Published online Aug 28, 2020. doi: 10.13105/wjma.v8.i4.292
Peer-review started: July 29, 2020
First decision: August 22, 2020
Revised: August 28, 2020
Accepted: August 28, 2020
Article in press: August 28, 2020
Published online: August 28, 2020
Helicobacter pylori (H. pylori) infection is predominantly acquired in childhood. When indicated, the most accepted treatment for H. pylori eradication in this age group is first-line triple therapy. However, the increasing resistance to clarithromycin and nitroimidazoles has been associated with treatment failure, and thus, alternative treatment regimens have been proposed.
To perform a systematic review of randomized controlled trials on treatment regimens for H. pylori infection in children.
We surveyed relevant articles published in English from 2010 to April 2020 in the PubMed and MEDLINE databases. Keywords included “Helicobacter pylori”/”children or childhood”/”treatment or eradication.” The risk of bias was evaluated according to the Cochrane Handbook of Systematic Reviews for Interventions.
Among the 1144 records identified through the database, 20 articles were selected. Four studies compared the eradication rates of H. pylori infection between standard triple therapies, changing only the main antibiotic used. Seven studies evaluated the effectiveness of standard triple therapy with the addition of probiotics. One study investigated the relationship between the effectiveness in the eradication rates of standard triple therapy and vitamin E levels. Six studies analyzed the eradication rates of sequential therapy.
The findings suggest that although standard triple therapy is the most recommended regimen for children by the current guidelines, other therapeutic schemes have shown promising results and may also be recommended for clinical practice in the future.
Core Tip: Helicobacter pylori (H. pylori) is a bacterium that infects more than 50% of the population worldwide. In the last several years, no significant changes in the treatment of infected children have been observed, mainly due to a lack of studies with satisfactory scientific evidence to support the indication of therapies in clinical practice. We performed a systematic review of randomized controlled trials on treatment regimens for H. pylori infection in children.
- Citation: da Silva FAF, de Brito BB, Santos MLC, Marques HS, Sampaio MM, da Silva Júnior RT, Apolonio JS, de Carvalho LS, Silva CS, de Sá Santos LK, Oliveira MV, Rocha GA, de Magalhães Queiroz DM, de Melo FF. Treatment of Helicobacter pylori infection in children: A systematic review. World J Meta-Anal 2020; 8(4): 292-308
- URL: https://www.wjgnet.com/2308-3840/full/v8/i4/292.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i4.292
Helicobacter pylori (H. pylori) is a gram-negative spiral bacterium that colonizes the gastric mucosa of more than 50% of the population worldwide. The infection is acquired predominantly in childhood, and is more prevalent in developing countries where about 70% of children are infected until 15-years-old[1,2], whereas it is disappearing in developed countries. Once acquired, the bacterium is rarely eliminated without adequate antibiotic therapy and individuals remain infected throughout life[3]. Most infected individuals do not develop complications, but gastric colonization can progress to chronic gastritis, duodenal ulcer, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma, and bacterial eradication is associated with the prevention of such diseases[4]. H. pylori infection has also been implicated in the pathogenesis of extra-gastric diseases, including iron deficiency anemia and chronic immune thrombocytopenic purpura.
The mechanisms by which the infection progresses to the above-mentioned diseases are not completely understood and depend on the relationship between host genetics and factors regarding the environment and bacterial virulence.
Severe H. pylori-associated diseases are more common in adults than in children. This phenomenon can be explained, in part, by the differences in immune response between the two age groups, which seems to be a relevant factor influencing mucosal damage and clinical outcomes[5]. In general, H. pylori infection induces a T helper type 1 (Th1)-polarized response with high levels of interferon-γ that stimulate gastric inflammation and mucosal damage in adults. Moreover, Th17 cells and interleukin (IL)-17 levels are important in that process because they stimulate the recruitment and activation of neutrophils in the gastric mucosa, increasing the inflammatory environment against the bacterium. In contrast to the pro-inflammatory response found in adults, children tend to exhibit an immune response pattern with a predominance of regulatory T cells that contribute to the persistence of the infection and milder clinical manifestations[6,7]. Children tend to have lower levels of Th1- and Th17-related cytokines as well as overexpression of IL-10 and transforming growth factor-β, resulting in a lower degree of polymorphonuclear cell activation in the acute phase of infection[8,9] and prominent mononuclear cell infiltration in chronic infection compared with adults[10].
H. pylori virulence factors also play an important role in the pathogenesis of infection in childhood. It is well documented that genes such as cytotoxin gene A (cagA) and vacuolating cytotoxin gene A (vacA) increase the risk of severe gastric diseases such as duodenal ulcer and gastric cancer[11].
H. pylori eradication prevents duodenal ulcer recurrence and prevention of gastric cancer. In addition, H. pylori eradication in children induces platelet and iron recovery in immune thrombocytopenic purpura and iron deficiency anemia, respectively.
Since eradication therapies show variable failure rates, retesting for H. pylori infection after an antimicrobial regimen is recommended to ensure that patients have been successfully treated[12]. There are various available therapeutic options aimed toward H. pylori eradication, and clarithromycin-based triple therapy, sequential therapy, bismuth-containing quadruple therapy or triple therapy, and hybrid therapy are the regimens most often used[13]. Moreover, various alternative approaches have been attempted in order to improve bacterial eradication such as susceptibility-guided therapies, probiotics, and vaccines[14-16]. In that context, a number of factors influence the choice of appropriate treatment, including antimicrobial susceptibility profile, economic factors, and importantly, individual characteristics such as previous exposure to antibiotics and age[17].
Performing H. pylori eradication in children demands specific precautions, of which avoiding regimens with unacceptable rates of adverse events (AEs) for this population is very important[18]. In this context, the number of treatment options usually available for children tends to be significantly lower compared to the range of treatments at hand for adults. Clarithromycin-based triple therapy is the most used therapeutic scheme for children, although other standard therapies have also been tried[19]. Moreover, to reduce the frequency and severity of side effects as well as to improve eradication rates, probiotics in association with standard therapy have been tested in children[20].
Therefore, we reviewed the randomized controlled trials (RCTs) of treatments for H. pylori eradication children.
In this systematic review, the criteria recommended by the PRISMA checklist were used[21].
Prospective RCTs, published in peer-reviewed journals from 2010 to April 2020 and reporting the results of antibiotic therapy and/or supplementation with other drugs for the H. pylori eradication in infected children under 18-years-old, were included. There was no restriction regarding the therapeutic schemes used. Excluded studies were those including adults or lacking their complete or free full text. Only English language studies were included. The inclusion criteria are outlined in Table 1.
| Criteria | Description |
| Date range | January 2010 to April 2020 |
| Language | Only English published articles |
| Location | No restriction of localization |
| Population | Children (< 18-years-old) |
| Type of study | Randomized controlled trials |
H. pylori-positive patients under 18-years-old diagnosed by any validated test accepted by the scientific community for H. pylori detection. Patients who had previous failed antibiotic therapies were included.
Prospective RCTs evaluating H. pylori eradication rates in children.
We collected outcomes of intention-to-treat (ITT), per protocol (PP), and simple percentages of H. pylori infection eradication.
We surveyed the relevant articles published in English language from 2010 to April 2020 in the PubMed and MEDLINE databases. The term strategies used for the search at both databases were: ((Helicobacter pylori [and] children [and] treatment) [OR] eradication) and (Helicobacter pylori [and] childhood [and] (treatment [OR] eradication)).
The eligibility of the articles was evaluated by two independent reviewers (Da Silva FAF and de Brito BB). Duplicate articles were excluded. The abstracts of the articles were evaluated and studies that were not prospective RCTs and/or did not evaluate H. pylori eradication rate in children were excluded. A third reviewer (de Melo FF) resolved disagreements between the two reviewers. To verify if the articles met all previously established criteria, each article was individually analyzed. To statistically evaluate the agreement between the reviewers, the Kappa coefficient (K) was calculated, which indicated a K = 0.752, considered as a substantially strong degree of agreement between the reviewers.
We developed a structured data extraction spreadsheet specifically for this review based on the criteria recommended by the Cochrane Handbook of Systematic Reviews for Interventions[42]. We independently reviewed the relevant study data and results of interest such as rates of eradication of H. pylori infection in childhood.
Information was extracted from each study including: General characteristics of the participants and studies, type of intervention, therapeutic regimen used, type of outcome measure, and positive and negative outcomes.
To assess the validity of RCTs, two authors independently analyzed the risk of bias criteria recommended by the Cochrane Handbook of Systematic Reviews for Interventions[42]: Generation of the random sequence, concealment of allocation, blinding of participants and professionals, blinding of outcome evaluators, incomplete outcomes, and reporting of the selective outcome. Then the risk of bias was categorized as high, low, or uncertain.
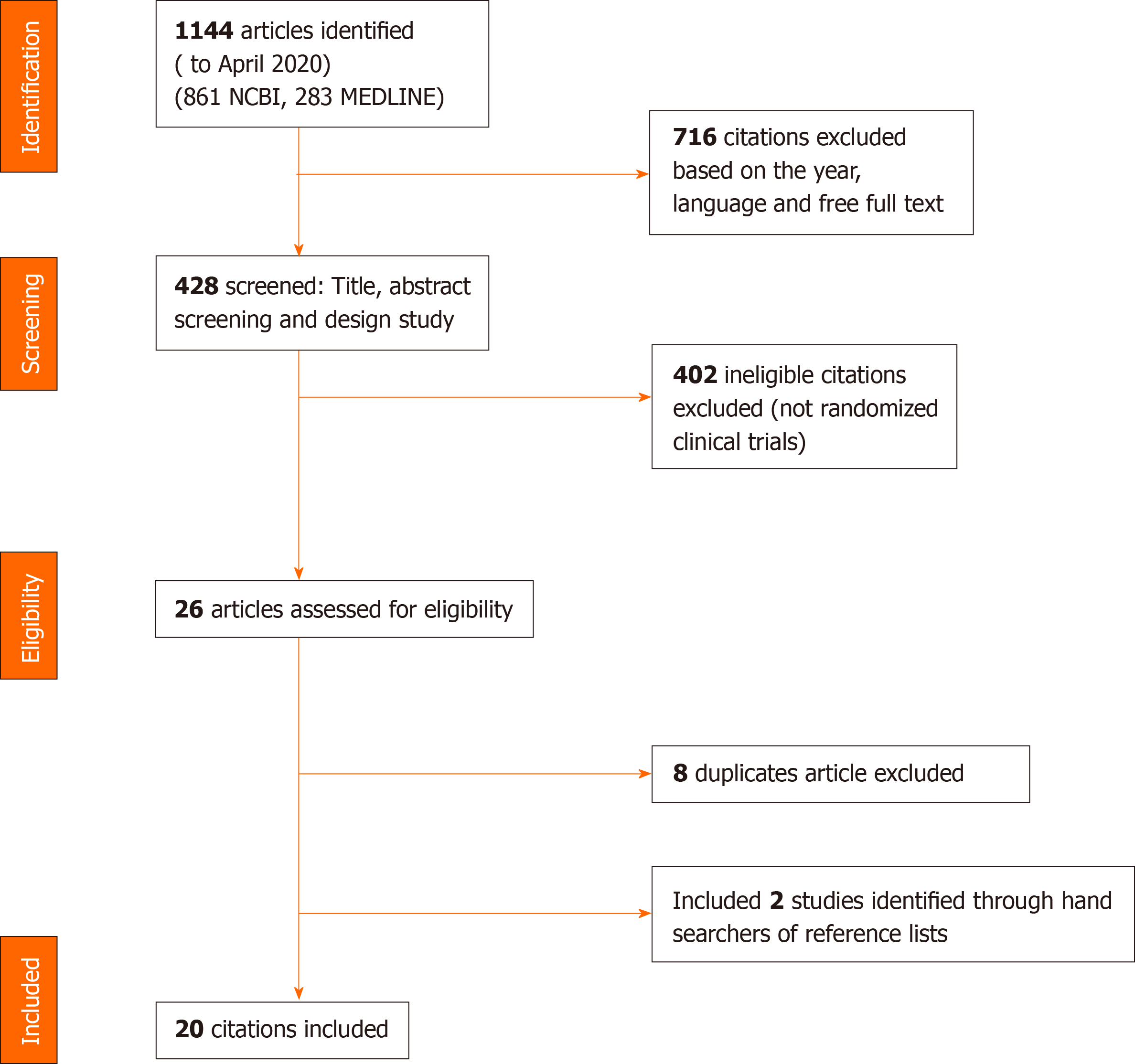
Of the 1144 articles reviewed (861 in NCBI and 283 in MEDLINE), 1118 were excluded using previously established inclusion criteria. Twenty-six articles were selected for complete analysis; however, eight were duplicate and thus were excluded. Finally, 2 studies were an additional reference list and 20 articles were included. Figure 1 shows the selection and distribution of articles according to the databases searched, from the first search to the application of all of the selection criteria.
The characteristics of the 20 selected studies are summarized in Table 2. A total of 2261 children aged 22 mo to 18 years were included. Regarding the geographic distribution of the studies, 25% of the articles were from Iran; 25% from Turkey; 10% from China; and 40% from Algeria, Italy, Poland, India, Kenya, United States, Belgium, and France. The studies had a follow-up average of 6 wk. In addition, the articles evaluated conventional eradication therapies, probiotics, or sequential therapies as well as the eradication rates of H. pylori infection in childhood.
| Ref. | Year | Country | Study design | Patients, n | Age range | Follow-up | Goal |
| Moubri et al[22] | 2018 | Algeria | RCT | 272 | 5-16 yr | 8-12 wk | To compare efficacy, side effects and influence of resistance of H. pylori strains between two different treatments in Algerian children. |
| Namkin et al[23] | 2016 | Iran | RCT | 28 | 9-12 yr | 4-8 wk | To evaluate the effect of S. boulardii supplementation on the eradication of H. pylori in children in the region. |
| Akcam et al[24] | 2015 | Turkey | RCT | 61 | 7-18 yr | 6 wk | To evaluate the effect of probiotics on eradication rates and side effects in association with standard triple therapy in H. pylori-positive children. |
| Tolone et al[25] | 2012 | Italy | RCT | 68 | 4-11 yr | 4 wk | To evaluate if addition of probiotics increases eradication rates and reduces side effects in children. |
| Farahmand et al[26] | 2016 | Iran | RCT | 66 | 7-15 yr | 4 wk | To compare the effect of ciprofloxacin and furazolidone on H. pylori eradication in combination with amoxicillin and omeprazole. |
| Ahmad et al[27] | 2013 | Iran | RCT | 66 | 3-14 yr | 4-8 wk | To evaluate the effect of probiotic supplementation with the combination of seven microorganisms on the treatment of H. pylori infection in childhood. |
| Bin et al[28] | 2015 | China | RCT | 205 | 22 mo-16 yr | 2 wk | To investigate the effects of Saccharomyces boulardii CNCM I-745 on eradication of H. pylori in children. |
| Kasiri et al[29] | 2017 | Iran | RCT | 82 | 1-15 yr | 4 wk | To compare the effect of amoxicillin and metronidazole in the triple therapy regimen to eradicate H. pylori infection in children aged 1 to 15 yr. |
| Iwańczak et al[30] | 2016 | Poland | RCT | 69 | 5-17 yr | 6-8 wk | To compare the efficacy of sequential therapy for 10 d with triple therapy for 7 d (PPI, amoxicillin and clarithromycin or PPI, amoxicillin and metronidazole) in children. |
| Tümgör et al[31] | 2014 | Turkey | RCT | 90 | 10-17 yr | 6 wk | To compare the treatment with lansoprazole, amoxicillin and clarithromycin (LAC) and with the combination of LAC + vitamin E (LACE) in Turkish children. |
| Ali Habib et al[32] | 2013 | India | RCT | 18 | 12-15 yr | 6 wk | To investigate which sequential or standard eradication regimen has the most effective improvement in the status of associated iron and iron deficiency in children. |
| Ustundag et al[33] | 2017 | Turkey | RCT | 69 | 6-16 yr | 4-6 wk | To evaluate the effects of the use of the symbiotic Bifidobacterium lactis B94 + inulin, together with standard triple therapy on the eradication rate, adherence, as well as in the symptoms of H. pylori infection in children. |
| Huang et al[34] | 2013 | China | RCT | 360 | 3-16 yr | 4 wk | To compare 10 d sequential therapy and standard triple therapy in Chinese children with H. pylori infection. |
| N Şirvan et al[35] | 2017 | Turkey | RCT | 104 | 5-17 yr | 4 wk | To evaluate the addition of symbiotics containing Bifidobacterium lactis to triple therapeutics in the rates of side effects, dyspeptic symptoms and H. pylori eradication in children. |
| Baysoy et al[36] | 2013 | Turkey | RCT | 61 | 4-18 yr | 6-8 wk | To compare ornidazole-based sequential therapy with standard triple therapy for the eradication of H. pylori in children. |
| Esmaeili-Dooki et al[37] | 2015 | Iran | RCT | 64 | 2-15 yr | 4-6 wk | To evaluate the effect of the classic triple therapy and azithromycin eradication regimen against H. pylori in children. |
| Laving et al[38] | 2013 | Kenya | RCT | 71 | 2-15 yr | 2-6 wk | To determine the effectiveness of a new 10 d sequential therapy compared to the standard 10 d triple therapy for the treatment of H. pylori infection in children. |
| Prieto-Jimenez et al[39] | 2011 | United States of America | RCT | 110 | 3-11 yr | 6 wk | To evaluate the efficacy of sequential quadruple eradication therapy for 10 d and observe the iron levels of positive H. pylori children. |
| Nguyen et al[40] | 2012 | Vietnam | RCT | 232 | 3–15 yr | 4 wk | To investigate the role of antibiotic resistance, drug dosage, and administration frequency in treatment of H. pylori infection in Vietnamese children. |
| Bontems et al[41] | 2011 | Belgium, France and Italy | RCT | 165 | 2,7 a 17 yr | 8 wk | To compare sequential vs tailored triple therapy regimens on H. pylori eradication rate and to assess the effect of antimicrobial susceptibility in children. |
All patients were H. pylori-positive children diagnosed by validated methods. Randomization methods and loss of follow-up were highlighted for analysis of the risk of bias. All articles were classified according to the criteria of the Oxford Center for Evidence-based Medicine - Levels of Evidence[43] (Table 3). Standard triple therapy was the main therapeutic scheme evaluated. The studies compared conventional triple therapy alone to triple therapy containing probiotics and to sequential therapies (Table 4).
| Ref. | Specific disease | Diagnosis | Groups | Randomization | Loss of follow-up | Level of evidence |
| Moubri et al[22] | No | Biopsy, RUT, culture, HpSA and 13C-UBT/(Biopsy, RUT, culture, HpSA and 13C-UBT) | 2 | The method chosen was to classify closed envelopes, which were randomly assigned to treatment | 12 patients | 1b |
| Namkin et al[23] | No | HpSA/(HpSA) | 2 | Limited information on the method used | 4 patients | 2b |
| Akcam et al[24] | No | Biopsy, histology, RUT and | 2 | Admission order | 5 patients | 1b |
| Tolone et al[25] | No | Biopsy, histology, RUT and | 2 | No reported | No loss | 2b |
| Farahmand et al[26] | No | Biopsy, RUT and HpSA/(HpSA) | 2 | Table of aleatory numbers | No loss | 1b |
| Ahmad et al[27] | No | RUT, histology, and HpSA/(HpSA) | 2 | Limited information on the method used | No loss | 1b |
| Bin et al[28] | No | Immunoglobulin G Antibody, histology and 13C-UBT/(13C-UBT) | 2 | No reported | 14 | 1b |
| Kasiri et al[29] | Dyspepsia, epigastric pain and GB | Biopsy, histology and RUT/(HpSA) | 2 | Limited information on the method used | 3 | 1b |
| Iwańczak et al[30] | Dyspepsia and gastric and/or duodenal ulcer | Histology and/or culture/(Biopsy and culture) | 3 | No reported | No loss | 1b |
| Tümgör et al[31] | Dyspepsia. | 14C-UBT and histology/(13C-UBT) | 2 | No reported | 2 | 2b |
| Ali Habib et al[32] | No | Immunoglobulin G Antibody and 13C-UBT/(13C-UBT) | 2 | No reported | 2 | 2b |
| Ustundag et al[33] | Gastrointestinal symptoms, severe regurgitation, gastrointestinal bleeding, unexplained weight loss or chronic diarrhea | Histology/(14C-UBT) | 2 | Double-blind randomization list | 5 | 2b |
| Huang et al[34] | No | RUT, HpSA, histology and culture/(HpSA) | 3 | Limited information on the method used | 42 | 1b |
| N Şirvan et al[35] | Chronic diseases, abdominal pain and dyspepsia | Histology/(HpSA) | 2 | Limited information on the method used | No loss | 2b |
| Baysoy et al[36] | No | Histology, RUT, 13C-UBT/(HpSA or Biopsy and histology) | 2 | Limited information on the method used | 8 | 1b |
| Esmaeili-Dooki et al[37] | No | Biopsy, histology and HpSA/(13C-UBT) | 2 | Table of aleatory numbers | No loss | 1b |
| Laving et al[38] | No | Histology and HpSA/(HpSA) | 2 | Computer-generated random numbers | 33 | 1b |
| Prieto-Jimenez et al[39] | No | 13C-UBT and urine antibody | 6 | Computer-generated random numbers | 20 | 1b |
| Nguyen et al[40] | No | Biopsy, RUT, histology and culture | 3 | No reported | 10 | 1b |
| Bontems et al[41] | Abdominal pain, nausea, vomiting, heartburn and anemia | Biopsy, histology and culture/(HpSA) | 2 | No reported | 15 | 1b |
| Ref. | Treatment schemes |
| Moubri et al[22] | Group A - Amoxicillin-based triple therapy: (1) Children > 30 kg: Omeprazole 40 mg/d, amoxicillin 50 mg/kg/d with a maximum of 2 g/d and clarithromycin 15 mg/kg/d for 7 d. All given in two daily doses; and (2) Children < 30 kg: omeprazole 2 × 10 mg/d b.i.d., amoxicillin 50 mg/kg/d b.i.d. with a maximum of 2 g/d, and clarithromycin 15 mg/kg/d b.i.d. for 7 d. |
| Group B - Metronidazole-based triple therapy: (1) Children > 30 kg: Omeprazole 2 × 20 mg/d, amoxicillin 50 mg/kg/d b.i.d. with a maximum of 2 g/d and metronidazole 40 mg/kg/d b.i.d. for 10 d; and (2) Children < 30 kg: omeprazole 2 × 10 mg/d, amoxicillin 50 mg/kg/d b.i.d. with a maximum of 2 g/d and metronidazole 40 mg/kg/d b.i.d., for 10 d, with a maximum of 1.5 g/d in children above 30 kg and 1 g/d in children below 30 kg body weight. | |
| Namkin et al[23] | Probiotic group: Yomogi® 250 mg lyophilizate - 1 capsule daily for 30 d. |
| Placebo group: placebo capsule 250 mg - lactose and powdered wheat starch - 1 capsule daily for 30 d. | |
| Akcam et al[24] | Standard triple therapy group: Omeprazole 1 mg/kg, before breakfast, amoxicillin 50 mg/kg b.i.d. after meals, clarithromycin 15 mg/kg b.i.d. after meals for 7 d. |
| Standard triple therapy group + probiotics: Omeprazole 1 mg/kg, before breakfast + amoxicillin 50 mg/kg b.i.d. after meals, clarithromycin 15 mg/kg b.i.d. after meals, PROBINUL® 5 g once a day for 7 d. | |
| Tolone et al[25] | Standard triple therapy group - lansoprazole 30 mg, before breakfast, amoxicillin 50 mg/kg/d b.i.d., clarithromycin 15 mg/kg/d b.i.d. for 14 d. |
| Standard triple therapy group + probiotics: Lansoprazol 30 mg, before breakfast + amoxicillin 50 mg/kg/d b.i.d., clarithromycin 15 mg/kg/d b.i.d., Maflor® plus 1 capsule, b.i.d. for 14 d. | |
| Farahmand et al[26] | Group A - Ciprofloxacin-based triple therapy: Ciprofloxacin 30 mg/kg/d b.i.d. and amoxicillin 50 mg/kg/d b.i.d. for 1 wk. Omeprazole 1 mg/kg/d b.i.d for 4 wk. |
| Group B - Furazolidone-based triple therapy: furazolidone 6 mg/kg/d single-dose over and amoxicillin 50 mg/kg/d b.i.d. for 1 wk. omeprazole 1 mg/kg/d b.i.d. for 4 wk. | |
| Ahmad et al[27] | Group A - Triple therapy + placebo: Amoxicillin 50 mg/kg/d b.i.d., as syrup or capsule, and furazolidone 6 mg/kg/d b.i.d. as syrup or tablet for 1 wk. Omeprazole 1 mg/kg/d plus placebo for 4 wk. |
| Group B - Triple therapy + probiotics: Amoxicillin 50 mg/kg/d b.i.d., as syrup or capsule, and furazolidone 6 mg/kg/d b.i.d. as syrup or tablet, for 1 wk. Omeprazole 1 mg/kg/d plus placebo for 1 sachet/d for 4 wk. | |
| Bin et al[28] | Group A - Triple therapy + Saccharomyces boulardii: Amoxicillin, omeprazole, clarithromycin and 2 sachet S. boulardii 250 mg per day, for 2 wk. Allergic to penicillin: metronidazole + omeprazole, clarithromycin and S. boulardii (dosages not reported). |
| Group B - Only triple therapy: Amoxicillin, omeprazole, clarithromycin. Allergic to penicillin: metronidazole + omeprazole, clarithromycin (dosages not reported). | |
| Kasiri et al[29] | Group A - Amoxicillin-based triple therapy: Omeprazole 1–2 mg/kg b.i.d. for 1 mo. Amoxicillin 50 mg/kg and clarithromycin 15 mg/kg b.i.d. for 2 wk. |
| Group B - Metronidazole-based triple therapy: Omeprazole 1 mg/kg b.i.d. divided into two doses for 1 mo. Metronidazole 15 mg/kg and clarithromycin 15 mg/kg b.i.d. for 2 wk. | |
| Iwańczak et al[30] | Group I - Amoxicillin-based triple therapy: Omeprazole, amoxicillin and clarithromycin for 7 d (dosages not reported). |
| Group II - Metronidazole-based triple therapy: Omeprazole, amoxicillin and metronidazole for 7 d (dosages not reported). | |
| Group III - Sequential therapy: omeprazole 1 mg/kg of body weight/d, max 20 mg b.i.d. and amoxicillin 50 mg/kg of body weight/d, max 1000 mg/24 h for 5 d, b.i.d. followed by 5 d treatment with omeprazole 1 mg/kg of body weight/d, max 20 mg/24 h twice daily, clarithromycin 15 mg/kg of body weight/d, max 500 mg/24 h twice daily and metronidazole 20 mg/kg of body weight/d, max 500 mg/24 h twice daily. | |
| Tümgör et al[31] | Group A - Clarithromycin-based triple therapy: Lansoprazole 1 mg/kg/d, amoxicillin 50 mg/kg/d, and clarithromycin 14 mg/kg/d, all medications b.i.d. for 14 d. |
| Group B - Clarithromycin-based triple therapy + vitamin E: Lansoprazole 1 mg/kg/d b.i.d., amoxicillin 50 mg/kg/d b.i.d., and clarithromycin 14 mg/kg/d b.i.d., vitamin E 200 IU/d for 14 d. | |
| Ali Habib et al[32] | Group A - Standard therapy: Rabeprazole 20 mg, clarithromycin 250 mg, and amoxicillin 500 mg each administered orally twice daily for 10 d. |
| Group B - Sequential therapy: Rabeprazole 20 mg and amoxicillin 500 mg for 5 d, followed by rabeprazole 20 mg clarithromycin 250 mg and tinidazole 500 mg for another 5 d, twice daily. | |
| Ustundag et al[33] | Group A - Standard triple therapy: Amoxicillin 50 mg/kg/d, clarithromycin 15 mg/kg/d twice daily for 14 d and omeprazole 1 mg/kg/d once daily for 1 mo. |
| Group B - Standard triple therapy + symbiotic: Amoxicillin 50 mg/kg/d and clarithromycin 15 mg/kg/d twice daily for 14 d. Omeprazole 1 mg/kg/d once daily for 1 mo and Maflor® a single dose for 14 d concurrently. | |
| Huang et al[34] | Group A – 1 d sequential therapy: Omeprazole 0.8–1.0 mg/kg/d, amoxicillin 30 mg/kg/d for the first 5 d, followed by omeprazole 0.8–1.0 mg/kg/d, clarithromycin 20 mg/kg/d, and metronidazole 20 mg/kg/d for the remaining 5 d. |
| Group B – 7 d triple standard therapy: Omeprazole 0.8–1.0 mg/kg/d, amoxicillin 30 mg/kg/d, and clarithromycin 20 mg/kg/d. | |
| Group C – 10 d triple standard therapy: Omeprazole 0.8–1.0 mg/kg/d, amoxicillin 30 mg/kg/d, and clarithromycin 20 mg/kg/d. | |
| N Şirvan et al[35] | Group 1 - Standard triple therapy + symbiotic: Amoxicillin 50 mg/kg/d b.i.d. for 7 d, clarithromycin 15 mg/kg/d b.i.d. for 14 d, and lansoprazole 1 mg/kg/d - single dose, in the morning for 14 d. Maflor® sachet - single dose for 14 d. |
| Group 2 - Standard triple therapy: Amoxicillin 50 mg/kg/d b.i.d. for 14 d, clarithromycin 15 mg/kg/d b.i.d. for 14 d, and lansoprazole 1 mg/kg/d - single dose, in the morning for 14 d. | |
| Baysoy et al[36] | Group A - Standard triple therapy: Amoxicillin 50 mg/kg/d, clarithromycin 15 mg/kg/d, and lansoprazole 1 mg/kg/d for 14 d. |
| Group B - Sequential therapy group: Amoxicillin 50 mg/kg/d and lansoprazole 1 mg/kg/d for the first 5 d and clarithromycin 15 mg/kg/d, ornidazole 30 mg/kg/d and lansoprazole 1 mg/kg/d for another 5 d. | |
| Esmaeili-Dooki et al[37] | Group 1 - Clarithromycin-based triple therapy: Clarithromycin 7.5 mg/kg/d b.i.d. and amoxicillin 50 mg/kg/d every b.i.d. for 10 d, and omeprazole 1 mg/kg/d b.i.d. for 2 wk. |
| Group 2 - Amoxicillin-based triple therapy: Azithromycin 10 mg/kg/d once a day, before meal, for 6 d along with amoxicillin and omeprazole. | |
| Laving et al[38] | Conventional therapy group: Omeprazole plus 1 mg/kg/d, amoxicillin plus 50 mg/kg/d and clarithromycin 15 mg/kg/d for 10 d. |
| 10 d sequential therapy group: Omeprazole plus 1 mg/kg/d, amoxicillin plus 50 mg/kg/d for 5 d followed by omeprazole plus 1 mg/kg/d, clarithromycin 15 mg/kg/d, and tinidazole 20 mg/kg/d for the next 5 d. | |
| Prieto-Jimenez et al[39] | Group I - Quadruple sequential eradication and placebo: Lansoprazole, once per day before breakfast, plus an oral solution containing amoxicillin 50 mg/kg/d for 5 d followed by lansoprazole, once per day, plus an oral solution containing clarithromycin 15 mg/kg/d and an oral solution containing tinidazole 20 mg/kg/d for another 5 d. 6 wk of a placebo matched to iron supplementation. |
| Group II - Quadruple sequential eradication and iron: Lansoprazole, once per day before breakfast, plus an oral solution containing amoxicillin 50 mg/kg/d for 5 d followed by lansoprazole, once per day, plus an oral solution containing clarithromycin 15 mg/kg/d and an oral solution containing tinidazole 20 mg/kg/d for another 5 d. 6 wk of iron supplementation. | |
| Group III: Iron and placebo: A 10 d course of a placebo matched to H pylori sequential eradication therapy plus 6 wk of iron supplementation. | |
| Group IV: Placebo alone: A 10 d course of placebo matched to H. pylori sequential eradication therapy plus 6 wk of a placebo matched to iron supplementation. | |
| Nguyen et al[40] | Group A: Lansoprazole, amoxicillin and clarithromycin: (1) Children weighing 13–22 kg: lansoprazole 15 mg once daily, amoxicillin 500 mg twice daily and clarithromycin 250 mg once daily; and (2) Children weighing 23–45 kg: lansoprazole 15 mg, amoxicillin 750 mg and clarithromycin 250 mg, all given twice daily. |
| Group B: Lansoprazole, amoxicillin and metronidazole: (1) Children weighing 13-22 kg: lansoprazole 15 mg once daily, amoxicillin 500 mg and metronidazole 250 mg twice daily; and (2) Children weighing 23–45 kg: Lansoprazole 15 mg, amoxicillin 750 mg and metronidazole 500 mg, all given twice daily. | |
| Bontems et al[41] | Group A: 10 d sequential treatment: 5 d therapy with a combination of omeprazole (10 mg b.i.d. below 30 kg body weight or 20 mg b.i.d. above 30 kg) and amoxicillin 25 mg/kg b.i.d.—maximum 2 g/d), followed by 5 d of omeprazole, clarithromycin (7.5 mg/kg b.i.d.—maximum 1 g/d), and metronidazole (10 mg/kg b.i.d.—maximum 1.5 g/d). |
| Group B: 7 d triple therapy: 7 d treatment tailored comprising omeprazole and amoxicillin with clarithromycin in cases of H. pylori strains susceptible to clarithromycin or with clarithromycin in cases of H. pylori strains susceptible to metronidazole and resistant to clarithromycin. |
Table 5 summarizes the positive and negative outcomes of the studies.
| Ref. | Adverse events | Deaths | Eradication rates | Conclusion |
| Moubri et al[22] | Mild and moderate symptoms, mainly gastrointestinal. | No | (1) ITT: Group A - Amoxicillin - based triple therapy: 68%; Group B - Metronidazole - based triple therapy: 80%; and (2) PP: Group A - Amoxicillin - based triple therapy: 71%; Group B - Metronidazole - based triple therapy: 88%. | The group B eradication rates were higher than Group A rates. The differences were only significant for PP (P < 0.03). |
| Namkin et al[23] | Loss of appetite. | No | Probiotic group: 0.40 ± 0.32 to 0.21 ± 0.27 average HpSA title; P = 0.005. Placebo group: 0.24 ± 0.2 to 0.24 ± 0.27 average HpSA title; P = 0.89. | There was no significant difference between the two groups in relation to the eradication rate of H. pylori infection (P = 0.16), however the decrease in the concentration of HpSA was significantly greater in the group treated with probiotics (P = 0.005 vs P = 0.89). |
| Akcam et al[24] | Abdominal pain, nausea, vomiting, constipation, belching, changes in taste, poor appetite and diarrhea. | No | Standard triple therapy group: 68.9%; Standard triple therapy group + probiotics: 66.6%; P = 0.78. | There was no statistically significant difference between the two groups. |
| Tolone et al[25] | Epigastric pain, nausea, vomiting, diarrhea and constipation. | No | Group A standard therapy group: 76.4%; Group B standard therapy + probiotics: 88.2%; P = 0.1. | There was no significant difference in the rate of H. pylori eradication between group A and group B, however, the side effects were significantly greater (P < 0.05) in group A than in group B. |
| Farahmand et al[26] | Vomiting, abdominal pain, iron deficiency, anemia and gastrointestinal bleeding. | No | Group A - Ciprofloxacin-based triple therapy: 87.9%; Group B - Furazolidone-based triple therapy: 60.6%; P = 0.011. | This study concludes that triple therapy consisting of ciprofloxacin, amoxicillin and omeprazole is highly valuable as H. pylori is not resistant to the antimicrobials. |
| Ahmad et al[27] | Diarrhea, nausea, vomiting and abdominal swelling. | No | Group A - Triple therapy + placebo: 69.69%; Group B - Triple therapy + probiotics: 90.09%; P = 0.04. | The group treated with the combination of probiotic and standard therapeutic regimen showed a more significant eradication rate. Supplementation with probiotics has a positive effect on H. pylori and decreases adverse events and effectiveness. |
| Bin et al[28] | Diarrhea. | 11 | Group A - Triple therapy + S. boulardii: 71.4%; Group B - Only triple therapy: 61.9%; P = 0.51 | The probiotic prevented diarrhea associated with triple H. pylori eradication therapy. In addition, when diarrhea developed, it was less severe and of shorter duration in the S. boulardii group. The probiotic increased the adherence to H. pylori eradication therapy, which may be related to a small increase in H. pylori eradication by 10 percent. |
| Kasiri et al[29] | Intolerance to clarithromycin. | No | Group A - Amoxicillin-based triple therapy: 87.2%; Group B - Metronidazole-based triple therapy: 92.5%; P = 0.43. | There was no significant difference in the complete recovery and eradication of H. pylori between the two regimens. Both therapeutic regimens were considered to be effective, since both have similar rates of eradication, recovery and side effects. |
| Iwańczak et al[30] | - | - | Group I - Amoxicillin-based triple therapy: 78.2%; Group II Metronidazole-based triple therapy: 78.2%; Group III - Sequential therapy: 91.3%; P > 0.5. | For strains susceptible to clarithromycin: treatment with amoxicillin-based triple therapy was the most effective. For regions with clarithromycin resistance greater than 20%, quadruple therapy or therapy based on the susceptibility of the strains is recommended. |
| Tümgör et al[31] | Nausea, headache, vomiting, abdominal pain and diarrhea. | - | Group A - Clarithromycin-based triple therapy: 46.6%; Group B - Clarithromycin-based triple therapy + vitamin E: 64.4%; P = 0.13. | Although, Group B showed a higher rate of eradication, no statistically significant difference was observed between the two groups. |
| Ali Habib et al[32] | - | No | Group A - Standard therapy: 55.6%; Group B - Sequential therapy: 57.1%; P = 0.949. | The rates of H. pylori eradication were not significantly different in sequential vs standard therapy. In addition, serum ferritin was not significantly different between the two therapies and in the same therapy group before and after treatment. |
| Ustundag et al[33] | Abdominal pain and nausea. | No | ITT: Group A - Standard triple therapy: 58.8%; Group B - Standard triple therapy + symbiotic: 77.1%; P = 0.16. PP: Group A: 64.5%; Group B: 81.8%; P = 0.19. | The results of the study demonstrated that the addition of Bifidobacterium lactis B94 (5 × 109 CFU/dose) plus inulin once daily to standard triple therapy did not show superiority in eradication rates compared to standard triple therapy administered alone. |
| Huang et al[34] | Nausea, vomiting and diarrhea. | - | (1) ITT: Group A – 10-d sequential therapy: 81.4%; Group B – 7 d triple standard therapy: 61.9%; Group C – 10 d triple standard therapy: 67.7%; P < 0.05; and (2) PP: Group A – 10 d sequential therapy: 89.7%; Group B – 7 d triple standard therapy: 70.8%; Group C – 10 d triple standard therapy: 77.8%; P < 0.05. | The 10 d sequential regimen was significantly more effective than the 7 or 10 d triple regimens in eradicating Chinese children. In addition, the adverse events between the three groups were also similar, with no statistical differences P > 0.05. |
| N Şirvan et al[35] | Abdominal pain, nausea and diarrhea. | - | Group 1 - Standard triple therapy + symbiotic: 88%; Group 2 - Standard triple therapy: 72%; P = 0.046. | The rate of eradication was statistically higher in group I. The addition of probiotics to triple therapy is effective in eradicating H. pylori infection in children and is generally useful in reducing or eliminating dyspeptic symptoms, such as abdominal pain, diarrhea and vomiting. |
| Baysoy et al[36] | Metallic taste sensation, abdominal pain, diarrhea, vomiting, rash, itching. | - | (1) ITT: Group A- Standard triple therapy: 46.0%; Group B - Sequential therapy group: 40.9%; and (2) PP: Group A - Standard triple therapy: 54.2%; Group B - Sequential therapy group: 48.6%. | Sequential ornidazole therapy did not show superiority compared to standard triple treatment in children with H. pylori infection. |
| Esmaeili-Dooki et al[37] | - | - | (1) ITT: Group 1 - Clarithromycin-based triple therapy: 62.5%; Group 2 - Amoxicillin-based triple therapy: 56.2%; P = 0.4; and (2) PP: Group 1 - Clarithromycin-based triple therapy: 69%; Group 1 - Amoxicillin-based triple therapy: 61.9%; P = 0.431. | The therapeutic response was observed in more than half of the patients treated with triple therapy of the H. Pylori eradication regimen, including azithromycin or clarithromycin, and there was no significant difference between the two treatment groups. |
| Laving et al[38] | - | - | Conventional therapy Group: 48.8%; 10 d sequential therapy Group: 84.6%; P = 0.02. | The sequential treatment had a significantly higher rate of H. pylori eradication than conventional treatment. |
| Prieto-Jimenez et al[39] | Abdominal pain, nausea, diarrhea, rash. | No | (1) ITT: Group I - Quadruple sequential eradication and placebo: 44.8%; Group II - Quadruple sequential eradication and iron: 43.7%; Group III: Iron and placebo: 17.4%; Group IV: Placebo alone: 7.7%; P < 0.001; and (2) PP: Group I - Quadruple sequential eradication and placebo: 56.5%; Group II - Quadruple sequential eradication and iron: 50%; Group III: Iron and placebo: 20%; Group IV: Placebo alone: 10.5%; P < 0.001. | A sequential quadruple regimen eradicated H. pylori in only half of asymptomatic children who received this treatment. Eradication rates did not differ between patients who received iron supplementation and those who received placebo. |
| Nguyen et al[40] | No reported. | No | (1) Group A: Antibiotic sensitive - 79% using high medication dosages, P = 0.278; 75% using lansoprazole twice daily, P = 0.096. Antibiotic resistant - 67.5% using high medication dosages, P = 0.006; 69.2% using lansoprazole twice daily, P = 0.004; and (2) Group B: Antibiotic sensitive - 85.2% using high medication dosages, P = 0.278; 87.5% using lansoprazole Twice daily, P = 0.096. Antibiotic resistant - 45.3% using high medication dosages, P = 0.096; 50.0% using lansoprazole Twice daily, P = 0.004. | The prevalence of resistance to clarithromycin, metronidazole, and amoxicillin was 50.9%, 65.3% and 0.5%, respectively. The two treatment regimens used did not successfully eradicate H. pylori in Vietnamese children, mainly because of the unexpectedly high prevalence of antibiotic resistance. |
| Bontems et al[41] | Abdominal pain, diarrhea, nausea and vomiting. | (1) ITT: Group A – 10 d sequential treatment: 81.9%; Group B: 7 d triple therapy: 71.9%; and (2) PP: Group B – 10 d sequential treatment: 88.3%, Group B: 7 d triple therapy: 80.8%. | The sequential treatment is greatly effective for eradicating H. pylori in children except in clarithromycin-resistant strains. Sequential treatment can be used as a first-line therapy, but only in areas with a low clarithromycin resistance rate. |
Using the Cochrane risk of bias tool[44], seven RCTs[23,26,27,34,37-39] had a low risk of bias for the following criteria: Generation of the random sequence, allocation concealment, blinding of participants and professionals, and blinding of outcome evaluators. Only the work by Akcam et al[24] was classified as high risk of bias for generating the random sequence. All other RCTs had an uncertain or low rating for all of the criteria mentioned above. In general, we observed some risks of biases through deviations from the intended interventions, represented by the concealment of how the drugs were distributed and how the researchers made recommendations to the participants. In addition, interaction with a healthcare professional can improve symptoms and treatment adherence, becoming a possible bias for all the analyzed results.
In pediatric clinical practice, H. pylori infection is common, especially in developing countries and certain populations such as ethnic minorities and migrant communities living in developed countries. In this systematic review, standard triple therapies, given for 7, 10, or 14 d were compared with sequential, third-line, and quadruple therapies for H. pylori eradication. In addition, some studies evaluated the efficacy of probiotics as adjuvant therapy for triple therapy.
Currently, triple therapies recommended by the main guidelines for H. pylori eradication include a proton pump inhibitor (PPI) or ranitidine, amoxicillin, and either clarithromycin or metronidazole, considered the first-line regimen, and bismuth, administered for 7, 10, or 14 d[45]. The desirable target of anti-H. pylori treatment regimens is to reach an eradication rate of at least 90% in the per-protocol analysis whereas antibiotic use eradication rate below 80% is considered unacceptable[46]. However, few studies have achieved this goal. The low efficacy of triple therapy observed in diverse geographic areas has been attributed to the rising resistance of H. pylori strains to clarithromycin and metronidazole, poor compliance, duration of treatment, and inadequate dosage and number of daily doses. The growing H. pylori resistance is due to the previous exposition of children to these antimicrobials that are overused to treat upper and lower respiratory diseases that are very common in childhood. Eradication rates of standard triple therapies are often below 80% in various regions of the world[47]. In this study, extremely low eradication rates were observed in China, India, Kenya, and Turkey. The frequency of H. pylori-resistant strains was 16.4%, 75.2%, and 0.06% to clarithromycin, metronidazole, and amoxicillin, respectively, in children from the southeast regions of China[48]. The resistance to clarithromycin in Turkish children ranges from 9.5% to 27%[49]. No data are available on clarithromycin resistance in Indian and Kenyan children. Concerning resistance to metronidazole, no data are available on those countries.
A trial comparing amoxicillin-based with metronidazole-based triple therapy, by ITT analysis, found that the latter showed a significantly higher eradication rate (68% vs 80%, respectively)[22]. The 7 and 10 d triple therapy failed to eradicate H. pylori infection in most of the studies[22,26,27,30,34,37-39]. In some of them, the eradication rates were less than 60%.
Esmaeili-Dooki et al[37] compared a triple therapy consisting of azithromycin once daily plus amoxicillin and omeprazole given twice daily for 6 d with clarithromycin in association with amoxicillin and omeprazole twice daily for 10 d. Based on the ITT analysis, the eradication rates in the azithromycin and clarithromycin groups were 56.2% and 62.5%, respectively (P = 0.40)[37]. AEs were 15.6% in the omeprazole, clarithromycin, and amoxicillin group and 3.1% in the omeprazole, azithromycin, and amoxicillin group (P = 0.19). Per protocol, the eradication rate was 61.9% in the azithromycin group and 69% in the clarithromycin group (P = 0.431).
High resistance of H. pylori to clarithromycin and metronidazole, poor compliance, and a short duration of treatment may explain these findings, in part. European Society for Paediatric Gastroenterology, Hepatology and Nutrition and North American Society for Paediatric Gastroenterology, and Hepatology and Nutrition guidelines recommend when antimicrobials susceptibility profiles are either unknown or H. pylori is susceptible to clarithromycin or metronidazole, a high-dose triple therapy with PPI, amoxicillin and triple for 14 d or bismuth-base quadruple therapy. In this review, the effectiveness of a triple therapy for 14 d was evaluated in eight studies. Eradication rate superior to 80% was observed in two of them[29,40]. Kasiri et al[29] did not observe a significant difference in the eradication rate between 14 d amoxicillin-based (87.2%) and for metronidazole-based triple therapy (92.5%) in Iranian children.
A study from Vietnam, triple therapy consisting of lansoprazole amoxicillin and either clarithromycin or metronidazole (LAM) therapy given once or twice daily for 14 d were compared[40]. Eradication success was associated with the strain susceptibility to clarithromycin (78.2% vs 29.3%, P = 0.0001). PPI and clarithromycin given twice daily was superior to once-daily dosage for resistant strains (50.0% vs 14.7%, P = 0.004) and tended to be effective so also for sensitive strains (87.5% vs 65.2%, P = 0.051). The differences were less pronounced with LAM when PPI was given twice daily in comparison with PPI once a day (69.2% vs 50.0%, P = 0.096). The reported resistance to clarithromycin, metronidazole, and amoxicillin was 50.9%, 65.3%, and 0.5%, respectively. The authors found that resistance to clarithromycin was an important cause for treatment failure[40]. Higher doses of PPI improve the success of eradication rate of clarithromycin and amoxicillin based-therapy[46]. Moreover, younger children need a higher PPI dose per kg of bodyweight compared to adolescents and adults to obtain sufficient acid suppression[46].
Eradication rate with triple therapy for 14 d ranged from 46% to 76.4% in five studies[24,25,31,33,36].
One study evaluated the efficacy of a third-line therapy. Farahmand et al[26] compared a regimen consisting of ciprofloxacin, amoxicillin, and omeprazole, third-line therapy, with the standard triple therapy, amoxicillin, and omeprazole twice a day plus furazolidone once a day. Both regimens given for 1 wk reported that the eradication rate was significantly higher (P = 0.011) in the group treated with ciprofloxacin (87.9%) than in that receiving furazolidone (60.6%)[32].
Probiotics have been proposed as an adjuvant to triple therapy to improve the efficacy and diminish AEs in both children and adults. Diarrhea, nausea, and vomiting are the most frequent side effects of eradication therapy and are an important cause of poor compliance and treatment failure.
Several studies did not show an increase in the eradication rate when triple therapy was supplemented with probiotics[23,24,25,33], by contrast, an increase in the H. pylori eradication rate was observed by others[27,28,35]. Although the beneficial effects of probiotics depend on the strains of the microorganisms selected, more robust studies and meta-analyses on this issue should be performed to clarify these discrepant results[50]. Of note, some studies demonstrated a statistically significant decrease in adverse gastrointestinal effects during the treatment[25,27,28,35]. This result was also observed in a meta-analysis using multiple strains to eradicate H. pylori and prevent AEs, in children and adults[51]. Another meta-analysis observed that the addition of Lactobacillus, Bifidobacterium, and Saccharomyces to standard triple therapy improved medication tolerance and patient compliance due to the decrease in side effects, both in children and adults[52]. In the studies included in this review, commercial probiotics were used; however, the use in clinical practice is not economically accessible for many countries with high prevalence rates of H. pylori.
Tümgör et al[31] evaluated the use of vitamin E with clarithromycin-based triple therapy in children and found no statistically significant difference between its eradication rate and triple therapy alone. Although the addition of antioxidant vitamins in the eradication treatments has been evaluated[53,54], no statistically significant differences were observed. It is important to highlight that the available studies on this therapeutic alternative have a small sample size and to moderate methodological design[53].
Sequential therapy has been related to high H. pylori eradication rates[47], and some articles included here corroborated this, showing success rates of 91.3%, 81.4%, and 84.6%, whereas the effectiveness of standard triple therapy ranged from 48.8% to 78.2%[30,34,38]. However, two studies did not observe a statistically significant increase in eradication rates when this regimen was used[32,36]. A meta-analysis that evaluated sequential therapy compared to triple therapy in 13 RCTs also found a higher rate of H. pylori eradication in children when using sequential therapy, in accordance with the results of the articles analyzed in this review[55]. Although it is a therapeutic regimen with encouraging rates of H. pylori eradication, more robust studies are necessary to prove its effectiveness and safety to substitute triple therapy in high clarithromycin and/or nitroimidazole resistance settings[56].
This study had some limitations due to the small number of available studies evaluating each therapeutic regimen in children. The quality of included studies, the great diversity of treatment regimens, and the duration of treatment, doses, and administration frequency of the drugs as well as lack of antimicrobial susceptibility tests limit the comparison of the results. Most studies had an uncertain degree of bias for concealing the processes of allocation of patients as well as the blinding of participants, professionals, and outcome evaluators.
In summary, the eradication rate associated with current treatments is not satisfactory in many geographical areas. Unfortunately, many of the published works on H. pylori eradication in children have weaknesses in their methods and do not meet the ideal scientific criteria to be indicated in practice. It has to be emphasized that, because H. pylori is disappearing in developed, studies investigating H. pylori treatment are scarce in adults and children. Otherwise, a number of countries that have a high prevalence of H. pylori infection face difficulties in conducting research with a good level of evidence due to the lack of structural and financial supports. Finally, the studies were limited to a few countries (n = 10), and good results observed in some studies may not work well in other geographical areas.
In conclusion, although some studies support the use of new therapeutic regimens in the treatment of H. pylori infection in children, more methodologically reliable prospective studies evaluating the most promising new therapeutic regimens are needed to assess the applicability of these treatments in pediatric clinical practice.
Helicobacter pylori (H. pylori) is a gram-negative microaerophilic bacterium that infects the gastric epithelium and whose acquisition occurs mainly during childhood. In the last several years, no significant changes in the treatment of infected children have been observed, mainly due to the lack of studies with satisfactory scientific evidence to support the indication of new therapies in clinical practice. This systematic review evaluated the eradication rates of H. pylori infection using various therapeutic regimens and their positive and negative outcomes in pediatric patients.
Standard triple therapy for the eradication of H. pylori infection has been used as first-line treatment in children worldwide. However, the effectiveness of standard triple therapy in eradicating H. pylori is decreasing in various geographical areas as a consequence of increasing bacterial resistance to clarithromycin and nitroimidazoles.
To compare the eradication rates of H. pylori infection in childhood in controlled, randomized, and prospective studies evaluating different therapeutic schemes during the last 10 years.
We systematically reviewed in PubMed and MEDLINE relevant publications from 2010 to April 2020. Twenty studies were shortlisted. This systematic review uses guidance from the PRISMA checklist.
The results were quite heterogeneous. Standard triple therapy is still the most used regimen and its eradication rates vary according to the H. pylori susceptibility profiles in different world regions. The addition of probiotics to therapeutic schemes shows discrepant results in eradication rate, but decrease the incidence of side effects and increases the treatment adherence. Sequential therapy has been associated with higher eradication rates than triple therapies and is a promising therapeutic regimen for this population.
Currently, standard triple therapy is the most recommended H. pylori eradication regimen for children worldwide. However, other therapeutic schemes have shown promising results in controlled trials and in a near future may be included in the guidelines recommendations.
There are still few studies with satisfactory evidence levels evaluating the eradication of H. pylori infection in children, mainly due to the difficulties to conduct controlled clinical trials as well as to the low availability of sources for research in many developing countries where the prevalence of H. pylori infection remain elevated. Well-designed studies evaluating treatments for H. pylori eradication in children are needed to further evaluate new therapeutic options in pediatric clinical practice in high bacterial resistance settings.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kanižaj TF S-Editor: Wang JL L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1816] [Article Influence: 82.5] [Reference Citation Analysis (3)] |
| 2. | Sherman PM. Appropriate strategies for testing and treating Helicobacter pylori in children: when and how? Am J Med. 2004;117 Suppl 5A:30S-35S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kuipers EJ, Peña AS, van Kamp G, Uyterlinde AM, Pals G, Pels NF, Kurz-Pohlmann E, Meuwissen SG. Seroconversion for Helicobacter pylori. Lancet. 1993;342:328-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 157] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Pacifico L, Anania C, Osborn JF, Ferraro F, Chiesa C. Consequences of Helicobacter pylori infection in children. World J Gastroenterol. 2010;16:5181-5194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 5. | Ortiz-Princz D, Daoud G, Salgado-Sabel A, Cavazza ME. Helicobacter pylori infection in children: should it be carefully assessed? Eur Rev Med Pharmacol Sci. 2016;20:1798-1813. [PubMed] [Cited in This Article: ] |
| 6. | Razavi A, Bagheri N, Azadegan-Dehkordi F, Shirzad M, Rahimian G, Rafieian-Kopaei M, Shirzad H. Comparative Immune Response in Children and Adults with H. pylori Infection. J Immunol Res. 2015;2015:315957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, Fonseca de Castro LP, Carvalho SD, Bittencourt PF, de Oliveira CA, Corrêa-Oliveira R, Magalhães Queiroz DM. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect. 2012;14:341-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Camorlinga-Ponce M, Muñoz L, Fuentes-Panana E, Torres J. Clinical consequences of Helicobacter pylori infection in children and its relation with the response of the gastric mucosa to the infection. Boletín médico del Hospital Infantil de México. 2014;71:2-7. [Cited in This Article: ] |
| 9. | Queiroz DM, Mendes EN, Carvalho AS, Rocha GA, Oliveira AM, Soares TF, Santos A, Cabral MM, Nogueira AM. Factors associated with Helicobacter pylori infection by a cagA-positive strain in children. J Infect Dis. 2000;181:626-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Yang HR. Updates on the Diagnosis of Helicobacter pylori Infection in Children: What Are the Differences between Adults and Children? Pediatr Gastroenterol Hepatol Nutr. 2016;19:96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Biernat MM, Gościniak G, Iwańczak B. Prevalence of Helicobacter pylori cagA, vacA, iceA, babA2 genotypes in Polish children and adolescents with gastroduodenal disease. Postepy Hig Med Dosw (Online). 2014;68:1015-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 12. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1745] [Article Influence: 249.3] [Reference Citation Analysis (1)] |
| 13. | Zamani M, Zamani V, Derakhshan MH, Shokri-Shirvani J. The efficacy of first-line regimens for Helicobacter pylori eradication in different continents: A systematic review and network meta-analysis protocol. Medicine (Baltimore). 2018;97:e13682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Goderska K, Agudo Pena S, Alarcon T. Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol. 2018;102:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 15. | Chen Q, Long X, Ji Y, Liang X, Li D, Gao H, Xu B, Liu M, Chen Y, Sun Y, Zhao Y, Xu G, Song Y, Yu L, Zhang W, Liu W, Graham DY, Lu H. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther. 2019;49:1385-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Lehours P, Ferrero RL. Review: Helicobacter: Inflammation, immunology, and vaccines. Helicobacter. 2019;24 Suppl 1:e12644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 475] [Article Influence: 59.4] [Reference Citation Analysis (2)] |
| 18. | Okuda M, Lin Y, Wang C, Kakiuchi T, Kikuchi S. Metronidazole for Helicobacter pylori eradication therapy among children and adolescents in Japan: Overcoming controversies and concerns. Helicobacter. 2019;24:e12575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Erdur B, Ozturk Y, Gurbuz ED, Yilmaz O. Comparison of sequential and standard therapy for Helicobacter pylori eradication in children and investigation of clarithromycin resistance. J Pediatr Gastroenterol Nutr. 2012;55:530-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Zhu XL, Liu Z, Wu ZQ, Li D, Jiang AP, Yu GX. [Clinical effects of different therapeutic regimens for Helicobacter pylori infection in children]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:672-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 21. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3566] [Cited by in F6Publishing: 3859] [Article Influence: 257.3] [Reference Citation Analysis (0)] |
| 22. | Moubri M, Kalach N, Larras R, Berrah H, Mouffok F, Guechi Z, Cadranel S. Adapted first-line treatment of Helicobacter pylori infection in Algerian children. Ann Gastroenterol. 2019;32:60-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Namkin K, Zardast M, Basirinejad F. Saccharomyces Boulardii in Helicobacter Pylori Eradication in Children: A Randomized Trial From Iran. Iran J Pediatr. 2016;26:e3768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Akcam M, Koca T, Salman H, Karahan N. The effects of probiotics on treatment of Helicobacter pylori eradication in children. Saudi Med J. 2015;36:286-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Tolone S, Pellino V, Vitaliti G, Lanzafame A, Tolone C. Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Ital J Pediatr. 2012;38:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Farahmand F, Mohammadi T, Najafi M, Fallahi G, Khodadad A, Motamed F, Mahdi Marashi S, Shoaran M, Nabavizadeh Rafsanjani R. Comparison of Ciprofloxacin-Based Triple Therapy with Conventional Triple Regimen for Helicobacter pylori Eradication in Children. Acta Med Iran. 2016;54:395-400. [PubMed] [Cited in This Article: ] |
| 27. | Ahmad K, Fatemeh F, Mehri N, Maryam S. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23:79-84. [PubMed] [Cited in This Article: ] |
| 28. | Bin Z, Ya-Zheng X, Zhao-Hui D, Bo C, Li-Rong J, Vandenplas Y. The Efficacy of Saccharomyces boulardii CNCM I-745 in Addition to Standard Helicobacter pylori Eradication Treatment in Children. Pediatr Gastroenterol Hepatol Nutr. 2015;18:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Kasiri KA, Khoshdel A, Karimi A, Sedehi M, Kasiri N. Comparison of amoxicillin and metronidazole effect on three-drug regimen for the treatment of Helicobacter pylori infection in children. J Adv Pharm Technol Res. 2017;8:63-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 30. | Iwańczak BM, Borys-Iwanicka A, Biernat M, Gościniak G. Assessment of Sequential and Standard Triple Therapy in Treatment of Helicobacter pylori Infection in Children Dependent on Bacteria Sensitivity to Antibiotics. Adv Clin Exp Med. 2016;25:701-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Tümgör G, Baran M, Çakır M, Yüksekkaya HA, Aydoğdu S. Comparison of standard and standard plus vitamin E therapy for Helicobacter pylori eradications in children. Turk J Gastroenterol. 2014;25 Suppl 1:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Ali Habib HS, Murad HA, Amir EM, Halawa TF. Effect of sequential versus standard Helicobacter pylori eradication therapy on the associated iron deficiency anemia in children. Indian J Pharmacol. 2013;45:470-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Ustundag GH, Altuntas H, Soysal YD, Kokturk F. The Effects of Synbiotic "Bifidobacterium lactis B94 plus Inulin" Addition on Standard Triple Therapy of Helicobacter pylori Eradication in Children. Can J Gastroenterol Hepatol. 2017;2017:8130596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Huang J, Zhou L, Geng L, Yang M, Xu XW, Ding ZL, Mao M, Wang ZL, Li ZL, Li DY, Gong ST. Randomised controlled trial: sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Aliment Pharmacol Ther. 2013;38:1230-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | N Şirvan B, K Usta M, U Kizilkan N, Urganci N. Are Synbiotics added to the Standard Therapy to eradicate Helicobacter pylori in Children Beneficial? A Randomized Controlled Study. Euroasian J Hepatogastroenterol. 2017;7:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Baysoy G, Saltık Temızel İN, Uslu N, Balamtekın N, Demır H, Gürkan F, Özen H, Akyön Y, Yüce A. Ornidazole-based sequential therapy is not effective in Helicobacter pylori eradication in children. Turk J Gastroenterol. 2013;24:382-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Esmaeili-Dooki MR, Shirdel H, Hajiahmadi M. Eradication of Helicobacter pylori in Children by Triple Therapy Regimens of Amoxicillin, Omeprazole, and Clarithromycin or Azithromycin. Iran J Pediatr. 2015;25:e2360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Laving A, Kamenwa R, Sayed S, Kimang'a AN, Revathi G. Effectiveness of sequential v. standard triple therapy for treatment of Helicobacter pylori infection in children in Nairobi, Kenya. S Afr Med J. 2013;103:921-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Prieto-Jimenez CA, Cardenas VM, Fischbach LA, Mulla ZD, Rivera JO, Dominguez DC, Graham DY, Ortiz M. Double-blind randomized trial of quadruple sequential Helicobacter pylori eradication therapy in asymptomatic infected children in El Paso, Texas. J Pediatr Gastroenterol Nutr. 2011;52:319-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Nguyen TV, Bengtsson C, Yin L, Nguyen GK, Hoang TT, Phung DC, Sörberg M, Granström M. Eradication of Helicobacter pylori in children in Vietnam in relation to antibiotic resistance. Helicobacter. 2012;17:319-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Bontems P, Kalach N, Oderda G, Salame A, Muyshont L, Miendje DY, Raymond J, Cadranel S, Scaillon M. Sequential therapy versus tailored triple therapies for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:646-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Cochrane Training. Cochrane Handbook for Systematic Reviews of Interventions. 2019 [cited 20 May 2020]. In: Cochrane website [Internet]. Cochrane Training. Available from: URL: https://training.cochrane.org/handbook/current. [Cited in This Article: ] |
| 43. | Centre for Evidence-Based Medicine. Oxford Centre for Evidence-based Medicine â Levels of Evidence. 2009 Mar [Cited 27 April 2020]. In: CEBM website [Internet]. Oxford: CEBM. Available from: URL: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. [Cited in This Article: ] |
| 44. | Cochrane Training. Assessing risk of bias in a randomized trial. 2019 [cited 20 May 2020]. In: Cochrane website [Internet]. Cochrane training. Available from: URL: https://training.cochrane.org/handbook/current/chapter-08. [Cited in This Article: ] |
| 45. | Gold BD, Colletti RB, Abbott M, Czinn SJ, Elitsur Y, Hassall E, Macarthur C, Snyder J, Sherman PM; North American Society for Pediatric Gastroenterology and Nutrition. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000;31:490-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 46. | Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, Czinn S, Gold BD, Guarner J, Elitsur Y, Homan M, Kalach N, Kori M, Madrazo A, Megraud F, Papadopoulou A, Rowland M, ESPGHAN, NASPGHAN. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017;64:991-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 242] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 47. | Rajindrajith S, Devanarayana NM, de Silva HJ. Helicobacter pylori infection in children. Saudi J Gastroenterol. 2009;15:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Kotilea K, Bontems P, Touati E. Epidemiology, Diagnosis and Risk Factors of Helicobacter pylori Infection. Adv Exp Med Biol. 2019;1149:17-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 49. | Güven B, Gülerman F, Kaçmaz B. Helicobacter pylori resistance to clarithromycin and fluoroquinolones in a pediatric population in Turkey: A cross-sectional study. Helicobacter. 2019;24:e12581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] [Cited in This Article: ] |
| 51. | Khurana R, Fischbach L, Chiba N, VAN Zanten SV, Sherman PM, George BA, Goodman KJ, Gold BD. Meta-analysis: Helicobacter pylori eradication treatment efficacy in children. Aliment Pharmacol Ther. 2007;25:523-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol. 2015;21:10644-10653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (3)] |
| 53. | McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4:546-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Lau CS, Ward A, Chamberlain RS. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis. Infect Drug Resist. 2016;9:275-289. [PubMed] [Cited in This Article: ] |
| 55. | Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069-79; quiz 1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 56. | Kate V, Kalayarasan R, Ananthakrishnan N. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a systematic review of recent evidence. Drugs. 2013;73:815-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |