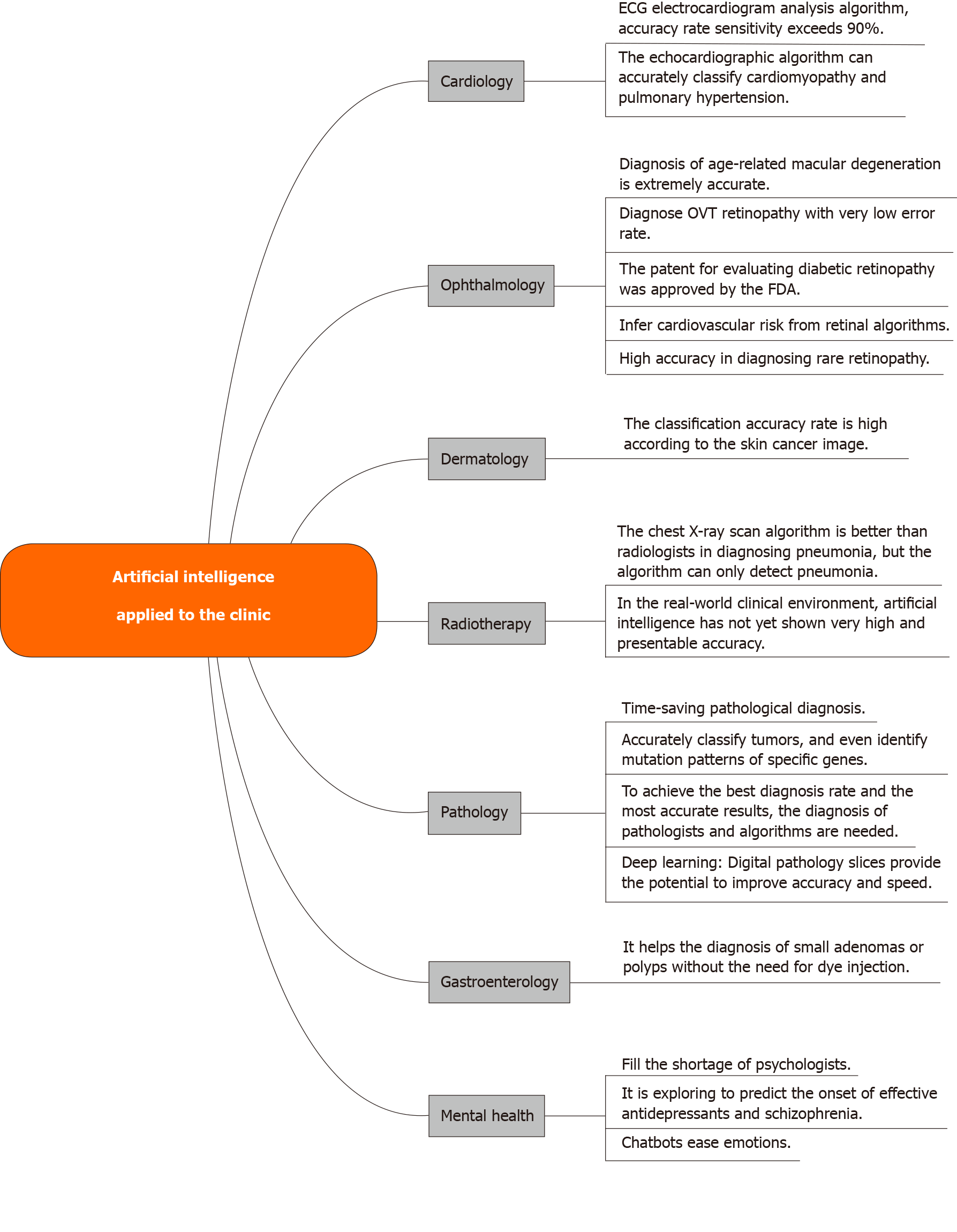
INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer death worldwide. The incidence of GC in East Asia has increased significantly in recent years[1], ranking second in incidence in China, representing the most common cause of cancer death[2]. In recent years, with the transformation of information technology, AI (AI) technology is gradually becoming an alternative to traditional technology or an integral part of an integrated system. AI has been used to solve complex practical problems in various fields and is becoming more and more popular today[3]. AI can learn from examples, has certain fault tolerance, can deal with noisy data and incomplete data, can deal with nonlinear problems, and can be predicted and summarized at high speed once it has been trained. AI-based systems are being widely developed and deployed worldwide, mainly because of their symbolic reasoning, flexibility, and interpretation capabilities. Thanks to the rapid development of large amounts of labeled data and computers, AI, especially deep learning, has begun to penetrate the medical field. AI is of great significance to medicine and has been partially applied in clinic. Topol[4] enumerates and analyzes the main aspects and functions of AI in clinical application at present (refer to Figure 1 for details). AI can make accurate judgments on diseases through large-scale learning, and can assist clinicians in the diagnosis and treatment of GC. As such, AI-assisted diagnosis has become an important direction for the diagnosis of GC.
Figure 1 Artificial intelligence applied to the clinic.
APPLICATION OF AI IN GASTROSCOPE
Gastrointestinal endoscopy is the most important and potential direction for AI-assisted diagnosis. In previous studies, much of the initial work of endoscopic AI technology has focused on the detection and optical diagnosis of colonic polyps[5]. Esophagogastric duodenoscope (EGD) is widely regarded as one of the standard methods for diagnosing gastric diseases. However, a study[6] has shown that the missed rate of endoscopy in the 3 years before diagnosis of gastrointestinal tumors is 11.3%. Two other studies[7,8] showed that the proportion of missed GCs was 9.4% and 25.8%. AI-based detection’s potential usefulness in GC was first reported by Hirasawa et al[9]. For gastroenterology, AI is another important direction for the diagnosis of GC.
Application of AI in the diagnosis of early GC by endoscopy
Topol[4] thinks that AI can be help make clinical diagnosis fast and accurate, optimizing processes in the health-care system to reduce diagnostic errors and malpractice. More than this, it can benefit the patient's daily life, helping in observing and analyzing their health data to accelerate rehabilitation. Gastrointestinal endoscopy is an important and rapidly developing research field in the application of AI in gastrointestinal surgery, specifically in the diagnosis and treatment of early cancer. Endoscopic submucosal dissection (referred to as ESD) and endoscopic mucosal resection (referred to as EMR) are considered to be the most beneficial procedures for patients with early GC (EGC), and surgical treatment is considered when endoscopic treatment is not possible. The risk of lymph node metastasis in the mucosal layer (referred to here as “M”)/shallow submucosal layer (referred to here as “SM1”; < 500 mm from the muscularis mucosa) is very low but the potential of metastasis in the deep submucosal layer (referred to here as “SM2”; > 500 mm invasion) is quite high. As usual, for patients with EGC and an infiltration depth greater than 500 mm, surgery is considered the first choice. However, for patients with EGC whose depth of invasion is limited to the M or superficial submucosa (~ 500 mm from the muscularis mucosa), ESD/EMR should be provided.
The accuracy of endoscopists in using endoscopy, endoscopic ultrasonography, or both to predict the depth of invasion was only 69% to 85% in previous studies[10]. Therefore, it is an important clinical problem to accurately predict the invasion depth of EGC. Research has shown that machine vision can interpret specific medical images more accurately and faster than humans using high magnification[11]. In a separate study that is more accurate, Zhu et al[12] report significant progress in the use of endoscopy in EGC. They developed and validated an AI system model that uses deep learning algorithms to determine the depth of invasion of EGC. The model is called a convolutional neural network computer-aided detection (CNN-CAD) system, which can determine whether the intrusion depth is "M/SM1" and "SM2" or deeper. In the research results of Zhu et al[12], the AI machine learned a total of 790 GC images and tested 203 GC images, which are different and independent of the learning images. The result is that when the threshold of CNN-CAD system is 0.5, the sensitivity is 76.47%, the specificity is 95.56%, and the accuracy is 89.16%. The positive and negative predictive values were 89.66% and 88.97%, respectively. The sensitivity of the endoscopist was 87.80%, the specificity was 63.31%, the accuracy was 71.49%, and the positive and negative predictive values were 55.86% and 91.01%, respectively.
For experienced endoscopists, the CNN-CAD system has once again achieved higher accuracy and specificity. High specificity of 96% will help to enhance the accurate diagnosis of the depth of invasion, distinguishing EGC from deeply invasive submucosal layer cancer. However, there are still some limitations in the research of Zhu et al[12]. First there are relatively few materials for deep learning. Second, in AI learning algorithms, only high resolution and clear images are selected as learning and testing materials. These two points lead to a serious defect whereby AI models may show excellent performance in clean and clear images of GC, but the diagnostic accuracy may be greatly affected when faced with poor quality images which endoscopists often encounter in clinical practice. This disadvantage can be overcome by enabling AI to learn a large number of images which are common among clinical gastroscopic pictures, such as mucus on the surface of the lesion, the lesion not being concentrated, or the location being too narrow to be seen clearly.
On colonoscopy, it is considered very difficult to find small adenoma or pedicleless polyps. In a first prospective clinical trial of AI, in a real-time routine colonoscopy, a total of 466 images of 466 tiny polyps were analyzed, with an accuracy of 94% and a negative predictive value of 96%. The speed of AI optical diagnosis is 35 s, which is faster than that of clinical endoscopists[11]. The algorithm is equally effective for novices and gastroenterologists and does not require dye injection. This study and Zhu et al[12] reached similar conclusions and revealed the application potential of AI in gastrointestinal endoscopy.
With AI, it's like opening a third eye to an endoscopist. AI for the diagnosis of disease, especially for EGC, is not only reflected in high accuracy but also it the quick training of junior doctors, improved diagnosis rate of EGC, and reduced missed cases.
Application of AI in endoscopic diagnosis of advanced GC
Gastroscopy easily detects advanced GC but there is also a certain risk of missed diagnosis. Korean scholars[13] prospectively collected undiagnosed cases of advanced GC with recent endoscopies, from 1997 to 2008, and reviewed the medical records of advanced GC diagnosed before 1991 to 1996. In total, 2310 cases of GC were analyzed. In that study, more than one-third of patients with advanced GC were not found in the previous endoscopy and they were located around the cardia.
Wu et al[14] has developed a new deep (D)CNN for endoscopic vision. This DCNN system is used to screen for EGC without blind spots during gastroenteroscopy (i.e. EGD). As a result, DCNN identified EGC from non-malignant tumors with an accuracy of 92.5%, sensitivity of 94.0%, specificity of 91.0%, positive predictive value of 91.3%, negative predictive value of 93.8%; these results were better than any achieved by an endoscopist. The accuracy of EGC detection by endoscopists is surpassed by the DCNN system of Wu et al[14], and that can better identify the location of the stomach. The advantage of the system is that it can detect EGC actively and track suspicious cancer lesions during EGD. Although the above study was aimed at EGC, we can see that an AI system has great potential for accurate diagnosis of advanced GC. The accuracy of GC diagnosis will be improved because of the intervention of an AI system. The high rate of missed diagnosis of advanced GC in the cardia will also be overcome by an AI system.
The prevalence and incidence rates of advanced stage GC are high, and the diagnosis rate is about 2/3. This has prompted doctors and researchers from all over the world not only to improve the detection rate of EGC but also to optimize the clinical management of advanced GC[15].
Perspectives
Ishioka et al[16] believe that the application of a CNN system in video should be expanded, and the image is expected to improve the standard of early detection of GC. Luo et al[17] developed a gastrointestinal-AI diagnostic system. Seven validation sets were used in their multicenter study, with accuracy ranging from 91.5% to 97.7%. The diagnostic sensitivity of “griaids” was higher than that of endoscopists (85.8%) and interns (72.2%). Kanesaka et al[18] collected and randomly selected 66 EGC magnifying narrow-band imaging (m-Nbi) images and 60 non cancer m-Nbi images as training sets, and 61 EGC m-Nbi images and 20 non cancer m-Nbi images as test sets. The test shows that the cadx system has great potential in the real-time diagnosis and sketching of EGCS in m-Nbi images. The study by Horiuchi et al[19] also supports this conclusion.
Whether it is EGC or advanced GC, the invasion depth of the tumor is related to the prognosis of the patients. Accurate determination of the invasion depth is beneficial to the patients. The overall accuracy rate of using “WLis” to evaluate the invasion depth of Zhu et al[12] was 89.16%, which was significantly higher than that of endoscopists
Many research studies on AI and the stomach have been focused on Japan, China and South Korea. At present, the combination of GC and AI mainly focuses on the detection and diagnosis of GC. In addition, AI systems may have potential applications in other areas. There are also many research studies on the application of AI technology in the detection and diagnosis of GC.
AI has great potential in the field of digestive diseases. Using AI for accurate diagnosis can make more accurate optical biopsy and reduce unnecessary biopsy or endoscopic resection, which is beneficial to patients. This can reduce the risk of bleeding, the incidence of complications, and the economic expenditure caused by the disease.
APPLICATION OF AI IN LYMPH NODE METASTASIS OF GC
Just as AI is gradually changing gastroenterology and endoscopy, it has also changed imaging doctors greatly. Preoperative localization diagnosis of lymph nodes is an ongoing and substantial challenge for radiologists. At present, the detection of lymph nodes is mainly achieved by imaging methods, which extracts a variety of diagnostic features. Some feature extraction methods are used to extract the effective diagnosis features, and then to realize the diagnosis of lymph node metastasis. Lymph node metastasis is an important independent factor affecting the prognosis of GC. Before medical and surgical treatment, lymph nodes must be understood as accurately as possible to determine treatment options and evaluate prognosis. Lymph node metastasis is an important independent factor affecting the prognosis of GC. Some studies have shown that the diagnosis of lymph node metastasis is of great significance[20-22]. AI and the diagnosis of GC lymph nodes can be divided into two aspects. The former is the application of AI in the diagnosis of lymph nodes, and the latter is the application of AI in the diagnosis of lymph node metastases. Because artificial detection is time-consuming and laborious, AI detection of abdominal lymph nodes is considered to be one of the development trends.
Barbu et al[23] propose an automatic detection method based on learning, which can detect and segment axillary and pelvic lymph nodes at the same time. First, the learning-based method is used to detect the suspected lymph nodes; then, the segmentation model is used to extract the boundary of each suspected lymph node. Finally, some features of the lymph nodes are used to score all the suspected lymph nodes; ultimately, the portion with the highest score is the lymph nodes. Although there has been some work to achieve automatic or semi-automatic detection of lymph nodes, so far few have detected gastric lymph nodes in the treatment of GC. Due to the different structure of different parts of the gastric system, it is difficult to detect gastric lymph nodes, so it is necessary to use AI technology to detect gastric lymph nodes.
Application of AI in lymph node detection
Lymph nodes are mainly detected by the observation of radiologists. Although this method has high clinical value, it takes a lot of time to detect every lymph node, so it is difficult to detect every lymph node in clinical application. In addition, radiologists need continuous training to detect lymph nodes accurately. In order to improve the efficiency of imaging doctors, it is a potential direction to detect lymph nodes with the help of computer.
In the treatment of GC, it is necessary to resect the metastasis and the lesion at the same time. The abdominal lymph node is one of the main metastasis routes of GC. It is very important for the prognosis of patients to accurately determine the resection area.
AI can learn to distinguish lymph nodes better and greatly reduce the work burden of imaging doctors. There are few reports about the use of AI technology to locate lymph nodes in GC. However, lung cancer, breast cancer, prostate cancer[24-28] and other reports are more common.
Application of AI in detection of lymph node metastasis
The medical decision-making method mainly depends on the clinical practice experience of doctors, their own medical knowledge, and various kinds of doctors. The therapeutic instrument diagnoses the patient's examination results. On the one hand, this traditional decision-making method depends on the professional level and subjective factors of doctors, which will lead to misdiagnosis, missed diagnosis, and other wrong decisions. On the other hand, modern diseases usually have the characteristics of multi-attribute, instability, complexity and time-varying, which require the information in medical diagnosis to have the characteristics of timeliness, accuracy, acceptability and traceability. With the development of computer technology and the production of a large amount of medical data, it is imperative to use computers to realize auxiliary decision-making, which has a positive role in improving the accuracy of medical diagnosis, reducing missed diagnosis and improving work efficiency.
The most common path of GC metastasis is lymph node metastasis, which is due to the abundance of lymphatic vessels and lymph nodes around the stomach[29]. In most studies, lymph node metastasis has been judged by size alone[30,31]. However, large lymph nodes may be caused by inflammation, and small lymph nodes may also have metastases. In addition, some studies have shown that lymph node metastasis is related to multiple characteristics[32-34]. However, it is difficult for doctors to make final diagnosis with multiple features at the same time, so it is necessary to introduce a clinical decision support system[35].
According to National Comprehensive Cancer Network (commonly known as NCCN) guidelines[36], preoperative evaluation of metastatic lymph nodes is considered to be an indication of neoadjuvant chemotherapy. In our opinion, surgery is still the most effective way to treat GC. Radical resection of metastatic lymph nodes is recommended by NCCN guidelines and Japanese GC guidelines as the key to the success of radical gastrectomy[36,37]. In this regard, accurate standard dissection and dissection of metastatic lymph nodes can greatly improve the 5-year survival rate of patients[38]. Until now, enhanced computer tomography (CT) has been used to judge gastric lymph node metastasis and tumor stage, which is the most reliable and commonly used method for evaluating lymph nodes in GC[39]. However, for the CT diagnosis of GC lymph nodes, the false negative and false positive of perigastric metastatic lymph nodes are inevitable technical problems[40]. Gao et al[41] found that, through in-depth study, faster region-based CNNs have higher judgment efficiency and recognition accuracy for CT diagnosis of perigastric metastatic lymph nodes.
Perspectives
The number of gastric lymph node dissections has been shown to be an independent predictor of the prognosis of GC by most studies. Many guidelines and studies have recognized that the minimum standard is to clear more than 15 lymph nodes during operation[42-44]. An AI system is helpful to reduce the imbalance of image source distribution, to the diagnosis and treatment of GC, and to determining the location of lymph nodes and lymph node metastasis.
AI AND ROBOTIC SURGERY
During the operation of the robotic surgery system, the doctor controls the bedside robotic arm system through the console. There are a total of three robotic arms through which the surgery is completed; the imaging system follows the robotic arm to enter the body for imaging, providing a field of vision for the doctor's surgery. Compared with traditional surgical operations, the surgical trauma performed by surgical robots is less invasive and basically minimally invasive. In recent years, with the rise of rapid rehabilitation surgery and the popularization and application of the Leonardo da Vinci robotic surgery operating system in China, many medical institutions have carried out robotic surgery. For example, minimally invasive robotic surgery is used increasingly in interventional therapy of urinary tumors[45,46]. In the field of gastrointestinal surgery, robotic radical gastrectomy has also become one of the minimally invasive radical methods commonly used in central hospitals specializing in GC[47]. The implementation of robotic surgery emphasizes the concept of "precise surgery"[48]. Robotic surgery uses a technologically advanced platform, with the chief knife doctor sitting at the console and operating in the operating room or by remotely controlling the robot. With the increasing complexity of mechanical surgery technology, the accuracy and proficiency of robotic surgery will be increased only by developing advanced training modes[49,50].
AI in Da Vinci robotic GC surgery
Traditional laparotomy, laparoscopic surgery and robotic surgery are considered as three surgical treatments for GC. Laparoscopic surgery was first performed in 1991[51] because it caused less trauma to patients than traditional surgery, gradually replacing the former. Robotic surgery has the advantages of using wristed instruments, tremor filtering, and high-resolution 3D images over laparoscopic surgery[52], which were also reported in another article first[53].
Robot-assisted applications in minimally invasive surgery were first described in 1985, and this technology has evolved to its current state in the form of a Da Vinci surgical system (Intuitive Surgery, Sunnyvale, CA, United States)[54]. Studies have shown that prediction by deep learning systems combined with diagnosis by human pathologists has reduced the error rate by about 85%. It was demonstrated that medical professionals and machine deep learning significantly improved decision-making[55].
Machine learning (ML) is widely used in many fields, such as communication and engineering manufacturing, but rarely be used in medicine, especially in surgery[56]. The efficiency of doctors can be improved by ML. With the continuous development of medicine, efficiency is also increasingly valued by the public[57]. Before the birth of the laparoscopic technique, the surgeon's operation often brought great trauma to the patient, and it is a long process for a young doctor to accumulate experience and learn through laparotomy. Especially in some operations with high accuracy requirements, although the surgeons have undergone long professional training and repeated operations, there is also a risk of errors and the efficiency of doctors' diagnosis and treatment is a little low. However, after the birth of endoscopic technology, traditional laparotomy was gradually replaced because of its high trauma, and after learning the endoscopic surgical technology, the surgeon's clinical treatment efficiency has been greatly improved. The robotic surgery technology born after the endoscopic technology is even more so. Robotic surgery technology can greatly enhance the surgical efficiency of doctors who are lacking surgical experience and further reduce the trauma suffered by patients.
In addition to the above points, the robot can also be combined with AI in the following aspects. First of all, the level of operator can be distinguished by AI combined with robot. Fard et al[57] extracted eight global motion features for surgeons at novice and expert levels. The ability of AI to automatically classify experts and novice surgeons has been proved by research. Dai et al[58] developed and validated an integrated system to alert operators before suture breaks. The results show that this system can improve the results related to knotting tasks in robotic surgery and can reduce suture failure without reducing the quality of the resulting knots. Iranian scholars[59] used the Cox proportional hazard model and artificial neural network model to predict the survival rate of Iranian GC patients, and found that the prediction accuracy of the neural network was 83.1%, and that of the Cox regression model was 75.0%. Compared with the Cox proportional hazard regression model, the neural network model was deemed a more powerful statistical tool to predict the survival rate of GC patients.
Perspectives
Robotic surgery provides a good platform for the application of AI in surgical systems (gastrointestinal surgery). It is possible for a large number of clinical data to be evaluated and interpreted by ML methods. The rapid acquisition of surgical technology by junior doctors, the efficiency of a surgeons' operation, and the small trauma to patients are the results of the combination of ML method and robotic operation for the prognosis judgment and prediction of GC patients. However, there is a big flaw in this, which is the standardization of data.
AI affects and narrows the training growth cycle of robotic GC surgeons, reduces patient injury, changes the surgical results, and may even make GC surgery a robotic automated surgery in the future. To be honest, it is still difficult to do this, but we firmly believe that when surgeons, GC patients, robotic engineers and AI programmers cooperate in multiple disciplines, advanced robotic AI surgery for GC will be realized.
CONCLUSION
The problem of population aging has been increasing in East Asian countries in recent years, especially in China, where the incidence of cancer has increased year by year. As a new and comprehensive subject, AI will become an important means to promote the development of the medical industry. The application of AI in GC is mainly focused on digestive endoscopy, lymph node image positioning diagnosis, and working in combination with a robotic surgery system. In terms of gastrointestinal endoscopy, AI can detect EGC earlier and faster, with higher accuracy than clinical endoscopists. For advanced GC, AI can increase the detection rate of cancers in areas where gastroscopy is performed, such as pump-door cancer, which reduces the missed diagnosis rate. In the face of GC lymph nodes, the intervention of AI not only reduces the burden of radiologists, but also increases the accuracy of lymph node localization. Just as for the location of lymph nodes, it is also of great significance for detection of lymph node metastasis with higher accuracy. It also has important potential for robotic surgery of GC. AI has further revolutionized GC surgery by training young doctors to perform robotic GC surgery, improving surgical trauma of patients and predicting patient prognosis in timely and accurate manners - precision surgery was gradually promoted by AI to improve the relevant outcomes of GC disease and surgery without affecting patient survival and safety.
In addition to gastroscopic detection of GC or precise localization of lymph nodes, as well as use of Da Vinci robotic surgery to improve the patient's intraoperative experience and prognosis, the aim is to minimize trauma suffered by patients. The development of technology is constantly being updated, and the invention of laparotomy has saved the lives of many patients with GC but also brought great trauma to these patients. After the advent of endoscopic techniques, the concept of minimally invasive surgery began to gain popularity, and laparoscopic surgery gradually replaced open surgery because of its smaller damage. However, in the development of technology and times, the limitations of its operation are also increasingly exposed.
With the arrival of the big data era, AI technology has gradually matured, and its combination with robotic surgical systems has become a research hotspot. Robotic surgery, boasting accuracy that laparoscopic surgery does not have, is an emerging surgical system for the future. Through the deep integration of this system with AI, the trauma of the patient's operation is further reduced. In the future, there may even be a fully automated robotic surgical system controlled by AI, in which case the trauma of GC surgery will be very small and can be considered noninvasive. In other words, the change that AI will bring to GC is that the surgical treatment of GC will change from greater trauma to minimally invasive, and from minimally invasive to nearly noninvasive.