Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1705
Peer-review started: October 23, 2020
First decision: November 20, 2020
Revised: December 24, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: March 6, 2021
The coronavirus disease 2019 (COVID-19) caused by novel coronavirus 2019 in December 2019 has spread all around the globe and has caused a pandemic. There is still no current effective guidance on the clinical management of COVID-19. Mesenchymal stem cell therapy has been shown to be one of the therapeutic approaches to alleviate pneumonia and symptoms through their immunomo-dulatory effect in COVID-19 patients.
We describe the first confirmed case of COVID-19 in Hangzhou to explore the role of human menstrual blood-derived stem cells (MenSCs) in the treatment of COVID-19. Moreover, we review the immunomodulation effect including non-specific and specific immune functions of MenSCs for the therapy of COVID-19.
MenSCs can be helpful to find a promising therapeutic approach for COVID-19.
Core Tip: The coronavirus disease 2019 (COVID-19) is the word that certainly will not be forgotten by everybody who lives in the first half of the twenty-first century. It has led many researchers from different biomedical fields to find solutions or treatments to manage the pandemic. However, there is still no current effective guidance on the clinical management of COVID-19. Mesenchymal stem cells are widely used to treat tissue and organ injuries with effective immunomodulatory and repair capacities, which makes them ideal for allogenic adoptive transfer therapy. In this study, we describe the first confirmed case of COVID-19 in Hangzhou, China to explore the role of human menstrual blood-derived stem cells (MenSCs) in the treatment of COVID-19. Moreover, we review the immunomodulation effect including non-specific and specific immune functions of MenSCs for the therapy of COVID-19, which can be helpful to find a promising therapeutic approach for this disease.
- Citation: Lu J, Xie ZY, Zhu DH, Li LJ. Human menstrual blood-derived stem cells as immunoregulatory therapy in COVID-19: A case report and review of the literature. World J Clin Cases 2021; 9(7): 1705-1713
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1705.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1705
The outbreak of coronavirus disease 2019 (COVID-19) caused by novel coronavirus 2019 (2019-nCoV) in late December 2019 is increasing rapidly in an epidemic scale and has spread to over 200 countries[1,2]. It has rapidly transmitted and become a major concern all over the world. The epidemiology, clinical characteristics, and treatment of COVID-19 have been reported in many cases by many institutions[3]. However, there is still no current effective guidance from the World Health Organization on the clinical management of COVID-19[4].
Mesenchymal stem cells (MSCs) are widely used to treat tissue and organ injuries, with effective immunomodulatory and repair capacities and low immunogenicity. This makes them ideal for allogenic adoptive transfer therapy[5,6]. Human menstrual blood-derived stem cells (MenSCs) have become a promising alternative because they are easy to collect and isolate and do not involve ethical considerations[7,8]. Previously, our group revealed that MenSCs were effective for treating liver failure and lung injury[9,10]. Here, we present the first case of COVID-19 identified in Hangzhou, China, on January 19, 2020, and its treatment, including MenSC therapy, and review the immunoregulatory effect of MenSC in treatment of COVID-19.
A low-grade fever and fatigue accompanied by dizziness for 2 d.
On January 19, 2020, a 32-year-old man was referred to our hospital with a low-grade fever and fatigue accompanied by dizziness for 2 d. Given the COVID-19 outbreak in Wuhan and his symptoms, the local hospital immediately admitted him with suspected COVID-19. He was immediately placed in a quarantine ward and underwent examination. Sputum and throat swab specimens were collected at admission and tested by reverse transcription-polymerase chain reaction for SARS-Cov-2 RNA. The infection was confirmed the day of admission, in accordance with China Centers for Disease Control guidance.
He had no other underlying diseases. He worked and lived in Hangzhou, but had travelled to Wuhan for business one week earlier and contacted two colleagues there.
There was no noteworthy personal or family medical history.
After hospitalization, the patient’s chief symptoms were an occasional cough, shortness of breath, and chest pain. On physical examination, he had short rough breaths. His temperature was elevated to 38.7 ºC, with an arterial oxygen tension (PaO2) of 82 mmHg under ambient air.
Laboratory examinations showed normal leukocytes (9.3 × 109/L), neutrophils (6 × 109/L), and lymphocytes (2.3 × 109/L). The patient’s high-sensitivity C-reactive protein (hs-CRP) level was 10.8 mg/L. Laboratory testing showed sharply increased leukocytes (25.9 × 109/L) and neutrophils (23.2 × 109/L), and an hs-CRP level of 43.5 mg/L. On day 7, his fever reached 39.4 ºC and he developed chest tightness with a PaO2 of 68 mmHg, PaCO2 of 34 mmHg, and oxygenation index of 208 mmHg on nasal oxygen at 3 L/min. The levels of inflammatory cytokines were also increased, with interleukin (IL)-6 71.5 pg/mL, IL-10 7.75 pg/mL, tumor necrosis factor (TNF)-α 84.28 pg/mL, and TNF-γ 34.71 pg/mL on day 11. The sputum 2019-nCoV RNA test turned negative for the first time, although a repeat test was positive on day 20. The leukocyte count (14.6 × 109/L), neutrophil count (10.4 × 109/L), and inflammatory factor levels were all improved. Liver function tests showed elevated alanine aminotransferase (ALT, 190 U/L) and aspartate aminotransferase (AST, 41 U/L) levels on day 23.
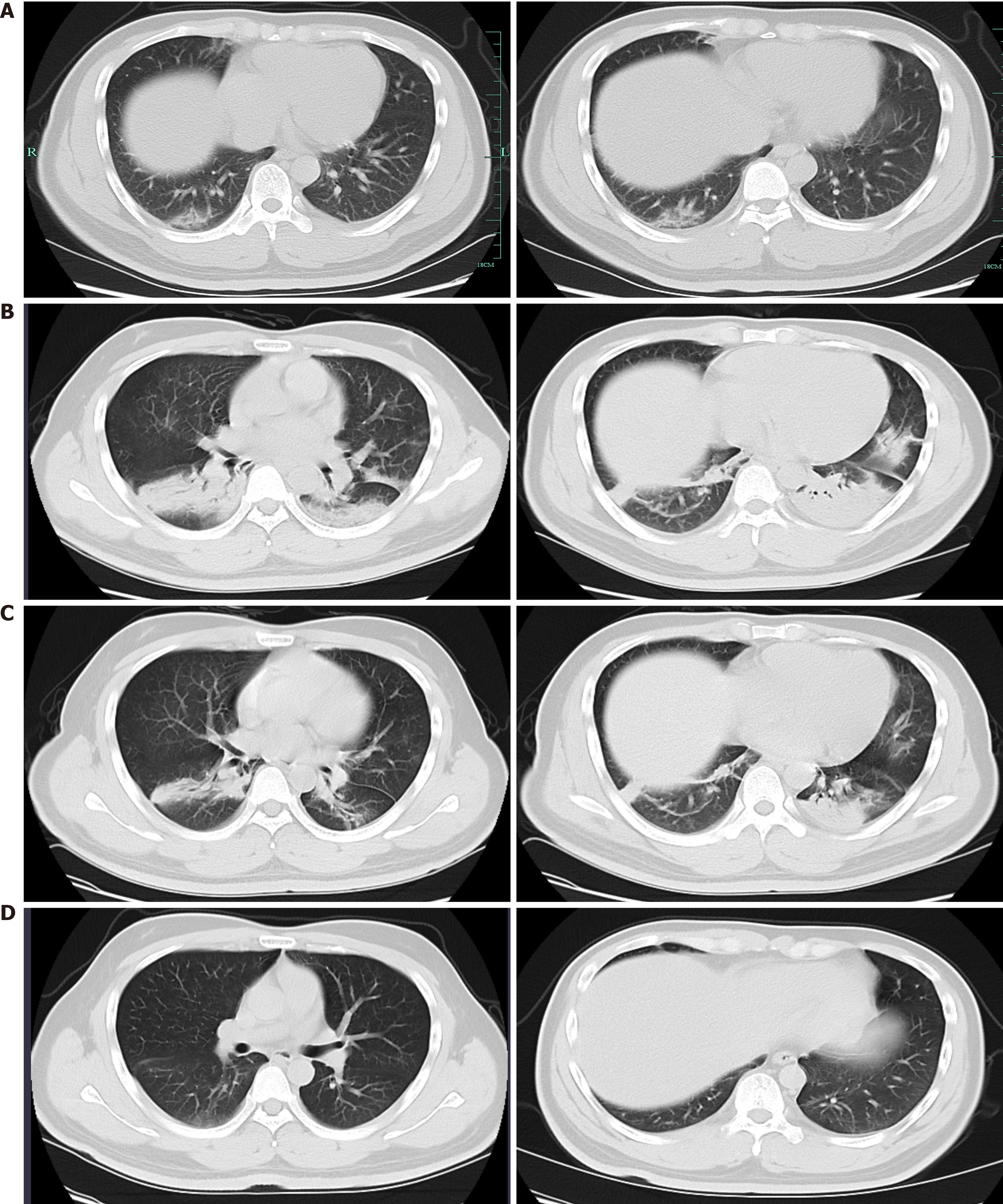
The initial chest computed tomography (CT) scan showed a few interstitial changes in both lungs and ground-glass opacities (GGOs) in the subpleural area of the right lower lobe (Figure 1A). Subsequent chest CT showed multifocal peripheral patchy areas of nodular consolidation and new GGOs in the left subpleural area on day 7. Progressive resolution of the parenchymal lesions was seen on follow-up CT, which showed basilar streaky opacities and patchy consolidations, but worsened GGOs in both lungs (Figure 1B) on day 11. Chest CT on day 17 revealed improvement of the infiltrates in both lungs (Figure 1C). On day 24, the patient was discharged on glycyrrhizin tablets for liver protection. No abnormalities were observed on chest CT 9 d post-discharge (Figure 1D).
Given the severe pulmonary injury caused by the inflammatory response and side effects, the glucocorticoid, antiviral, and antibiotic therapies were withdrawn. Under the guidance of a specialist group, MenSC therapy was proposed. The therapy was discussed and approved by the hospital ethics committee and the patient and family members provided informed consent before the therapy. Intravenous MenSCs were given at 3000, 2000, and 3000 U on hospital days 11, 12, and 14, respectively; no adverse events were observed in association with the infusion. Over the following 3 d, the patient’s breathing improved, with an intermittent dry cough and decreased chest stuffiness. His temperature decreased to 37.4 ºC.
The final diagnosis of the presented case was COVID-19 caused by 2019-nCoV.
The patient was given supportive care including immunoglobulin and antiviral treatment at the first stage of hospitalization. Supportive treatment was strengthened, and methylprednisolone and vaccination were given as empirical treatment during the progression of the disease. Finally, MenSC therapy was proposed while the glucocorticoid, antiviral, and antibiotic therapies were withdrawn.
The condition of the patient was significantly improved. Regular follow-up revealed that no abnormalities or recurrences were observed on the chest CT after the patient’s discharge (Supplementary Figure 1).
This was the first confirmed case of COVID-19 in Hangzhou, Zhejiang Province, China. Most of the initial cases had a history of exposure in the epidemic area and were infected with the virus via human-to-human transmission[11,12]. The main clinical manifestations of COVID-19 infection are cough, fever, fatigue, and gradual dyspnea in some cases and acute respiratory distress syndrome in severe cases, with a 1–14-d period from onset to admission[13]. Our patient developed a mild fever and fatigue 4 d after contact in the epidemic area. As his illness worsened, he developed obvious respiratory symptoms, including a cough and chest discomfort. With treatment, his temperature normalized and the other complaints were relieved.
Our patient showed changes in routine laboratory parameters and inflammatory cytokines consistent with other reports[14]. The elevated ALT and AST levels observed late in the patient’s illness were thought to be adverse outcomes of systemic inflammation due to the virus[15]. Of note, the stool specimen collected on day 23 was negative for 2019-nCoV RNA, consistent with the sputum specimens[16].
Chest CT can help diagnose patients suspected of having COVID-19[17]. Similar to viral pneumonia due to other etiologies, GGOs are the main CT findings in COVID-19. The initial CT showed basilar streaky opacities and GGOs in the right lower lobe[18,19]. As the illness progressed, the size and density of these GGOs or paving patchy consolidations increased. Only slight fibrotic changes remained when the patient recovered.
The most effective medical treatment against COVID-19 remains unknown, though several antiviral therapies were confirmed to be effective in published studies[20]. Antibiotic was also adopted to prevent secondary bacterial infection in this case. Apart from the large supportive treatment, stem cell therapy was considered with no side effect after three times of intravenous administration. After the third administration, inflammatory cytokines such as IL-6 and IL-10 decreased significantly. Moreover, patchy consolidations were absorbed in both lungs, accompanied with the gradually well-improved clinical conditions.
The superiority of MSC therapy over therapies based on bone marrow, adipose, umbilical cord, or embryonic tissue is due to the easily accessible source of the cells and their high rate of proliferation, the low invasiveness of the procedure, and the absence of ethical issues[21,22]. MenSCs can be isolated from female uterine blood with readily accessible materials, free of trauma or ethical concerns, and are thus of great potential for treating diseases[23,24]. In vitro, MenSCs can be differentiated into ectoderm and mesoderm, specifically, into fat, bone, cartilage, nerve, and endothelioid cells[5,9]. The expression by MenSCs of OCT-4, specific embryonic antigen SSEA-4, C-kit, and other embryonic stem cell markers suggests that MenSCs are more primitive and have a stronger multidirectional differentiation potential than other MSCs[25-27]. Moreover, with their strong paracrine and angiogenic potential, they may be useful in the repair of damaged tissues[28].
MSCs interact with inhibitory T cells, B cells, natural killer (NK) cells, and regulatory T cells to regulate the immune response, which also have direct effects on NK cells[29-32]. Recent studies indicate that acute respiratory distress syndrome (ARDS), characterized by acute inflammation and edema of the alveolar epithelial cells, is accompanied by the massive release of inflammatory cytokines, such as IL-1, IL-8, interferon, and TNF, which activate macrophages or NK cells, which in a “cytokine storm” trigger a strong immune response to damage alveoli[23]. COVID-19 is associated with abundant complications, with ARDS as the main cause of death[33,34]. By inhibiting over-activated NK cells, MenSCs could confer protection against the highly inflammatory environment while maintaining the NK activities that reduce inflammation during healing. Moreover, immunoregulation by MenSCs via IL-6 and IL-10 has been reported as well[23,35,36].
MenSCs are significant inhibitors of the inflammatory response[37,38]. Their ability to inhibit T lymphocyte proliferation results in stronger immunomodulatory effects than those exhibited by human umbilical cord mesenchymal stem cells or bone marrow mesenchymal stem cells (BM-MSCs)[39,40]. The inhibition of T lymphocyte proliferation by MenSCs is mediated by their secretion of prostaglandin (PGE2) and indoleamine2, 3-dioxygenase (IDO)[40]. PGE2 contributes to the transformation of classically activated macrophages (M1) to replace activated macrophages (M2) and may therefore be of interest in ameliorating the over-active inflammatory response in COVID-19[41]. It has also been shown that MenSCs promote the proliferation of CD4+ T lymphocytes in a density-dependent manner that is not affected by the concentration of IDO, in contrast to BM-MSCs, which are sensitive to IDO[42].
In addition to interleukins or PGE2, MenSCs release hepatocyte growth factor, granulocyte-macrophage colony stimulating factor, and keratinocyte growth factor (KGF), which may protect respiratory epithelial cells in lung injury via activities that include the increased clearance of alveolar fluid, the promotion of endothelial repair, and the inhibition of the inflammatory response[10,43,44]. MenSCs cov-2downregulate caspase-3 and IL-1β expression and upregulate KGF expression to improve microvascular permeability. In turn, KGF activates alveolar type II epithelial cells to stimulate the synthesis of pulmonary surfactant[10,32,45].
These abilities of MenSCs suggest a capacity to regulate the inflammatory response and thereby overcome the severe cytokine storm associated with COVID-19, while restoring the normal function of immune cells and tissues. Based on their homing potential, MenSCs may enhance the repair properties of immune and other cells, thus allowing the reconstruction of damaged tissues by receptor-mediated interactions[46,47]. By protecting alveolar epithelial cells and improving the pulmonary microen-vironment, MSCs can contribute to preventing lung dysfunction and the development of COVID-19 pneumonia[48]. Through mediators such as interleukin receptors, PGE2, and KGF, MSCs can significantly inhibit the inflammatory response and repair the vascular-alveolar epithelial cell barrier to promote the elimination of alveolar fluid and thereby the infection[49,50].
This was the first COVID-19 case seen in Hangzhou, China. During the patient’s hospitalization, laboratory abnormalities and chest CT findings changed in synchrony with the clinical illness. The immune system of COVID-19 patients produces a drastic inflammatory response, causing a cytokine storm that includes the overproduction of cytokines or immune cells[51]. MSCs have been reported to enhance cellular reparative and immunomodulatory properties and thereby prevent or reduce the cytokine storm that develops in COVID-19 patients[52]. After the intravenous injection of MSCs, a portion of the cells accumulate in the lungs via the circulation, where they may contribute to the recovery of the pulmonary microenvironment by protecting alveolar epithelial cells, reducing the amount of effusion, and preventing pulmonary fibrosis, thus ameliorating tissue dysfunction and COVID-19 pneumonia[33]. Pulmonary fibrosis is a serious complication of COVID-19[53], with the first step in its pathogenesis consisting of the intra-alveolar infiltration of proliferating fibroblasts. Proliferating fibroblasts infiltrating intra-alveolar zone was considered the first step in pathogenesis of pulmonary fibrosis[54]. Edema, fibroblast invasion of the pulmonary interspaces, and type II pneumocyte hyperplasia with inflammatory cytokine infiltrations have been seen in the lung sections of patients with COVID-19[55]. MenSCs, alone or combined with other therapeutic agents, might be a promising treatment for COVID-19.
The authors would like to acknowledge Jiong Yu for their assistance in the conduct of the study.
Manuscript source: Unsolicited manuscript
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakraborty C S-Editor: Liu M L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Li J, Gong X, Wang Z, Chen R, Li T, Zeng D, Li M. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020;286:198043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Lee SS. The 2019 novel coronavirus disease (COVID-19) pandemic: A zoonotic prospective. Asian Pac J Trop Med. 2020;13:242-246. [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Hadi AG, Kadhom M, Hairunisa N, Yousif E, Mohammed SA. A Review on COVID-19: Origin, Spread, Symptoms, Treatment, and Prevention. Biointerface Res App. 2020;10:7234-7242. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee SS, Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol. 2020;92:618-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 247] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 5. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 6. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2044] [Cited by in F6Publishing: 1970] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 7. | Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Rodrigues MC, Voltarelli J, Sanberg PR, Allickson JG, Kuzmin-Nichols N, Garbuzova-Davis S, Borlongan CV. Recent progress in cell therapy for basal ganglia disorders with emphasis on menstrual blood transplantation in stroke. Neurosci Biobehav Rev. 2012;36:177-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int J Mol Sci. 2017;18:689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28495] [Article Influence: 7123.8] [Reference Citation Analysis (3)] |
| 12. | Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N Engl J Med. 2020;382:872-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 673] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 13. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18987] [Cited by in F6Publishing: 16536] [Article Influence: 4134.0] [Reference Citation Analysis (0)] |
| 14. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6159] [Cited by in F6Publishing: 5256] [Article Influence: 1314.0] [Reference Citation Analysis (0)] |
| 15. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4155] [Cited by in F6Publishing: 3698] [Article Influence: 924.5] [Reference Citation Analysis (1)] |
| 16. | Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4118] [Cited by in F6Publishing: 4613] [Article Influence: 1153.3] [Reference Citation Analysis (1)] |
| 17. | Lei J, Li J, Li X, Qi X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 391] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 18. | Pan Y, Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020;30:3612-3613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 19. | Chakraborty C, Sharma AR, Sharma G, Bhattacharya M, Lee SS. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci. 2020;24:4016-4026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 118] [Reference Citation Analysis (0)] |
| 20. | Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14:69-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 717] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 21. | Golchin A, Farahany TZ, Khojasteh A, Soleimanifar F, Ardeshirylajimi A. The Clinical Trials of Mesenchymal Stem Cell Therapy in Skin Diseases: An Update and Concise Review. Curr Stem Cell Res Ther. 2019;14:22-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11:169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Bozorgmehr M, Moazzeni SM, Salehnia M, Sheikhian A, Nikoo S, Zarnani AH. Menstrual blood-derived stromal stem cells inhibit optimal generation and maturation of human monocyte-derived dendritic cells. Immunol Lett. 2014;162:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Khanjani S, Khanmohammadi M, Zarnani AH, Talebi S, Edalatkhah H, Eghtesad S, Nikokar I, Kazemnejad S. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J Tissue Eng Regen Med. 2015;9:E124-E134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 363] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 26. | Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143-3154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907-6912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 358] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Jiang Z, Hu X, Yu H, Xu Y, Wang L, Chen H, Chen H, Wu R, Zhang Z, Xiang C, Webster KA, Wang JA. Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J Cell Mol Med. 2013;17:1247-1260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Fayyad-Kazan H, Faour WH, Badran B, Lagneaux L, Najar M. The immunomodulatory properties of human bone marrow-derived mesenchymal stromal cells are defined according to multiple immunobiological criteria. Inflamm Res. 2016;65:501-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Chatterjee D, Marquardt N, Tufa DM, Hatlapatka T, Hass R, Kasper C, von Kaisenberg C, Schmidt RE, Jacobs R. Human Umbilical Cord-Derived Mesenchymal Stem Cells Utilize Activin-A to Suppress Interferon-γ Production by Natural Killer Cells. Front Immunol. 2014;5:662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351:114-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Peteranderl C, Morales-Nebreda L, Selvakumar B, Lecuona E, Vadász I, Morty RE, Schmoldt C, Bespalowa J, Wolff T, Pleschka S, Mayer K, Gattenloehner S, Fink L, Lohmeyer J, Seeger W, Sznajder JI, Mutlu GM, Budinger GR, Herold S. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest. 2016;126:1566-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 730] [Cited by in F6Publishing: 798] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 34. | Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 35. | Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of "inflame-aging". Inflamm Res. 2020;69:825-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 36. | Saha RP, Sharma AR, Singh MK, Samanta S, Bhakta S, Mandal S, Bhattacharya M, Lee SS, Chakraborty C. Repurposing Drugs, Ongoing Vaccine, and New Therapeutic Development Initiatives Against COVID-19. Front Pharmacol. 2020;11:1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 37. | Khoury M, Alcayaga-Miranda F, Illanes SE, Figueroa FE. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front Immunol. 2014;5:205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, Aguila-Díaz C, Fernandez A, Figueroa FE, Khoury M. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Cuenca J, Le-Gatt A, Castillo V, Belletti J, Díaz M, Kurte G M, Gonzalez PL, Alcayaga-Miranda F, Schuh CMAP, Ezquer F, Ezquer M, Khoury M. The Reparative Abilities of Menstrual Stem Cells Modulate the Wound Matrix Signals and Improve Cutaneous Regeneration. Front Physiol. 2018;9:464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Luz-Crawford P, Torres MJ, Noël D, Fernandez A, Toupet K, Alcayaga-Miranda F, Tejedor G, Jorgensen C, Illanes SE, Figueroa FE, Djouad F, Khoury M. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft vs host diseases. Stem Cells. 2016;34:456-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Liu J, Feng B, Xu Y, Zhu J, Feng X, Chen W, Sheng X, Shi X, Pan Q, Yu J, Zeng X, Cao H, Li L. Immunomodulatory effect of mesenchymal stem cells in chemical-induced liver injury: a high-dimensional analysis. Stem Cell Res Ther. 2019;10:262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Aleahmad M, Ghanavatinejad A, Bozorgmehr M, Shokri MR, Nikoo S, Tavakoli M, Kazemnejad S, Shokri F, Zarnani AH. Menstrual Blood-Derived Stromal Stem Cells Augment CD4+ T Cells Proliferation. Avicenna J Med Biotechnol. 2018;10:183-191. [PubMed] [Cited in This Article: ] |
| 43. | Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 44. | Chen L, Qu J, Cheng T, Chen X, Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10:406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Horie S, Laffey JG. Recent insights: mesenchymal stromal/stem cell therapy for acute respiratory distress syndrome. F1000Res. 2016;5:F1000 Faculty Rev-1532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, Ye P, Chen X, Wang B, Xu Z, Zhang Q, Xu X, Gao H, Wu X, Li D, Jiang W, Qu J, Xiang C, Li L. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14:664-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 47. | Gupta A, Kashte S, Gupta M, Rodriguez HC, Gautam SS, Kadam S. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum Cell. 2020;33:907-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 730] [Cited by in F6Publishing: 798] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 49. | Antebi B, Mohammadipoor A, Batchinsky AI, Cancio LC. The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome. J Trauma Acute Care Surg. 2018;84:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 542] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 51. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6366] [Cited by in F6Publishing: 6357] [Article Influence: 1589.3] [Reference Citation Analysis (0)] |
| 52. | Sun X, Wang T, Cai D, Hu Z, Chen J, Liao H, Zhi L, Wei H, Zhang Z, Qiu Y, Wang J, Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 296] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 53. | Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Chakraborty C. Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: Need to Know More. Arch Med Res. 2020;51:585-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 54. | El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, Bellusci S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell. 2017;21:166-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 55. | Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15:700-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 927] [Cited by in F6Publishing: 969] [Article Influence: 242.3] [Reference Citation Analysis (0)] |