Published online Jul 16, 2018. doi: 10.4253/wjge.v10.i7.125
Peer-review started: March 23, 2018
First decision: April 13, 2018
Revised: April 27, 2018
Accepted: June 8, 2018
Article in press: June 9, 2018
Published online: July 16, 2018
Pancreatic cysts are increasingly diagnosed due to expanding use of cross-sectional imaging, but current diagnostic modalities have limited diagnostic accuracy. Recently, a novel through-the-needle microbiopsy forceps has become available, offering the possibility of obtaining cyst-wall biopsies. We present a case of 41-year-old male with chronic pancreatitis and a 2-cm pancreatic cyst, initially considered a pseudocyst. Subsequently, endoscopic ultrasound guided microbiopsies were successfully obtained, which surprisingly revealed an intraductal papillary mucinous neoplasm of mixed subtype with low grade dysplasia. In conclusion, obtaining biopsies from the wall of the pancreatic cystic lesions with this novel instrument is feasible and, as demonstrated in this case, can possibly alter the clinical outcome. Microbiopsies offered enough cellular material, allowing supplemental gene mutation analysis, which combined with other modalities could lead to a more individual approach when treating pancreatic cysts. However, prospective studies are warranted before routine clinical implementation.
Core tip: We present a case of a pancreatic cyst with an initial diagnosis of a pseudocyst altered to an intraductal papillary mucinous neoplasm of mixed type with low grade dysplasia through the use of endoscopic ultrasound guided microbiopsies obtained with a novel through-the-needle microbiopsy forceps, rendering the possibility of microscopic evaluation and genetic analysis of the cyst.
- Citation: Rift CV, Kovacevic B, Karstensen JG, Plougmann J, Klausen P, Toxværd A, Kalaitzakis E, Hansen CP, Hasselby JP, Vilmann P. Diagnosis of intraductal papillary mucinous neoplasm using endoscopic ultrasound guided microbiopsies: A case report. World J Gastrointest Endosc 2018; 10(7): 125-129
- URL: https://www.wjgnet.com/1948-5190/full/v10/i7/125.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i7.125
Pancreatic cysts are increasingly diagnosed due to expanding use of cross-sectional imaging[1]. Whereas some of the cysts are completely benign, others are considered malignant or pre-malignant [intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasia (MCN)]. Endoscopic ultrasound (EUS) represents a cornerstone in preoperative diagnosis of these cysts, but cannot be used as a stand-alone modality[2]. EUS-guided fine needle aspiration (EUS-FNA) cytology has a relatively high specificity of 90.6%, but low sensitivity (64.8%), which is mainly due to absence of sufficient cellular material in the cyst fluid for a definite diagnosis[3,4]. A novel through-the-needle microbiopsy forceps (Moray™, US Endoscopy, Mentor, United States) has recently become available, offering a possibility of obtaining cyst-wall biopsies[5].
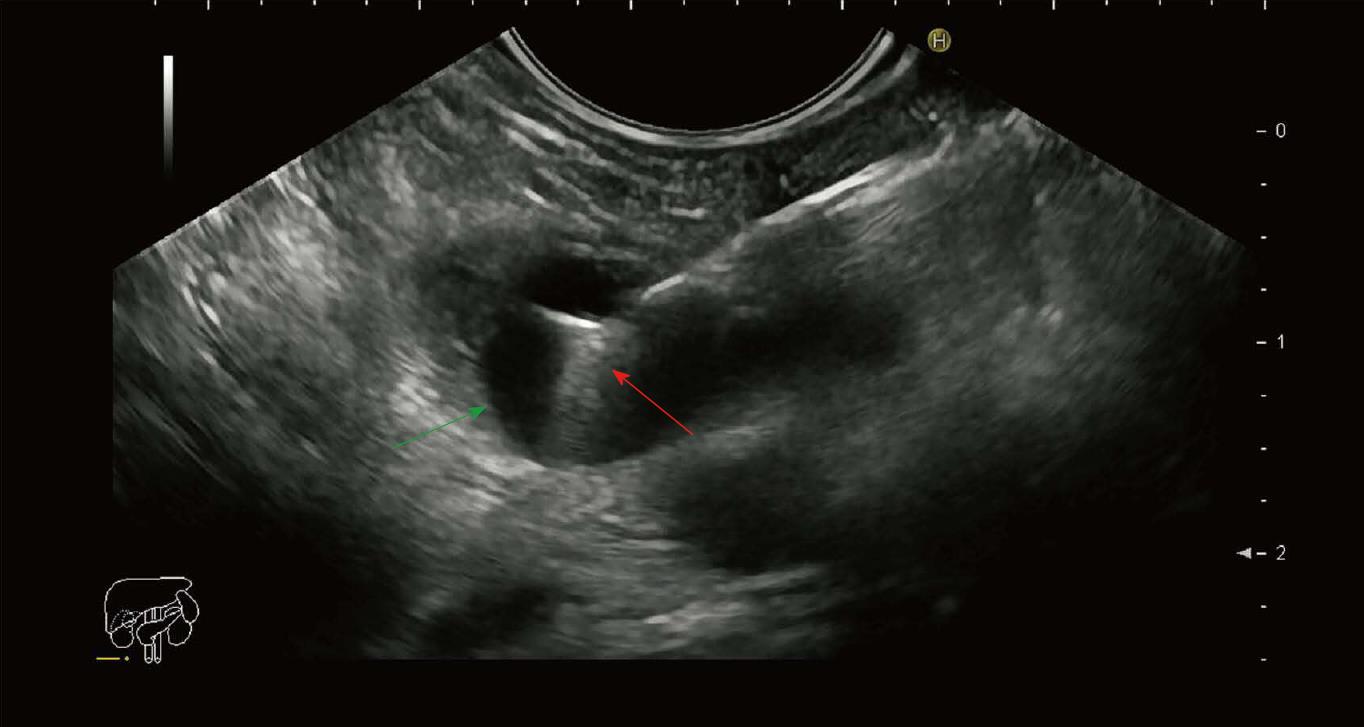
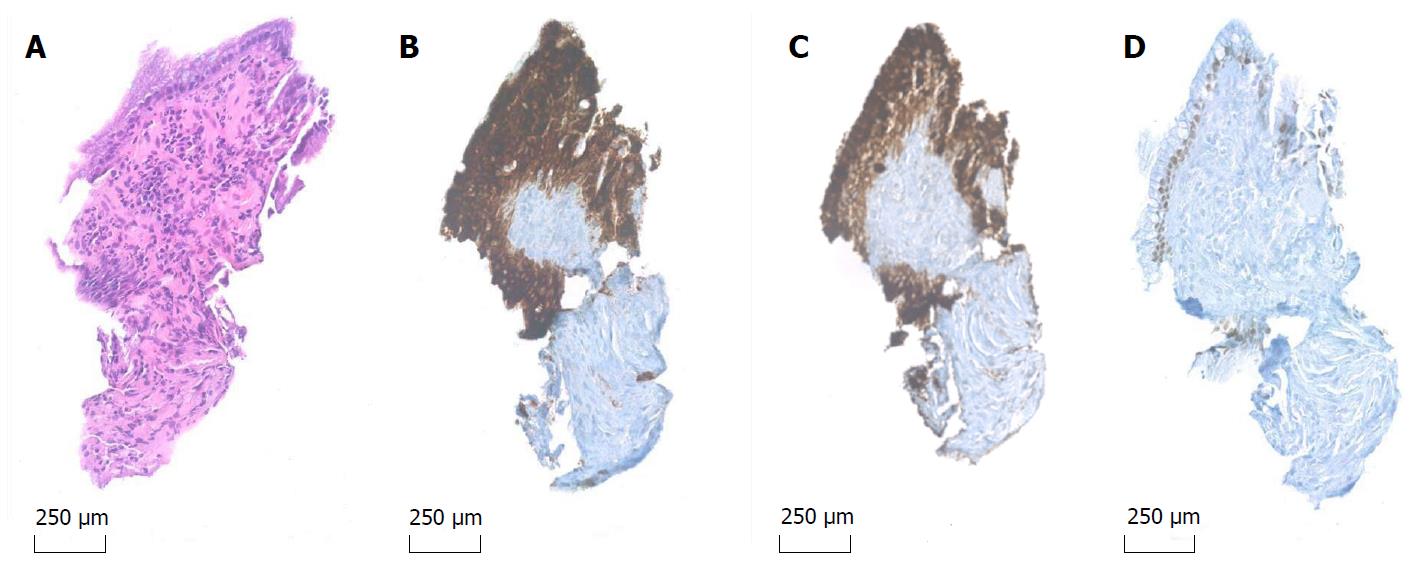
A 41-year-old male, with substantial alcohol abuse and chronic pancreatitis, was referred to the gastroenterological department due to long-lasting abdominal pain and a 2 cm cyst located in the body of the pancreas. After an initial EUS and subsequent multi-disciplinary conference, the lesion was classified as a pseudocyst, which was considered asymptomatic due to its small size. Due to persisting symptoms and the fact that the cyst had not decreased in size, another EUS was performed six months later, and the cyst was punctured through the stomach wall with a 19 Gauge needle (Expect-Flex™, Boston Scientific, Marlborough, United States). Subsequently, the Moray™ micro-biopsy forceps was introduced though the needle and two microbiopsies from the cyst wall were obtained (Figure 1). Lastly, the cyst was drained and the fluid was sent for carcinoembryonic antigen (CEA) analysis. The total procedural time was 12 min and no adverse events were observed. Cyst-fluid CEA value was 12 µg/L. The microbiopsies were fixed in formalin and processed for histology and immunohistochemical (IHC) staining for MUC1, MUC2, MUC6, MUC5AC, and CDX2. Surprisingly, the biopsies revealed fragments of mucinous epithelium with goblet cells. The nuclei where found basally oriented with focally distinct nucleoli. No mitoses where present. The epithelial cells where positive for MUC1 and MUC5AC and focally positive for CDX2 and MUC2. The underlying stroma consisted of fibroblasts and collagen tissue without any bleeding. Conclusively, the features seen on the biopsies were consistent with IPMN of mixed type: pancreatobiliary and intestinal subtype. Subsequently, the biopsies were examined by next generation sequencing (NGS) using the Ion AmpliSeq Cancer Hotspot Panel v2 (Life Technologies, Carlsbad, United States). The multigene panel explores selected regions of 50 cancer-associated genes, among others KRAS, GNAS, CDKN2A and SMAD4 genes. We were able to obtain adequate and fine quality DNA for NGS analysis, which was performed with approximately 2 million mapped reads and a uniformity of about 97%. No mutations were found. The patient was planned for follow-up in 6 mo.
Obtaining biopsies from the wall of the pancreatic cystic lesions with the use of a through-the-needle microbiopsy forceps seems feasible. The jaws of the forceps with an opening width of 4.3 mm are easily identified on EUS and as such can be guided to obtain biopsies from different areas of interest (Figure 1). Needle-based confocal laser endomicroscopy (nCLE) is a comparative technique, also used in conjunction with a 19G needle. Several studies have shown that the procedure has a high specificity (close to 100%), but the rather low sensitivity of approximately 60% for predefined epithelial structures[6,7]. There are currently no data on the efficacy of nCLE to predict dysplasia. On the other hand, acquirement of tissue samples with the use of the microbiopsy forceps enables further diagnostic possibilities (e.g., immunohistochemistry, next-generation sequencing). Conventional EUS-FNA is considered safe with a low associated risk of hemorrhage (0.69%)[8]. Furthermore, most cases of intracystic hemorrhage resolve spontaneously and do not require further management. Microbiopsy forceps procedure appears safe with no adverse events reported, although the use has been limited[9,10]. This novel instrument requires the use of a larger 19G needle, which possibly is associated with a higher risk of adverse events[8]. This should be however seen in the light of an increased diagnostic yield. Obtaining samples from lesions in the head of the pancreas or the uncinate process can be technically challenging with a 19G needle, especially when the forceps is introduced. However, the procedure seems to be associated with rather high technical success rates[9,10].
In this case, the biopsies offered adequate tissue for histology and IHC staining and secured a diagnosis of IPMN with low grade dysplasia. The lesion was presumably of a mixed type: Pancreatobiliary subtype due to positivity in MUC1 and MUC5AC stains and the intestinal subtype due to positivity in CDX2 and MUC2 stains with the presence of goblet cells (Figure 2). When compared to gastric and oncocytic subtypes, pancreatobiliary and intestinal subtypes are associated with progression to high grade dysplasia and invasive carcinoma[11]. Previously, surgical series have shown that the clinical behavior of an invasive carcinoma derived from the pancreatobiliary type IPMN has a significantly poorer prognosis than those associated with the intestinal subtype[12,13]. On the other hand no mutations in genes controlling cell cycle and arrest (KRAS, CDKN2A, SMAD4, PIK3CA) or DNA repair (TP53) were found, the presence of which would also have rendered a poorer prognosis[14]. Even though GNAS and concomitant KRAS mutations is considered specific of IPMN, the sensitivity is low[15], as to why a negative result of the NGS analysis does not rule out underlying pathology as seen in this case.
As a new diagnostic tool, the Moray™ microbiopsy forceps offers new diagnostic challenges for both endoscopists and pathologists, one of these being the issue of contamination of the tissue obtained by the microbiopsy forceps during the procedure. As EUS-guided FNA-needle either passes through the duodenum or the stomach, contamination with gastric or duodenal epithelium is possible and commonly seen in cytology specimens. In this case the cyst was punctured through the stomach wall, indicating the presence of goblet cells in the microbiopsy is not due to contamination from the duodenum. The endoscopist should therefore always note the location of the echo-endoscope when puncturing the lesion. Nuclear atypia such as high nuclear to cytoplasmic ratio, irregular nuclear membranes, and prominent nucleoli are also helpful characteristics for the pathologist to distinguish contamination from neoplasia in cytology specimens[16] and is by our experience applicable in the interpretation of microbiopsies.
Although calcification of the pancreas is usually considered to be one of the signs of chronic pancreatitis, it can also be associated with IPMN[17,18]. The exact pathogenic mechanism is unknown, but it is believed that extensive mucus production causes obstructing pancreatitis and formation of calcifications, the presence of which might lead to diagnostic confusion[19]. In the case mentioned above, the microbiopsy forceps has not only contributed to additional diagnostic information, but has also altered the initial diagnosis from a benign pseudocyst to a side branch IPMN with low grade dysplasia, causing a significant change in the treatment. Taken the patient’s history, EUS characteristics with no dilated pancreatic duct, and low CEA-concentration in the cyst fluid into account, without the micro-biopsies, no further follow-up would be instigated. After a multidisciplinary conference, the patient was instead referred for a surveillance program. Even though the use of the microbiopsy forceps seems feasible, at present there is only limited clinical experience with this instrument and further studies are warranted in order to determine its diagnostic value in pancreatic cysts.
A 41-year-old male with chronic pancreatitis and abdominal pain.
A 2 cm cyst located in the body of the pancreas.
Mucinous cyst.
A low level of cyst fluid carcinoembryonic antigen.
Endoscopic ultrasound suggested a pseudocyst.
Microbiopsies yielded the diagnosis of an intraductal papillary mucinous neoplasm of mixed type with low grade dysplasia.
Clinical follow-up.
Pancreatic cysts are increasingly diagnosed due to expanding use of cross-sectional imaging. Endoscopic ultrasound cannot be used as a stand-alone modality.
The wall of a pseudocyst has no epithelial lining, as to why presence of epithelial cells excludes the diagnosis of a pseudocyst.
Obtaining microbiopsies from the wall of a pancreatic cyst and subsequently performing microscopic evaluation as well as NGS-analysis has to our best knowledge not previously been reported. The technique seems feasible and, as demonstrated in this case, can possibly alter the clinical outcome.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript Source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hoff DAL, Krishna SG S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 409] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Suzuki R, Thosani N, Annangi S, Guha S, Bhutani MS. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: a systematic review and meta-analysis. Pancreatology. 2014;14:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Woolf KM, Liang H, Sletten ZJ, Russell DK, Bonfiglio TA, Zhou Z. False-negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: one institution’s experience. Cancer Cytopathol. 2013;121:449-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Oppong KW, Dawwas MF, Charnley RM, Wadehra V, Elamin K, White S, Nayar M. EUS and EUS-FNA diagnosis of suspected pancreatic cystic neoplasms: Is the sum of the parts greater than the CEA? Pancreatology. 2015;15:531-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Shakhatreh MH, Naini SR, Brijbassie AA, Grider DJ, Shen P, Yeaton P. Use of a novel through-the-needle biopsy forceps in endoscopic ultrasound. Endosc Int Open. 2016;4:E439-E442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Kadayifci A, Atar M, Basar O, Forcione DG, Brugge WR. Needle-Based Confocal Laser Endomicroscopy for Evaluation of Cystic Neoplasms of the Pancreas. Dig Dis Sci. 2017;62:1346-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Zhu H, Jiang F, Zhu J, Du Y, Jin Z, Li Z. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29:667-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Mittal C, Obuch JC, Hammad H, Edmundowicz SA, Wani S, Shah RJ, Brauer BC, Attwell AR, Kaplan JB, Wagh MS. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video). Gastrointest Endosc. 2018;87:1263-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Zhang ML, Arpin RN, Brugge WR, Forcione DG, Basar O, Pitman MB. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 393] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Kim J, Jang KT, Mo Park S, Lim SW, Kim JH, Lee KH, Lee JK, Heo JS, Choi SH, Choi DW. Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol. 2011;32:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Sadakari Y, Ohuchida K, Nakata K, Ohtsuka T, Aishima S, Takahata S, Nakamura M, Mizumoto K, Tanaka M. Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery. 2010;147:812-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Kuboki Y, Shimizu K, Hatori T, Yamamoto M, Shibata N, Shiratori K, Furukawa T. Molecular biomarkers for progression of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2015;44:227-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 16. | Layfield LJ, Jarboe EA. Cytopathology of the pancreas: neoplastic and nonneoplastic entities. Ann Diagn Pathol. 2010;14:140-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Origuchi N, Kimura W, Muto T, Esaki Y. Pancreatic mucin-producing adenocarcinoma associated with a pancreatic stone: report of a case. Surg Today. 1998;28:1261-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Zapiach M, Yadav D, Smyrk TC, Fletcher JG, Pearson RK, Clain JE, Farnell MB, Chari ST. Calcifying obstructive pancreatitis: a study of intraductal papillary mucinous neoplasm associated with pancreatic calcification. Clin Gastroenterol Hepatol. 2004;2:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kalaitzakis E, Braden B, Trivedi P, Sharifi Y, Chapman R. Intraductal papillary mucinous neoplasm in chronic calcifying pancreatitis: egg or hen? World J Gastroenterol. 2009;15:1273-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |