Published online Aug 26, 2013. doi: 10.13105/wjma.v1.i2.90
Revised: May 23, 2013
Accepted: June 1, 2013
Published online: August 26, 2013
AIM: To determine the relationship between subclinical hypothyroidism (SCH) and the metabolic syndrome (MS).
METHODS: We performed a systematic search of databases [MEDLINE (July 1950 to July 2012), EMBASE (July 1966 to July 2012)] and the references of identified studies. Completely published cross-sectional studies of a general population involving SCH and the MS were included. The pooled odds ratio and weighted mean difference (WMD) for the outcomes were calculated using random-effects models.
RESULTS: Six cross-sectional studies with 19546 participants were included. In total, 398 of 1324 participants (30.06%) in the SCH group had the MS compared with 4975 of 18222 participants (27.30%) in the euthyroid group [OR = 1.20; 95%CI: 1.05-1.36; P = 0.004; χ2 = 2.53 (P = 0.773); I2 = 0%]. Further analysis of the components of the MS showed that SCH was associated with increased body mass index (WMD, 0.32 kg/m2; 95%CI: 0.04-0.61; P = 0.026), systolic blood pressure (WMD, 2.62 mmHg; 95%CI: 1.35-3.89; P < 0.001) and triglyceride (WMD, 0.25 mmol/L; 95%CI: 0.23-0.28; P < 0.001).
CONCLUSION: Based on the cross-sectional data, SCH may be associated with an increased risk of the MS, which could be attributed to the increased risk of metabolic components.
Core tip: A recent meta-analysis of individual data concluded that subclinical hypothyroidism (SCH) is associated with an increased risk of coronary heart disease (CHD) and CHD mortality. Meanwhile, it has been well recognized that the metabolic syndrome (MS) is associated with increased cardiovascular events and all-cause mortality. Our meta-analysis of cross-sectional data demonstrated that SCH may be associated with an increased risk of the MS, which may explain the relationship between SCH and increased risk of CHD.
- Citation: Ye YC, Xie HZ, Zhao XL, Zhang SY. Subclinical hypothyroidism and the metabolic syndrome: A meta-analysis of cross-sectional studies. World J Meta-Anal 2013; 1(2): 90-96
- URL: https://www.wjgnet.com/2308-3840/full/v1/i2/90.htm
- DOI: https://dx.doi.org/10.13105/wjma.v1.i2.90
Subclinical thyroid disease is defined biochemically and subclinical hypothyroidism (SCH) occurs when serum thyroid-stimulating hormone (TSH) concentrations are raised and serum thyroid hormone concentrations are normal[1]. Over the past several decades, a plethora of publications has established that SCH is associated with cardiovascular disease and a meta-analysis of individual data by Rodondi et al[2] concluded that SCH is associated with an increased risk of coronary heart disease (CHD) and CHD mortality in those with higher TSH levels, particularly in those with a TSH concentration of 10 mIU/L or greater.
The metabolic syndrome (MS) is a cluster of multiple cardiovascular risk factors, including central obesity, glucose intolerance, diabetes, dyslipidemia and elevated blood pressure[3]. It has been well recognized that the MS is associated with increased cardiovascular events and all-cause mortality[4]. Recently, several cross-sectional studies have investigated a potential relationship between SCH and the MS, with conflicting findings[5-10]. To clarify this issue, we performed a meta-analysis of the published cross-sectional data in a general population to determine the association between SCH and the MS.
We designed a protocol that detailed the objective of our analysis, the criteria for study inclusion/exclusion, the assessment of study quality, the primary outcome and the statistical methods in accordance with the meta-analysis of observational studies in epidemiology[11] and PRISMA statements[12].
We conducted a search of MEDLINE (1950 to July 2012) and EMBASE (1966 to July 2012) via EMBASE.com to identify all cross-sectional studies of a general population involving SCH and the MS [search strategy: (1) “hypothyroidism”/exp OR hypothyroidism; (2) “thyroid dysfunction”/exp OR thyroid dysfunction; (3) “thyroid disease”/exp OR thyroid disease; (4) “thyrotropin”/exp OR thyrotropin; (5) “thyroid stimulation hormone”/exp OR thyroid stimulation hormone; (6) “MS x”/exp OR MS x; (7) “MS”/exp OR MS; (8) 1 OR 2 OR 3 OR 4 OR 5; (9) 6 OR 7; (10) 8 AND 9], as well as a search of the Cochrane Database for Systemic Reviews. In addition, we performed a manual search of the literature using the references of original manuscripts, reviews and meta-analyses.
Two reviewers (Ye YC and Xie HZ) independently determined the study eligibility. Disagreements were resolved by consensus. The eligibility criteria for study inclusion were: (1) cross-sectional studies; (2) studies of a general population; (3) studies that reported the numbers of participants with the MS from among subjects with SCH and euthyroid; and (4) completely published studies (exclusive of unpublished material and abstracts). No language restriction was imposed. The κ value between two reviewers (Ye YC and Xie HZ) was 0.97 for the first screen (based on title and abstract) and 1.0 for the full-text screen.
Data extraction was carried out independently by two authors in duplicate (Ye YC and Xie HZ). Disagreements were resolved by discussion between the two reviewing authors. From each included study, information was extracted on: (1) the characteristics of the study population; (2) the criteria for the MS; (3) TSH reference; (4) the numbers of participants with the MS in SCH and euthyroid groups; and (5) the components of the MS in SCH and euthyroid groups [including waist circumference, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose, triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C)].
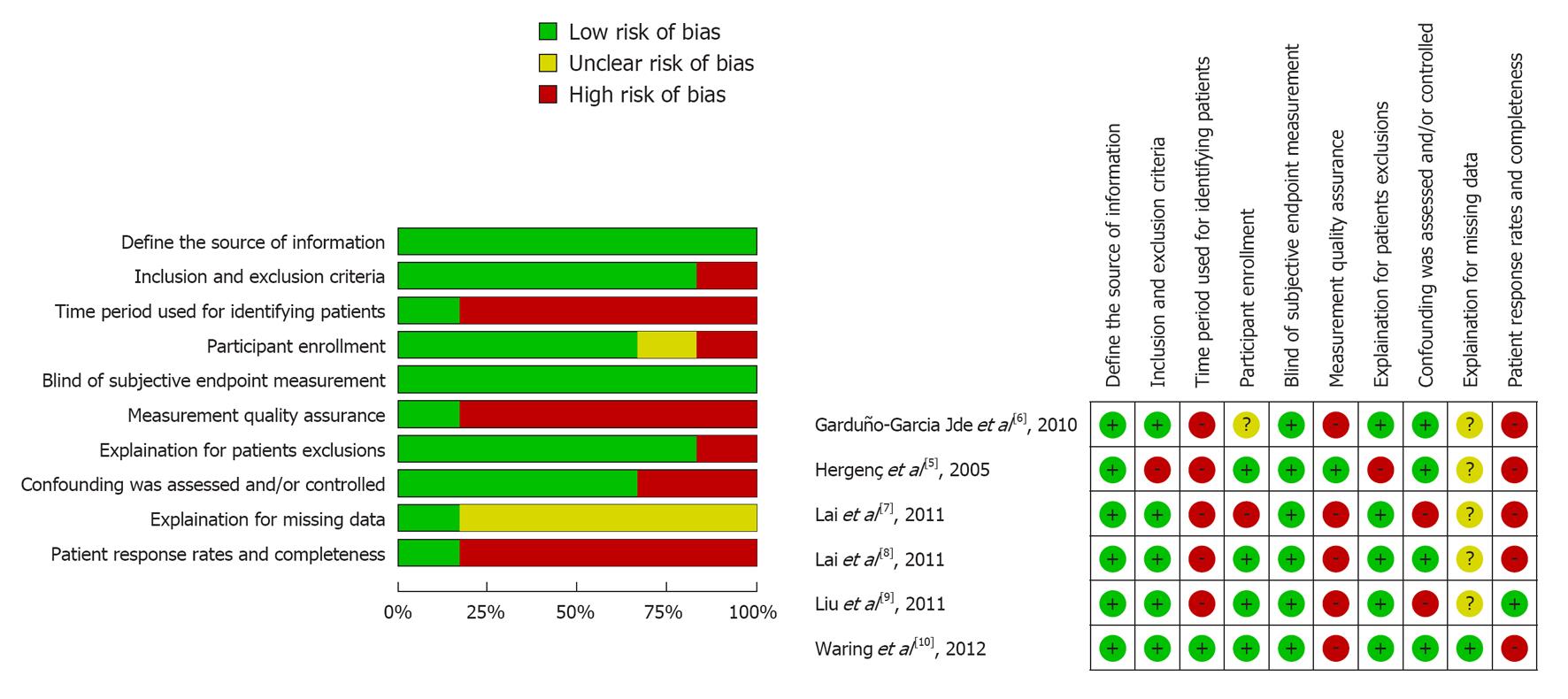
Risk of bias was described and judged in following domains[13]: (1) Define the source of information (survey, record review); (2) List inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications; (3) Indicate time period used for identifying individuals; (4) Indicate whether or not subjects were consecutively enrolled if not population-based; (5) Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants; (6) Describe any assessments undertaken for quality assurance purposes (e.g., test/retest of primary outcome measurements); (7) Explain any individual exclusions from analysis; (8) Describe how confounding was assessed and/or controlled; (9) If applicable, explain how missing data were handled in the analysis; and (10) Summarize individual response rates and completeness of data collection. The judgments were assessed independently by two authors (Ye YC and Xie HZ) based on the published report and protocols of the studies. Disagreements were resolved by consensus. The judgments involved the answers “yes” (indicating a low risk of bias), “no” (indicating a high risk of bias), and “unclear” (if risk of bias is unknown or if an entry is not relevant to the study).
The primary outcome was the unadjusted OR for the MS between the SCH and the euthyroid group. The secondary outcomes were the weighted mean difference (WMD) of the components of the MS between the SCH and the euthyroid group. The pooled unadjusted ORs and WMD for the outcomes were calculated using fixed effects models since there may be potential heterogeneity among the studies. The heterogeneity between the results of different studies was examined using χ2 tests for significance (a P-value < 0.10 was considered to be statistically significant) and the inconsistence was examined using I2 tests. Subgroup analysis was used to explore the potential race heterogeneity (Chinese population and non Chinese population). Sensitivity analysis was used to explore the degree to which the main findings of our meta-analysis were affected by individual studies. Publication bias was assessed by the Begg’s funnel plot and the Egger weighted regression statistic, with a value of P < 0.10 indicating significant publication bias among the included studies[14,15]. All statistical analyses were performed using STATA 11.0 (STATA, TX, United States).
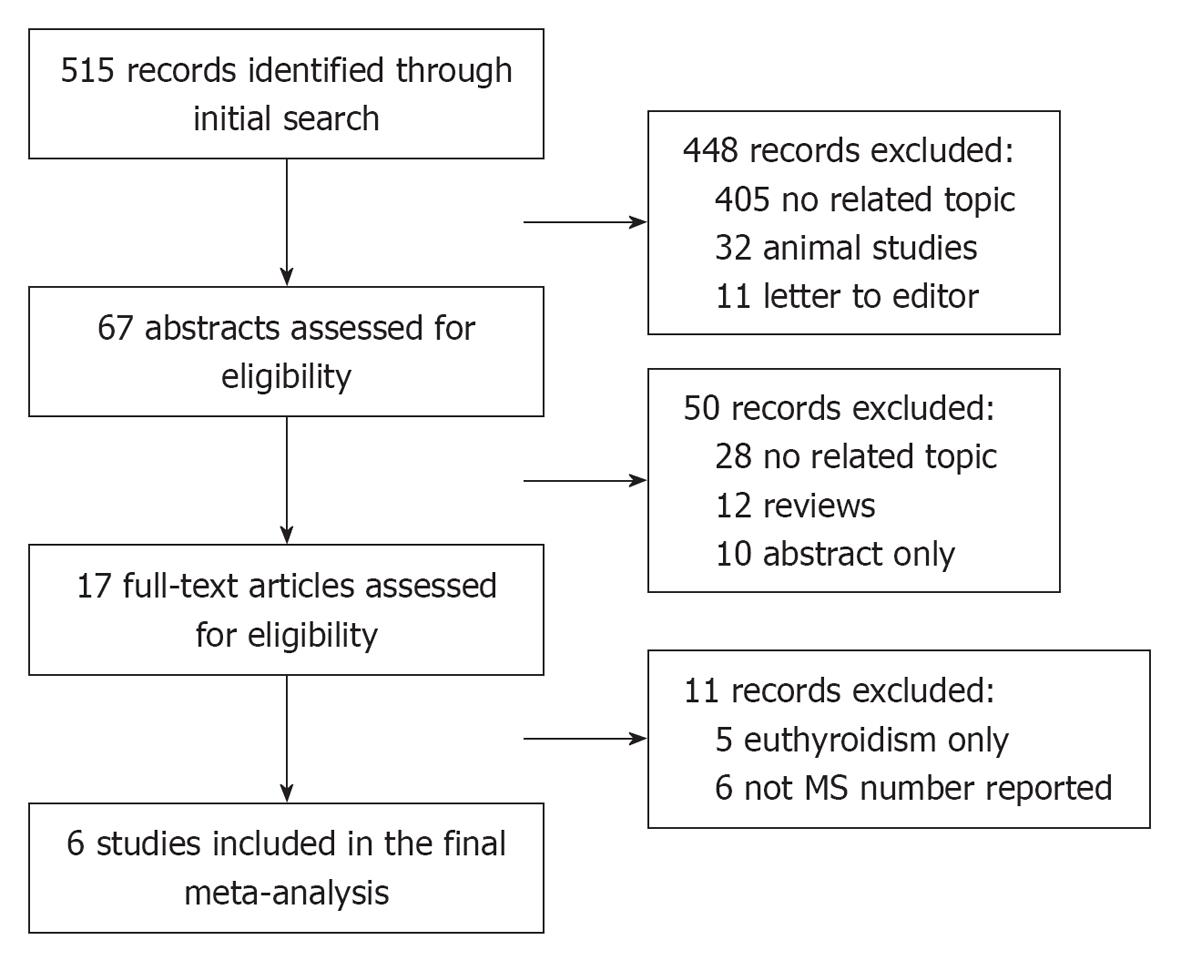
In total, 515 studies were retrieved from the initial search and 17 studies were reviewed in full text. Six cross-sectional studies investigating a total of 19546 participants were included in the meta-analysis (Figure 1).
The analysis included a total of 1324 SCH and 18222 euthyroid is imparticipants. In the individual studies, the sample size ranged from 488 to 6339. The reported mean age of the participants ranged from 42.3 to 73.6 years. Each study included both males and females. Table 1 presents a summary of the characteristics of the study population, the criteria for the MS, the TSH reference and the prevalence of the MS in SCH and euthyroid groups. Of the six studies included, four provided data on the components of the MS in SCH and euthyroid groups (Table 2). The results of quality assessment of included studies (risk of bias) were summarized and are presented in Figure 2.
| Studies | Age (yr) | Male (%) | MS criteria | Sample size | Definitionof SCH | Euthyroid | SCH | Inclusion criteria | Exclusion criteria | ||||
| No. | MS | mTSH | No. | MS | mTSH | ||||||||
| Hergenç et al[5] | 51.99 | 46.70 | NCEP /ATPIII | 488 | TSH > 4.2 μU/mL | 465 | 193 | 1.12 | 23 | 10 | 7.54 | Age > 34 yr | NA |
| Garduño-Garcia Jde et al[6] | 42.3 | 48.80 | NCEP /ATPIII | 3033 | TSH: 4.5-10 mIU/L | 2771 | 876 | NA | 262 | 84 | NA | Aged 18-70 yr | Thyroid disease, diabetes, cardiovascular disease, cerebral vascular disease, amputations, pregnancy, corticosteroid use, active liver disease, and renal dysfunction |
| FT4: 10-25 pmol/L | |||||||||||||
| Lai et al[7] | NA | NA | NCEP /ATPIII | 6254 | TSH > 5.0 μU /mL | 6123 | 1871 | NA | 131 | 43 | 8.5 | Age > 65 yr | Taking medications for control of glucose, blood pressure, dyslipidemia, and thyroid function |
| Normal FT4 | |||||||||||||
| Lai et al[8] | 45.1 | NA | China Diabetes Society | 1385 | TSH > 4.8 mIU/L | 1283 | 238 | NA | 102 | 25 | NA | Aged 18-85 yr | History of thyroid disease; Taking medication such as thyroxine/antithyroid drugs, glucocorticoid, antiepileptic and contraceptive drugs; Pregnant/within the first year of postpartum period; Overt hypothyroidism/hyperthyroidism |
| Normal FT3/FT4 | |||||||||||||
| Liu et al[9] | 48.9 | 38.20 | International Diabetes Federation | 6339 | TSH > 4.5 mIU/L | 5801 | 1236 | 2 | 538 | 138 | 6.5 | Aged 20-88 yr | Pregnant; Severe renal, liver or heart failure, or abdominal ascites; Taking medicines influencing thyroidal function |
| Normal FT3/FT4 | |||||||||||||
| Waring et al[10] | 73.6 | 47 | NCEP /ATPIII | 2047 | TSH > 0.35 mIU/L | 1779 | 561 | NA | 268 | 98 | NA | Aged 70-79 yr | Diabetes; Taking medication such as amiodarone, lithium and antithyroid medications |
| Studies | Thyroid function | n | Waist(cm) | BMI(kg/m2) | SBP(mmHg) | DBP(mmHg) | FBG(mmol/L) | TG(mmol/L) | HDL-C(mmol/L) |
| Hergenç et al[5] | Euthyroid | 465 | 95.4 ± 10.5 | 29.3 ± 4.5 | 126.5 ± 20.6 | 80.9 ± 10.9 | 5.77 ± 2.30 | 1.92 ± 1.24 | 1.14 ± 0.32 |
| SCH | 23 | 93.0 ± 10.5 | 29.0 ± 4.2 | 121.6 ± 23.8 | 78.8 ± 13.2 | 5.66 ± 1.51 | 1.81 ± 1.11 | 1.16 ± 0.30 | |
| Garduño-Garcia Jde et al[6] | Euthyroid | 2771 | 94.21 ± 11.18 | 28.69 ± 4.5 | 115 ± 15.12 | 77.29 ± 10.68 | 4.90 ± 1.16 | 2.29 ± 1.73 | 1.10 ± 0.29 |
| SCH | 262 | 94.06 ± 11.18 | 28.98 ± 5.1 | 118 ± 16.23 | 78.22 ± 10.87 | 4.91 ± 0.973 | 2.59 ± 4.03 | 1.13 ± 0.29 | |
| Lai et al[8] | Euthyroid | 1283 | 80.8 ± 10.3 | 24.3 ± 3.6 | 123 ± 18 | 79 ± 11 | 5.22 ± 0.03 | 1.47 ± 0.03 | 1.33 ± 0.37 |
| SCH | 102 | 80.7 ± 9.6 | 24.5 ± 3.3 | 122 ± 19 | 77 ± 11 | 5.12 ± 0.11 | 1.73 ± 0.12 | 1.26 ± 0.27 | |
| Liu et al[9] | Euthyroid | 5801 | 80.4 ± 11.1 | 24.4 ± 6.6 | 126.8 ± 19.2 | 81.9 ± 11.3 | 5.2 ± 1.7 | 1.5 ± 1.6 | 1.3 ± 0.4 |
| SCH | 538 | 81.1 ± 12.4 | 24.8 ± 3.9 | 130.2 ± 20.8 | 83.4 ± 12.0 | 5.3 ± 1.5 | 1.6 ± 1.7 | 1.3 ± 0.6 |
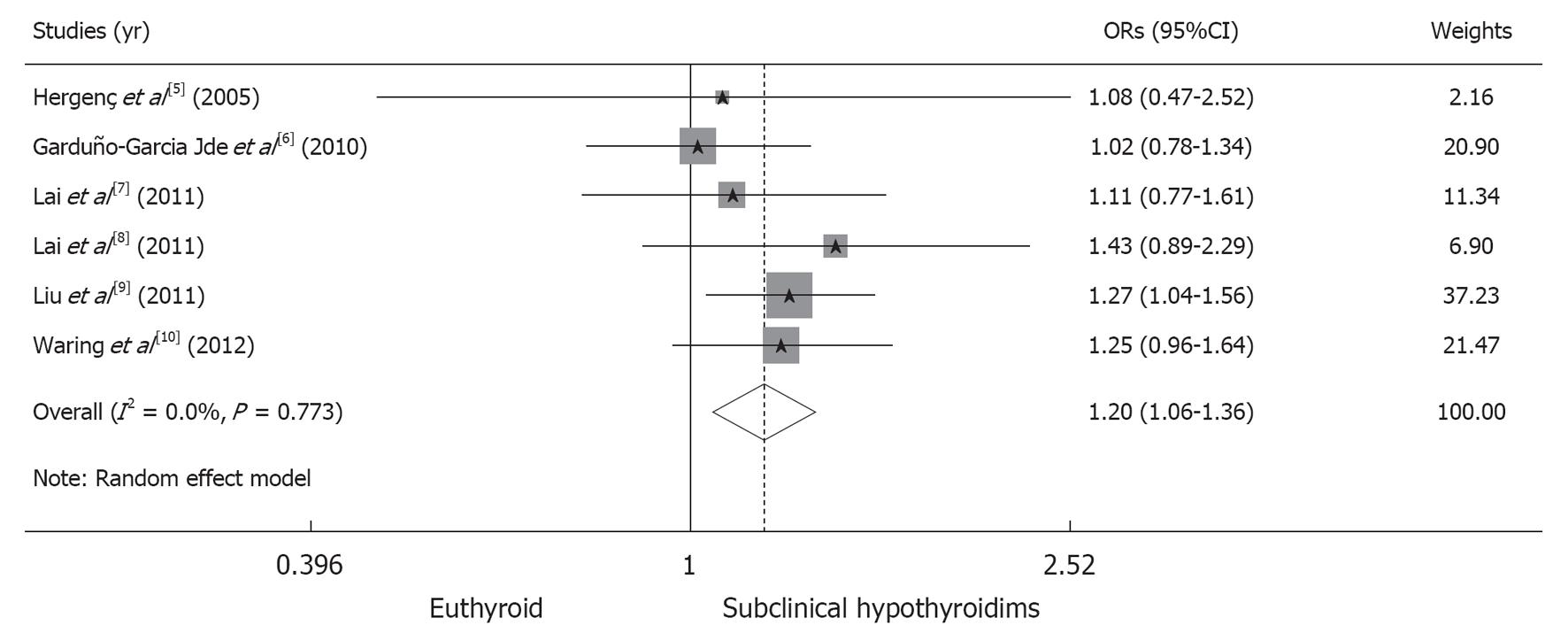
In total, 4975 of the 18222 participants (27.30%) in the euthyroid group fulfilled the criteria for the MS, compared to 398 of the 1324 participants (30.06%) in the SCH group. The pooled risk of the MS is significantly higher in the SCH group than in the euthyroid group (OR, 1.20; 95%CI: 1.05-1.36; P = 0.004). We obtained a χ2 value of 2.53 (P = 0.773) and an I2 value of 0%, indicating the absence of heterogeneity among the studies analyzed (Figure 3).
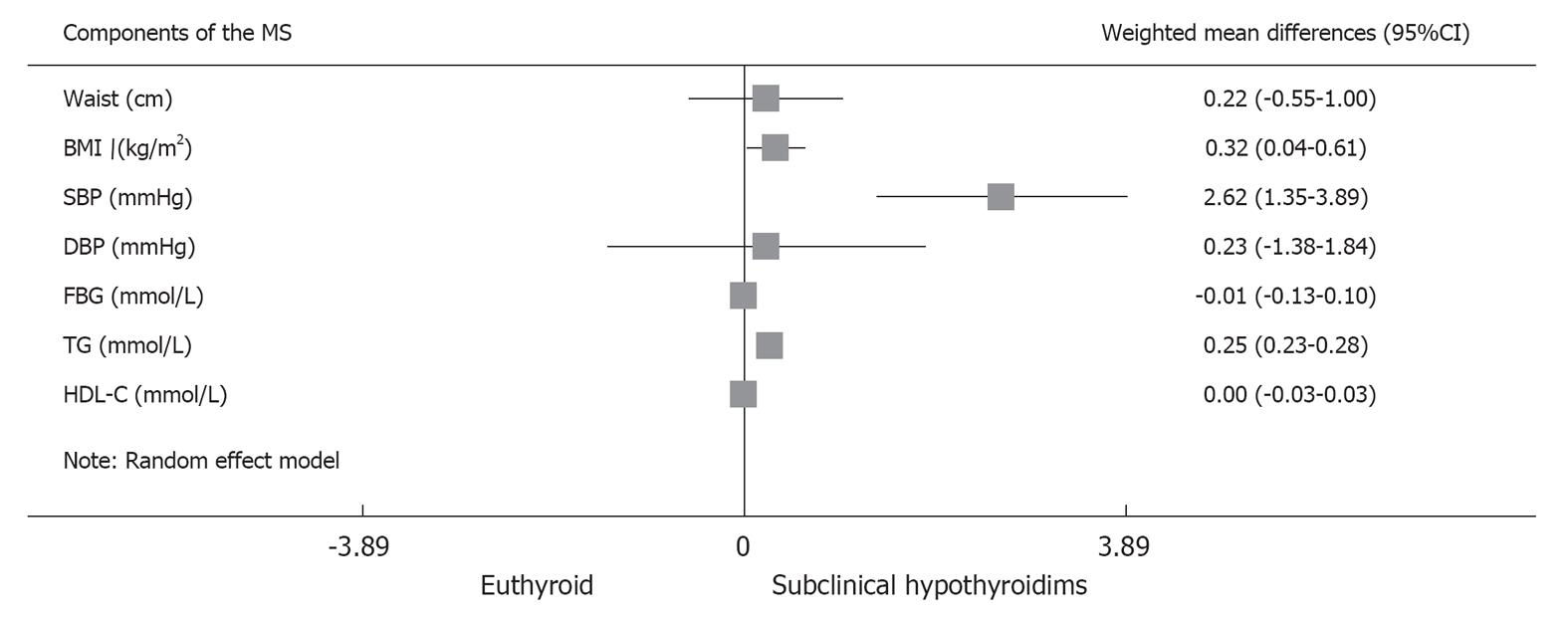
Further meta-analysis of the components of the MS showed that SCH was associated with increased BMI (WMD, 0.32 kg/m2; 95%CI: 0.04-0.61; P = 0.026), SBP (WMD, 2.62 mmHg; 95%CI: 1.35-3.89; P < 0.001) and TG (WMD, 0.25 mmol/L; 95%CI: 0.23-0.28; P < 0.001). The rest of the components, including waist circumference, DBP, fasting blood glucose and HDL-C, were not significantly different between the two groups (Figure 4).
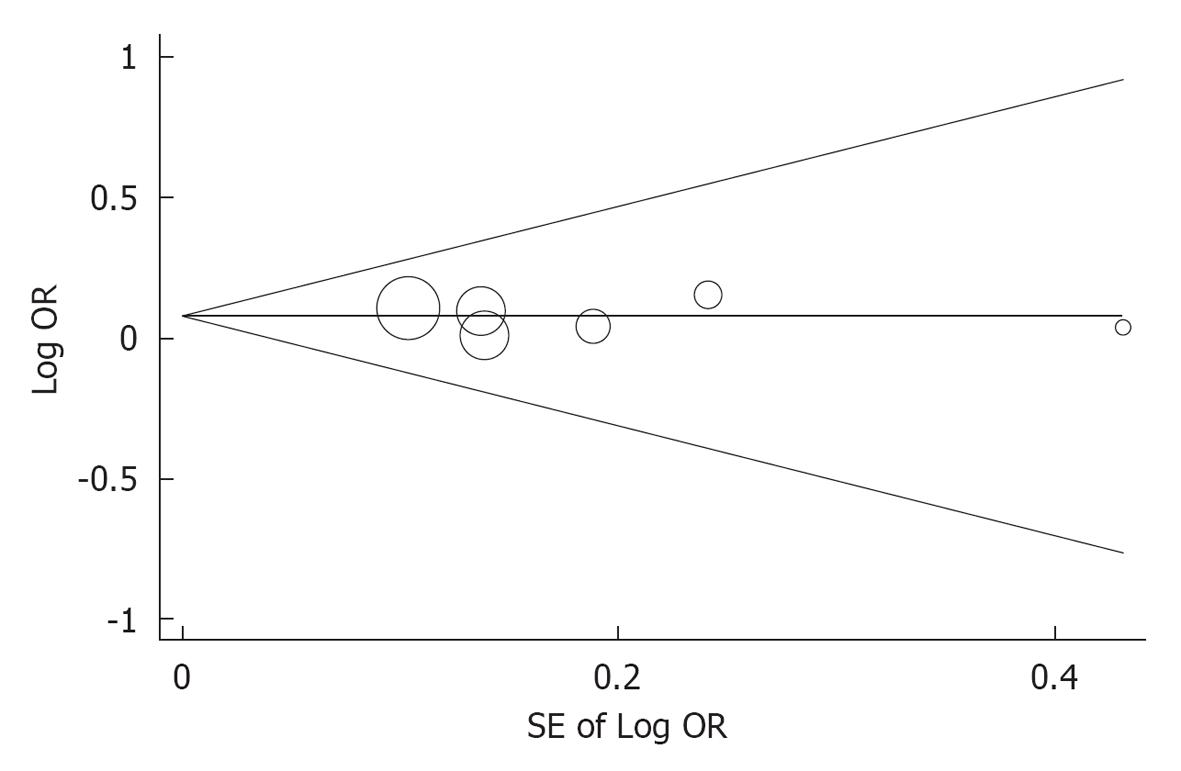
The subgroup analysis indicated that the OR for the MS in Chinese studies did not significantly differ from that in non Chinese studies (OR for MS in Chinese studies: 1.256; 95%CI: 1.063-1.484; OR for MS in non Chinese studies: 1.130; 95%CI: 0.938-1.360, P for interaction = 0.404). The sensitivity analysis indicated that the pooled ORs excluding each individual study were comparable (Table 3). No significant publication bias was found after assessment using the Begg’s funnel plot (P = 0.573) and the Egger weighted regression statistic (P = 0.833) (Figure 5).
| Studies excluded | Pooled ORs of remaining studies (95%CI) | P for OR | I2 (%) | χ2 | P for χ2 |
| Hergenç et al[5] | 1.20 (1.06-1.36) | 0.004 | 0 | 2.47 | 0.650 |
| Garduño-Garcia Jde et al[6] | 1.25 (1.09-1.44) | 0.002 | 0 | 0.84 | 0.934 |
| Lai et al[7] | 1.21 (1.06-1.38) | 0.005 | 0 | 2.34 | 0.673 |
| Lai et al[8] | 1.18 (1.04-1.35) | 0.011 | 0 | 1.97 | 0.742 |
| Liu et al[9] | 1.15 (0.99-1.35) | 0.071 | 0 | 1.97 | 0.742 |
| Waring et al[10] | 1.20 (1.03-1.36) | 0.018 | 0 | 2.40 | 0.663 |
In this meta-analysis of 19546 participants from six cross-sectional studies of a general population, SCH was associated with an increased risk of the MS compared with being euthyroid. To our best knowledge, this is the first meta-analysis showing the relationship between SCH and the MS. The previous meta-analysis demonstrated a possible association between SCH and increase risk of CHD[2]. This association might be mediated by the effect of these risk factors because the MS is one of the established risk factors for cardiovascular disease[4,16]. However, whether this association between SCH and coronary heart is independent or dependent of MS is still unclear and further investigation is needed.
Even in euthyroid populations, the relationship between thyroid function and the MS has been well investigated, with results consistent with those of our meta-analysis. In a cross-sectional study in the Netherlands, free T4 levels within the normal reference range were significantly related to four of the five components of the MS (P for waist circumference = 0.038, P for TGs = 0.023, P for HDL-C = 0.007 and P for SBP = 0.019), independent of insulin resistance[17]. Park et al[18] found a close relationship between TSH and the MS in euthyroid postmenopausal women (adjusted OR for the MS 1.55; 95%CI: 1.26-1.89; P < 0.001).
It was previously reported that overt hypothyroidism was associated with the MS[19], presumably because of the associated hypercholesterolemia and hypertension[20]. The reason why SCH is associated with the MS could be also attributed to the increased risk of the components of the MS. In a study evaluating 27097 individuals > 40 years of age without a diagnosis of thyroid disease, serum TSH, even within the normal range, was positively associated with BMI (P for trend in all BMI groups < 0.001)[21]. The relationship between blood pressure and thyroid function has also been well investigated but with conflicting findings[22-25]. A recent meta-analysis of seven cross-sectional studies concluded that SCH is associated with increased blood pressure, whereas subclinical hyperthyroidism is not[26]. Hypothyroidism has been associated with dyslipidemia, which is characterized by increased levels of total cholesterol and low-density lipoprotein cholesterol[27]. In the Colorado thyroid disease prevalence study, the total cholesterol, low-density lipoprotein cholesterol and TG levels of SCH subjects were significantly greater than the corresponding lipid levels in euthyroid subjects (P < 0.001 for total cholesterol and low-density lipoprotein cholesterol; P = 0.02 for TG), whereas no difference was found in HDL-C level[28]. Our meta-analysis of metabolic components has further confirmed these conclusions.
Another important issue is whether SCH should be treated with thyroid hormones or whether hormone replacement therapy could reduce the cardiovascular risk and mortality in SCH. A systematic review of randomized controlled trials showed that thyroid hormone replacement in SCH could improve the lipid profile (WMD for low-density lipoprotein cholesterol: -10.77 mg/dL; 95%CI: -21.57-0.04; P = 0.051)[29] and recent data from a cohort study indicated that treatment of SCH with levothyroxine was associated with less ischemic heart disease in younger individuals compared to those without treatment (adjusted hazard ratio 0.61; 95%CI: 0.39-0.95)[30]. Since our meta-analysis indicated a higher risk of the MS in SCH, screening and treatment of these metabolic components are necessary to further reduce the risk of cardiovascular events.
It is notable that the included study from Waring et al[10] reported not only cross-sectional data but also prospective data, making it the only prospective study on SCH and the MS in the literature. After 6 years of follow-up, the risk of the MS in SCH individuals with a TSH > 10 mIU/L was similar to that in the baseline cross-sectional analysis but with a wider confidence interval (OR for cross-sectional analysis, 2.3; 95%CI: 1.0-5.0; OR for prospective analysis, 2.2; 95%CI: 0.6-7.5). However, this study seemed to be underpowered because a very small number of participants with marked SCH were included[10].
Our study has several limitations. Firstly, although our heterogeneity test showed no statistically significant heterogeneity among studies, it is still impossible that our final results would be confounded by the different definitions of SCH and MS among the included studies, as well as the different inclusion/exclusion criteria. Secondly, this is a study-level and we used unadjusted OR instead of adjusted OR; thus, it is impossible to exclude the confounding effects of age, sex and race. Finally, although no statistically significant publication bias was detected in our analysis, we believe there may be data relevant to this topic that have never been published.
Our meta-analysis indicated that SCH may be associated with an increased risk of the MS, which is attributed to the increased risk of metabolic components. A large prospective cohort study is needed to further confirm the conclusion of our study.
Over the past several decades, a plethora of publications has established that subclinical hypothyroidism (SCH) is associated with cardiovascular disease. Meanwhile, it has been well recognized that the metabolic syndrome (MS) is associated with increased cardiovascular events and all-cause mortality.
Is SCH associated with a higher risk of the MS
This meta-analysis of 6 cross-sectional studies with 19546 participants indicated that SCH may be associated with increased risk of the MS, which is attributed to the increased risk of metabolic components.
It may be necessary to screen for the MS in SCH individuals in clinical practice.
SCH occurs when thyroid-stimulating hormone levels are elevated but thyroxine (T4) and triiodothyronine (T3) levels are normal. MS is a name for a group of risk factors that occur together and increase the risk for coronary artery disease, stroke and type 2 diabetes.
This paper reports the results of a meta-analysis of observational studies evaluating the association between SCH and MS and its individual components. Overall methodology is correct and the results are very interesting and valuable.
P- Reviewers Chao M, Cheung CL, Mariani J, Singh-Franco D, Treglia G S- Editor Wen LL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 585] [Cited by in F6Publishing: 534] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 2. | Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 722] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 3. | Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 738] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 4. | Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1623] [Cited by in F6Publishing: 1746] [Article Influence: 124.7] [Reference Citation Analysis (0)] |
| 5. | Hergenç G, Onat A, Albayrak S, Karabulut A, Türkmen S, Sari I, Can G. TSH levels in Turkish adults: Prevalences and associations with serum lipids, coronary heart disease and metabolic syndrome. Turk J Med Sci. 2005;35:297-304. [Cited in This Article: ] |
| 6. | Garduño-Garcia Jde J, Alvirde-Garcia U, López-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Lai CC, Tang SH, Pei D, Wang CY, Chen YL, Wu CZ, Hsiao FC, Chen HS, Wang JY. The prevalence of subclinical thyroid dysfunction and its association with metabolic syndrome in Taiwanese elderly. Int J Gerontol. 2011;5:25-29. [Cited in This Article: ] |
| 8. | Lai Y, Wang J, Jiang F, Wang B, Chen Y, Li M, Liu H, Li C, Xue H, Li N. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Liu C, Scherbaum WA, Schott M, Schinner S. Subclinical hypothyroidism and the prevalence of the metabolic syndrome. Horm Metab Res. 2011;43:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I, Satterfield S, Newman AB, Bauer DC. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf). 2012;76:911-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14425] [Cited by in F6Publishing: 15643] [Article Influence: 651.8] [Reference Citation Analysis (0)] |
| 12. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3566] [Cited by in F6Publishing: 3859] [Article Influence: 257.3] [Reference Citation Analysis (0)] |
| 13. | Rostom A, Dubé C, Cranney A. Celiac Disease. Rockville, MD: Agency for Healthcare Research and Quality (US), 2004. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: http://www.ncbi.nlm.nih.gov/books/NBK35156/. [Cited in This Article: ] |
| 14. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10586] [Cited by in F6Publishing: 11210] [Article Influence: 386.6] [Reference Citation Analysis (0)] |
| 15. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34245] [Cited by in F6Publishing: 36085] [Article Influence: 1336.5] [Reference Citation Analysis (1)] |
| 16. | Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769-1778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1195] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 17. | Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 18. | Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, Hur JY, Kim YT, Lee KW, Kim SH. Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas. 2009;62:301-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Erdogan M, Canataroglu A, Ganidagli S, Kulaksızoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest. 2011;34:488-492. [PubMed] [Cited in This Article: ] |
| 20. | Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1308] [Cited by in F6Publishing: 1296] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 21. | Asvold BO, Bjøro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94:5023-5027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Iqbal A, Figenschau Y, Jorde R. Blood pressure in relation to serum thyrotropin: The Tromsø study. J Hum Hypertens. 2006;20:932-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Duan Y, Peng W, Wang X, Tang W, Liu X, Xu S, Mao X, Feng S, Feng Y, Qin Y. Community-based study of the association of subclinical thyroid dysfunction with blood pressure. Endocrine. 2009;35:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Chen H, Xi Q, Zhang H, Song B, Liu X, Mao X, Li J, Shen H, Tang W, Zhang J. Investigation of thyroid function and blood pressure in school-aged subjects without overt thyroid disease. Endocrine. 2012;41:122-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Walsh JP, Bremner AP, Bulsara MK, O’Leary P, Leedman PJ, Feddema P, Michelangeli V. Subclinical thyroid dysfunction and blood pressure: a community-based study. Clin Endocrinol (Oxf). 2006;65:486-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Cai Y, Ren Y, Shi J. Blood pressure levels in patients with subclinical thyroid dysfunction: a meta-analysis of cross-sectional data. Hypertens Res. 2011;34:1098-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1513] [Cited by in F6Publishing: 1432] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 29. | Villar HC, Saconato H, Valente O, Atallah AN. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev. 2007;CD003419. [PubMed] [Cited in This Article: ] |
| 30. | Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |