of pancreas to decrease dumping syndrome, diarrhea, delayed gastric emptying and marginal ulceration[73]. Enucleation can be done for small SPN distant from pancreatic duct, but it is associated with high risk of pancreatic fistula[70,74]. Spleen preservation is contraindicated if there is splenomegaly, vascular (splenic artery and vein) and hilar involvement[69]. Incidence of lymph node metastasis is very rare in SPN and hence routine lymphadenectomy is not indicated[75]. Enbloc resection with microscopic clear margins is advocated especially when SPN involves portal vein, superior mesenteric vein/artery, spleen, duodenum and for locally progressed[70,76].
Metastasectomy of the liver is advocated at the time of primary resection or even for the recurrences when feasible[68,70,74]. The role of chemotherapy and radiation in SPN is not clear. Surgical treatment of metastasis in SPN is not standardized but debulking can be performed[77]. Hah et al[78] reported an unresectable SPN treated effectively by surgical resection of the primary tumor with preoperative chemotherapy (cisplatinum, ifosfamide, etoposide, and vincristine) followed by intraoperative radiofrequency ablation of metastatic liver lesions. Preoperative chemotherapy with fluorouracil and radiation followed by gemcitabine can be attempted in unresectable SPN to decrease the size of the tumor before definitive surgery[79]. Morikawa et al[80] reported patient treated with paclitaxel after she had recurrent liver and lymph node metastasis 3 mo after surgery. This patient was previously treated with partial liver resection and chemotherapy with S-1 and gemcitabine. The patient was alive after 20 mo of follow up without disease progression[80]. SPN is considered radiosensitive and radiotherapy can be attempted in tumors considered unresectable[81]. Peritoneal carcinomatosis (PC) can occur with intra operative rupture of SPN. Honore et al[82] showed that PC can be successfully treated with complete cytoreductive surgery (CCRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) with irinotecan and oxaliplatin. Extensive liver metastasis from SPN can be treated with trans arterial chemoembolization (TACE). Prasad et al[83] showed TACE with gemcitabine and lipoidal followed by gelfoam embolization is effective in treatment of multiple liver metastasis in SPN.
Postoperative complications include wound infection, bleeding, delayed gastric emptying, pseudomembranous colitis, pneumonitis, steatorrhea, pseudocyst, intestinal obstruction, pancreatic fistula, abscess, portal vein thrombosis and recurrence of the tumor[70,71,75,84,85]. Recurrence of SPN is not uncommon after surgery and it is variable. Papavramidis showed that 31/467 (6.6%) had recurrence after 1-10 years of follow up and the most common sites of metastasis were liver and lymph nodes[47]. Tipton et al[75] showed 2/14 (14%) had recurrence after a median follow up of 3 mo to 20 years. Machado et al[70] showed 2/34 (6%) had recurrence after a mean follow up of 84 mo. Tang et al[24] showed that most commonly metastasize to the liver and peritoneum.
Overall 5-year survival for SPN is about 97%, even in the presence of metastasis[24]. Pathological features indicative of aggressive behavior include diffuse growth pattern, high mitotic activity, nuclear atypia, tumor necrosis and component of sarcomatoid carcinoma[24]. There are no specific guidelines for follow up after surgery but SPN with pathologic features indicative of aggressive behavior may require extended period of follow up. Estrella et al[86] showed that muscular vessel invasion (tumor cells in the luminal spaces of blood vessels with circumferential smooth muscle layers), tumor (T) stage by European Neuroendocrine tumors society (ENETS) classification, ENETS stage grouping and stage grouping by the American joint committee on cancer (AJCC) were important predictors in disease specific survival of patients with SPN after surgical resection[86]. Recurrence rate was 5/39 (13%) after a median follow up of 76 mo. Ten-year disease specific survival was 96% and metastatic/recurrent disease was significantly associated with large tumor size (P < 0.001)[86]. Butte et al[7] showed that tumor size at presentation in SPN is associated with malignancy. Kato et al[87] showed that tumor doubling time of SPN according to the formula of Schwartz and coworkers is 765 d.
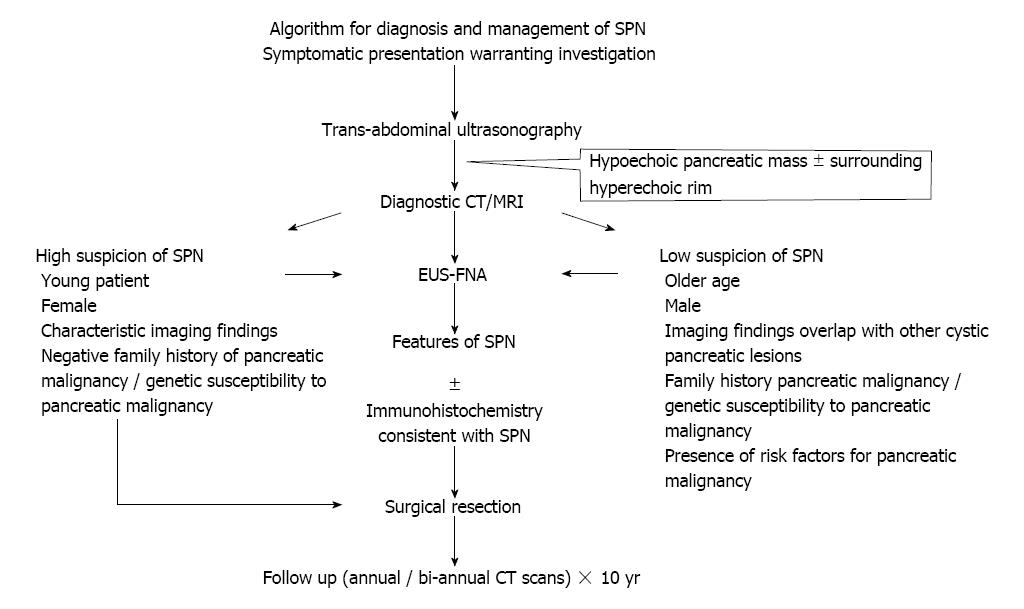
To our knowledge, we came across one review article from our literature search, which summarized the molecular pathogenesis and clinical features of SPN[88]. In our review article, we proposed algorithms for pathogenesis and management in addition to histology and morphology, immunohistochemistry, clinical presentation and diagnosis of SPN using currently available literature. We discussed EUS-FNA and EUS-FNB in preoperative diagnosis of SPN. SOX11, LEF1, TFE3, and AR that can be putative diagnostic markers in SPNs are discussed. Overall, this review article is comprehensive and guides in the management of SPN (Figure 8).
Over the last decade, there has been a tremendous increase in the number of SPN cases reported in the literature. A diagnosis of SPN inferred from imaging studies can be adequate to guide surgical resection without preoperative pathological assessment. Nonetheless, EUS-FNA with immunohistochemistry helps in establishing a preoperative diagnosis. A multidisciplinary team approach involving the radiologist, endoscopist, pathologist, oncologist as well as the surgeon improves treatment accuracy and helps in effective management of SPN. Surgical resection should be offered when feasible. Referral to experienced centers for surgery can minimize complications. Future studies should standardize the follow up duration, and the frequency as well as type of diagnostic imaging for surveillance after surgical resection. Overall prognosis of SPN is excellent even in the presence of metastasis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Fujino Y, Hanazaki K, Sakaguchi T, Tsolakis AV S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 2. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 346] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651-7; discussion 657-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131-136. [PubMed] [Cited in This Article: ] |
| 5. | Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Bouassida M, Mighri MM, Bacha D, Chtourou MF, Touinsi H, Azzouz MM, Sassi S. Solid pseudopapillary neoplasm of the pancreas in an old man: age does not matter. Pan Afr Med J. 2012;13:8. [PubMed] [Cited in This Article: ] |
| 7. | Butte JM, Brennan MF, Gönen M, Tang LH, D’Angelica MI, Fong Y, Dematteo RP, Jarnagin WR, Allen PJ. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15:350-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Reddy S, Cameron JL, Scudiere J, Hruban RH, Fishman EK, Ahuja N, Pawlik TM, Edil BH, Schulick RD, Wolfgang CL. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg. 2009;208:950-957; discussion 957-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Serra S, Chetty R. Revision 2: an immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas. J Clin Pathol. 2008;61:1153-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 11. | Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401-8404. [PubMed] [Cited in This Article: ] |
| 12. | Müller-Höcker J, Zietz CH, Sendelhofert A. Deregulated expression of cell cycle-associated proteins in solid pseudopapillary tumor of the pancreas. Mod Pathol. 2001;14:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Vlaeminck-Guillem V, Carrere S, Dewitte F, Stehelin D, Desbiens X, Duterque-Coquillaud M. The Ets family member Erg gene is expressed in mesodermal tissues and neural crests at fundamental steps during mouse embryogenesis. Mech Dev. 2000;91:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Mager AM, Grapin-Botton A, Ladjali K, Meyer D, Wolff CM, Stiegler P, Bonnin MA, Remy P. The avian fli gene is specifically expressed during embryogenesis in a subset of neural crest cells giving rise to mesenchyme. Int J Dev Biol. 1998;42:561-572. [PubMed] [Cited in This Article: ] |
| 16. | Tiemann K, Kosmahl M, Ohlendorf J, Krams M, Klöppel G. Solid pseudopapillary neoplasms of the pancreas are associated with FLI-1 expression, but not with EWS/FLI-1 translocation. Mod Pathol. 2006;19:1409-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Tiemann K, Heitling U, Kosmahl M, Klöppel G. Solid- pseudopapillary neoplasms of the pancreas show an interruption of the Wnt-signaling pathway and express gene products of 11q. Mod Pathol. 2007;20:955-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol. 1997;11:1593-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Said TK, Conneely OM, Medina D, O’Malley BW, Lydon JP. Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology. 1997;138:3933-3939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Adachi S, Jigami T, Yasui T, Nakano T, Ohwada S, Omori Y, Sugano S, Ohkawara B, Shibuya H, Nakamura T. Role of a BCL9-related beta-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res. 2004;64:8496-8501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Hallas C, Phillipp J, Domanowsky L, Kah B, Tiemann K. BCL9L expression in pancreatic neoplasia with a focus on SPN: a possible explanation for the enigma of the benign neoplasia. BMC Cancer. 2016;16:648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Cai H, Zhou M, Hu Y, He H, Chen J, Tian W, Deng Y. Solid-pseudopapillary neoplasms of the pancreas: clinical and pathological features of 33 cases. Surg Today. 2013;43:148-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Meriden Z, Shi C, Edil BH, Ellison T, Wolfgang CL, Cornish TC, Schulick RD, Hruban RH. Hyaline globules in neuroendocrine and solid-pseudopapillary neoplasms of the pancreas: a clue to the diagnosis. Am J Surg Pathol. 2011;35:981-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Schlosnagle DC, Campbell WG Jr. The papillary and solid neoplasm of the pancreas: a report of two cases with electron microscopy, one containing neurosecretory granules. Cancer. 1981;47:2603-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 27. | Ye J, Ma M, Cheng D, Yuan F, Deng X, Zhan Q, Shen B, Peng C. Solid-pseudopapillary tumor of the pancreas: clinical features, pathological characteristics, and origin. J Surg Oncol. 2012;106:728-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Tang WW, Stelter AA, French S, Shen S, Qiu S, Venegas R, Wen J, Wang HQ, Xie J. Loss of cell-adhesion molecule complexes in solid pseudopapillary tumor of pancreas. Mod Pathol. 2007;20:509-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ohara Y, Oda T, Hashimoto S, Akashi Y, Miyamoto R, Enomoto T, Satomi K, Morishita Y, Ohkohchi N. Pancreatic neuroendocrine tumor and solid-pseudopapillary neoplasm: Key immunohistochemical profiles for differential diagnosis. World J Gastroenterol. 2016;22:8596-8604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 31. | Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052-1070. [PubMed] [Cited in This Article: ] |
| 32. | Yang F, Yu X, Bao Y, Du Z, Jin C, Fu D. Prognostic value of Ki-67 in solid pseudopapillary tumor of the pancreas: Huashan experience and systematic review of the literature. Surgery. 2016;159:1023-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 33. | Vilar E, Salazar R, Pérez-García J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer. 2007;14:221-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Kim EK, Jang M, Park M, Kim H. LEF1, TFE3, and AR are putative diagnostic markers of solid pseudopapillary neoplasms. Oncotarget. 2017;8:93404-93413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Singhi AD, Lilo M, Hruban RH, Cressman KL, Fuhrer K, Seethala RR. Overexpression of lymphoid enhancer-binding factor 1 (LEF1) in solid-pseudopapillary neoplasms of the pancreas. Mod Pathol. 2014;27:1355-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 570] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 37. | Park M, Kim M, Hwang D, Park M, Kim WK, Kim SK, Shin J, Park ES, Kang CM, Paik YK. Characterization of gene expression and activated signaling pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol. 2014;27:580-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Julian LM, McDonald AC, Stanford WL. Direct reprogramming with SOX factors: masters of cell fate. Curr Opin Genet Dev. 2017;46:24-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Harrison G, Hemmerich A, Guy C, Perkinson K, Fleming D, McCall S, Cardona D, Zhang X. Overexpression of SOX11 and TFE3 in Solid-Pseudopapillary Neoplasms of the Pancreas. Am J Clin Pathol. 2017;149:67-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Foo WC, Harrison G, Zhang X. Immunocytochemistry for SOX-11 and TFE3 as diagnostic markers for solid pseudopapillary neoplasms of the pancreas in FNA biopsies. Cancer Cytopathol. 2017;125:831-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Mirminachi B, Farrokhzad S, Sharifi AH, Nikfam S, Nikmanesh A, Malekzadeh R, Pourshams A. Solid Pseudopapillary Neoplasm of Pancreas; A Case Series and Review Literature. Middle East J Dig Dis. 2016;8:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Belletrutti PJ, Allen PJ, Kurtz RC, DiMaio CJ. Education and imaging. Hepatobiliary and pancreatic: Recurrent pancreatitis caused by a solid pseudopapillary neoplasm of the pancreas. J Gastroenterol Hepatol. 2011;26:787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Zhu H, Xia D, Wang B, Meng H. Extrapancreatic solid pseudopapillary neoplasm: Report of a case of primary retroperitoneal origin and review of the literature. Oncol Lett. 2013;5:1501-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Takahashi Y, Fukusato T, Aita K, Toida S, Fukushima J, Imamura T, Tanaka F, Amano H, Takada T, Mori S. Solid pseudopapillary tumor of the pancreas with metastases to the lung and liver. Pathol Int. 2005;55:792-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Park JK, Cho EJ, Ryu JK, Kim YT, Yoon YB. Natural history and malignant risk factors of solid pseudopapillary tumors of the pancreas. Postgrad Med. 2013;125:92-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Ng KH, Tan PH, Thng CH, Ooi LL. Solid pseudopapillary tumour of the pancreas. ANZ J Surg. 2003;73:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 495] [Article Influence: 26.1] [Reference Citation Analysis (1)] |
| 48. | Huang Y, Feng JF. Clinicopathologic characteristics and surgical treatment of solid pseudopapillary tumor of the pancreas. Hippokratia. 2013;17:68-72. [PubMed] [Cited in This Article: ] |
| 49. | Jiang L, Cui L, Wang J, Chen W, Miao L, Jia J. Solid pseudopapillary tumors of the pancreas: Findings from routine screening sonographic examination and the value of contrast-enhanced ultrasound. J Clin Ultrasound. 2015;43:277-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, Chung JJ, Yoo HS, Lee JT, Kim KW. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178-W186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 231] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH, Lee JY, Han JK, Choi BI. Small (<or=3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010;257:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Park MJ, Lee JH, Kim JK, Kim YC, Park MS, Yu JS, Kim YB, Lee D. Multidetector CT imaging features of solid pseudopapillary tumours of the pancreas in male patients: distinctive imaging features with female patients. Br J Radiol. 2014;87:20130513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: emphasis on magnetic resonance imaging findings. World J Gastroenterol. 2007;13:1811-1815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Dong A, Wang Y, Dong H, Zhang J, Cheng C, Zuo C. FDG PET/CT findings of solid pseudopapillary tumor of the pancreas with CT and MRI correlation. Clin Nucl Med. 2013;38:e118-e124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Sato M, Takasaka I, Okumura T, Shioyama Y, Kawasaki H, Mise Y, Asato Y, Yoshimi F, Imura J, Nakajima K. High F-18 fluorodeoxyglucose accumulation in solid pseudo-papillary tumors of the pancreas. Ann Nucl Med. 2006;20:431-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, Hosotani R, Imamura M, Konishi J. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000;89:2547-2554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 58. | Stoita A, Earls P, Williams D. Pancreatic solid pseudopapillary tumours - EUS FNA is the ideal tool for diagnosis. ANZ J Surg. 2010;80:615-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, Brugge W, Lee K, Khalid A, McGrath K. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy. 2008;40:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Hosokawa I, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Takayashiki T, Ishihara T, Yokosuka O, Miyazaki M. Preoperative diagnosis and surgical management for solid pseudopapillary neoplasm of the pancreas. J Hepatobiliary Pancreat Sci. 2014;21:573-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Maimone A, Luigiano C, Baccarini P, Fornelli A, Cennamo V, Polifemo A, Fiscaletti M, de Biase D, Jaboli F, Virgilio C. Preoperative diagnosis of a solid pseudopapillary tumour of the pancreas by Endoscopic Ultrasound Fine Needle Biopsy: A retrospective case series. Dig Liver Dis. 2013;45:957-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 64. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Kita E, Yamaguchi T, Sudo K. A case of needle tract seeding after EUS-guided FNA in pancreatic cancer, detected by serial positron emission tomography/CT. Gastrointest Endosc. 2016;84:869-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Sakamoto U, Fukuba N, Ishihara S, Sumi S, Okada M, Sonoyama H, Ohshima N, Moriyama I, Kawashima K, Kinoshita Y. Postoperative recurrence from tract seeding after use of EUS-FNA for preoperative diagnosis of cancer in pancreatic tail. Clin J Gastroenterol. 2018;11:200-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Chen JY, Ding QY, Lv Y, Guo W, Zhi FC, Liu SD, Cheng TM. Slow-pull and different conventional suction techniques in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid lesions using 22-gauge needles. World J Gastroenterol. 2016;22:8790-8797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 39] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Romics L Jr, Oláh A, Belágyi T, Hajdú N, Gyurus P, Ruszinkó V. Solid pseudopapillary neoplasm of the pancreas--proposed algorithms for diagnosis and surgical treatment. Langenbecks Arch Surg. 2010;395:747-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | de Castro SM, Singhal D, Aronson DC, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Management of solid-pseudopapillary neoplasms of the pancreas: a comparison with standard pancreatic neoplasms. World J Surg. 2007;31:1130-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Panieri E, Krige JE, Bornman PC, Graham SM, Terblanche J, Cruse JP. Operative management of papillary cystic neoplasms of the pancreas. J Am Coll Surg. 1998;186:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Efron DT, Lillemoe KD, Cameron JL, Yeo CJ. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg. 2004;8:532-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Grace PA, Pitt HA, Longmire WP. Pylorus preserving pancreatoduodenectomy: an overview. Br J Surg. 1990;77:968-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999;23:1045-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 76. | Vollmer CM Jr, Dixon E, Grant DR. Management of a solid pseudopapillary tumor of the pancreas with liver metastases. HPB (Oxford). 2003;5:264-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Rebhandl W, Felberbauer FX, Puig S, Paya K, Hochschorner S, Barlan M, Horcher E. Solid-pseudopapillary tumor of the pancreas (Frantz tumor) in children: report of four cases and review of the literature. J Surg Oncol. 2001;76:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Hah JO, Park WK, Lee NH, Choi JH. Preoperative chemotherapy and intraoperative radiofrequency ablation for unresectable solid pseudopapillary tumor of the pancreas. J Pediatr Hematol Oncol. 2007;29:851-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Maffuz A, Bustamante Fde T, Silva JA, Torres-Vargas S. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2005;6:185-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 67] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 80. | Morikawa T, Onogawa T, Maeda S, Takadate T, Shirasaki K, Yoshida H, Ishida K, Motoi F, Naitoh T, Rikiyama T. Solid pseudopapillary neoplasms of the pancreas: an 18-year experience at a single Japanese Institution. Surg Today. 2013;43:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Fried P, Cooper J, Balthazar E, Fazzini E, Newall J. A role for radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer. 1985;56:2783-2785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 82. | Honore C, Goere D, Dartigues P, Burtin P, Dumont F, Elias D. Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz’s tumour) of the pancreas treated with HIPEC. Anticancer Res. 2012;32:1069-1073. [PubMed] [Cited in This Article: ] |
| 83. | Prasad TV, Madhusudhan KS, Srivastava DN, Dash NR, Gupta AK. Transarterial chemoembolization for liver metastases from solid pseudopapillary epithelial neoplasm of pancreas: A case report. World J Radiol. 2015;7:61-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Ren Z, Zhang P, Zhang X, Liu B. Solid pseudopapillary neoplasms of the pancreas: clinicopathologic features and surgical treatment of 19 cases. Int J Clin Exp Pathol. 2014;7:6889-6897. [PubMed] [Cited in This Article: ] |
| 85. | Kim MJ, Choi DW, Choi SH, Heo JS, Sung JY. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg. 2014;101:1266-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Estrella JS, Li L, Rashid A, Wang H, Katz MH, Fleming JB, Abbruzzese JL, Wang H. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol. 2014;38:147-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Kato T, Egawa N, Kamisawa T, Tu Y, Sanaka M, Sakaki N, Okamoto A, Bando N, Funata N, Isoyama T. A case of solid pseudopapillary neoplasm of the pancreas and tumor doubling time. Pancreatology. 2002;2:495-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Reddy S, Wolfgang CL. Solid pseudopapillary neoplasms of the pancreas. Adv Surg. 2009;43:269-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |