Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10616
Peer-review started: May 7, 2021
First decision: July 26, 2021
Revised: August 12, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: December 6, 2021
Abnormal bone metabolism and renal anemia seriously affect the prognosis of patients with chronic kidney disease (CKD). Existing studies have mostly addressed the pathogenesis and treatment of bone metabolism abnormality and anemia in patients with CKD, but few have evaluated their mutual connection. Administration of exogenous erythropoietin to CKD patients with anemia used to be the mainstay of therapeutic approaches; however, with the availability of hypoxia-inducible factor (HIF) stabilizers such as roxadustat, more therapeutic choices for renal anemia are expected in the future. However, the effects posed by the hypoxic environment on both CKD complications remain incompletely understood.
To summarize the relationship between renal anemia and abnormal bone metabolism, and to discuss the influence of hypoxia on bone metabolism.
CNKI and PubMed searches were performed using the key words “chronic kidney disease,” “abnormal bone metabolism,” “anemia,” “hypoxia,” and “HIF” to identify relevant articles published in multiple languages and fields. Reference lists from identified articles were reviewed to extract additional pertinent articles. Then we retrieved the Abstract and Introduction and searched the results from the literature, classified the extracted information, and summarized important information. Finally, we made our own conclusions.
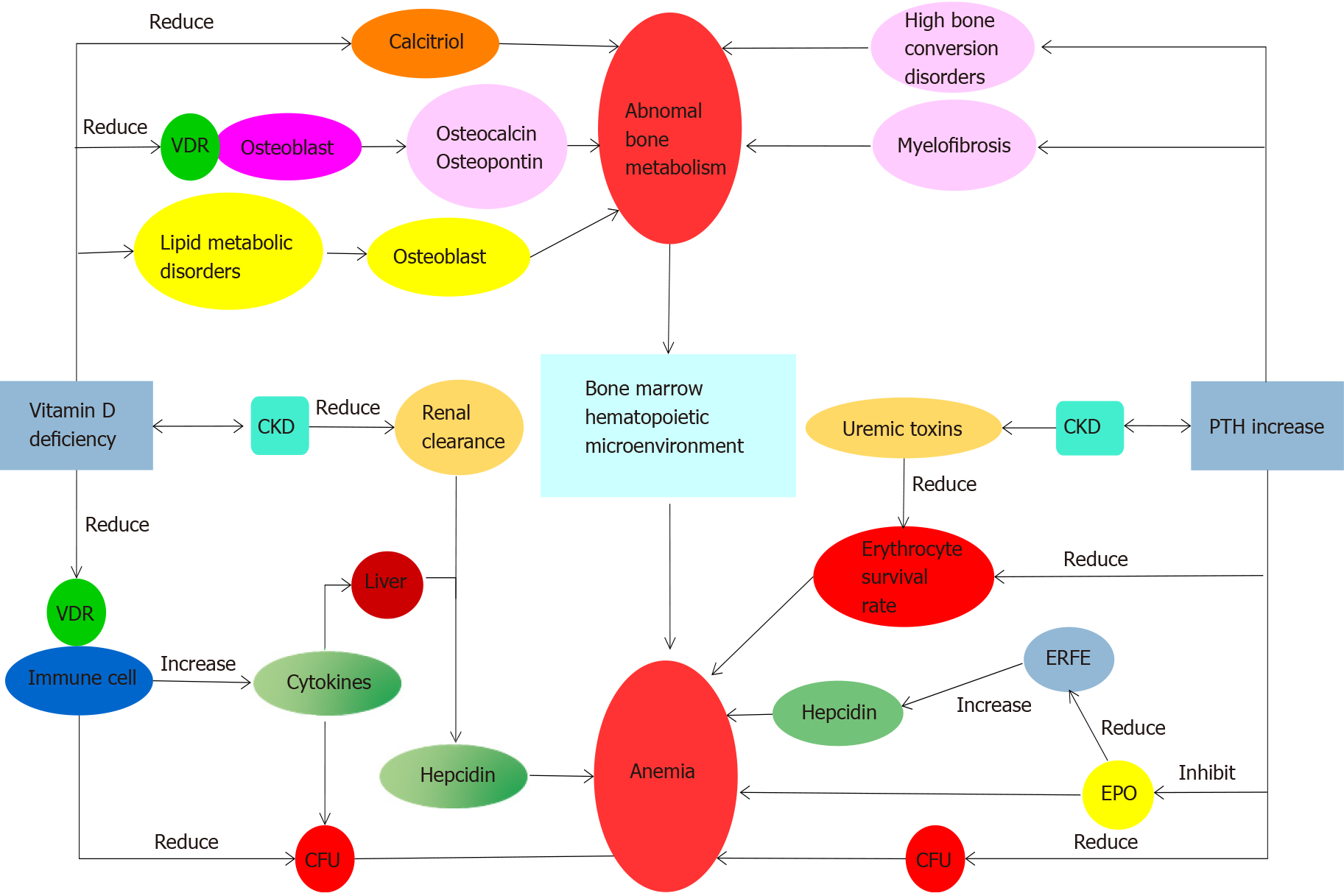
There is a bidirectional relationship between renal anemia and abnormal bone metabolism. Abnormal vitamin D metabolism and hyperparathyroidism can affect bone metabolism, blood cell production, and survival rates through mul
There is a bidirectional relationship between renal anemia and abnormal bone metabolism. Hypoxia may improve bone metabolism but the concentration and duration of hypoxia remain unclear and need further study.
Core Tip: Anemia and abnormal bone metabolism are complications in patients with chronic kidney disease (CKD), which seriously affect the prognosis of patients. This review summarizes the findings from recent studies on renal anemia and abnormal bone metabolism in patients with CKD. The bidirectional relationship between anemia and abnormal bone metabolism in patients with CKD is discussed. While studying the treatment of anemia with hypoxia-inducible factor (HIF), it was found that hypoxia can affect bone metabolism, but there is no consensus on the efficacy of HIF stabilizers in renal bone disease.
- Citation: Kan C, Lu X, Zhang R. Effects of hypoxia on bone metabolism and anemia in patients with chronic kidney disease. World J Clin Cases 2021; 9(34): 10616-10625
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10616.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10616
Chronic kidney disease (CKD) is defined according to the presence of kidney damage or an estimated glomerular filtration rate lower than 60 mL/min per 1.73 m2 for 3 mo or longer[1]. In addition to sustained kidney damage, patients with CKD are also at increased risk of developing multiple complications including renal anemia, abnormal mineral and bone metabolism, dyslipidemia, and malnutrition. The pathophysiology of anemia in CKD includes many important factors such as the presence of comor
Hypoxia-inducible factor (HIF) is a heterodimeric transcriptional factor that can induce the production of EPO and oxygen-sensitive genes under the hypoxic envi
Therefore, the present study aimed to clarify the bidirectional relationship between anemia and abnormal bone metabolism, search for evidence that hypoxia can improve bone metabolism, and provide a new research direction for the treatment of complications in patients with CKD.
CNKI and PubMed searches were performed using the key words “chronic kidney disease,” “abnormal bone metabolism,” “anemia,” “hypoxia,” and “HIF” to identify relevant articles published in multiple languages and fields. Reference lists from identified articles were reviewed to extract additional pertinent articles. Then we retrieved the Abstract and Introduction and searched the results from the literature, classified the extracted information, and summarized important information. Finally, we made our own conclusions. We have expanded the scope of literature search to reduce the risk of bias associated with article selection.
After reviewing 59 studies, we found that abnormal bone metabolism and renal bone disease were connected in the hematopoietic microenvironment. Abnormal vitamin D metabolism and hyperparathyroidism can affect bone metabolism, blood cell production, and survival rates through multiple pathways. Anemia will further attenuate the normal bone growth. According to the study of HIF in the treatment of renal anemia, HIF has more physiological potential. The hypoxic environment regulates bone morphogenetic protein, vascular endothelial growth factor, and neuropilin-1, and affects osteoblast/osteoclast maturation and differentiation through bone metabolic changes. Hypoxia preconditioning of mesenchymal stem cells (MSCs) can enhance their paracrine effects and promote fracture healing. Concurrently, hypoxia reduces the inhibitory effect on osteocyte differentiation by inhibiting the expression of fibroblast growth factor 23. Hypoxia potentially improves bone meta
Effects of impaired vitamin D metabolism: Inorganic phosphorus within the fluid of cortical tubules increases significantly in patients with CKD, and this increase in phosphorus significantly inhibits the synthesis of 1,25(OH)2D3. The injured kidney is unable to synthesize calcitrio[3,4], and even if calcitriol is synthesized, osteoblastic vitamin D receptors (VDRs) cannot bind to it effectively[9-11]. These pathologic changes serve as triggers of abnormal bone metabolism observed in CKD patients. Furthermore, abnormal lipid metabolism associated with decreased vitamin D stores can aggravate CKD-related osteoporosis in patients with specific physical conditions[12]. In addition, the hematopoietic system, especially hematopoietic stem cells (HSCs) in the bone marrow (BM), are vulnerable to the adverse effects of CKD[13-17]. Bony disorders can damage the BM hematopoietic microenvironment. VDR is also ex
Anemia and abnormal bone metabolism can be caused by secondary hyperparathyroidism: With the increase of parathyroid hormone (PTH) during CKD, the generation of early erythroid progenitor cells is inhibited. PTH potentially antagonizes EPO production[23], increases the osmotic brittleness of erythrocytes, and impairs their survival[24]. In patients with CKD, elevated PTH causes accelerated bone turnover and is associated with myelofibrosis[25,26], which reduces the production of EPO and aggravates anemia. Moreover, due to the positive correlation between erythroferrone (ERFE) and EPO and lower endogenous EPO production, the inhibition of hepcidin mediated by ERFE is reduced, which also aggravates anemia[27]. Cinacalcet, a calcimimetic for treating secondary hyperparathyroidism (SHPT), has been shown to attenuate the inhibitory effects on erythrocytes posed by PTH[6,28,29], reduce the amount of EPO required for correcting anemia in patients with CKD[30], and improve bone integrity in such patients[31]. Cinacalcet can simultaneously optimize their BM hematopoietic microenvironment[32]. After parathyroidectomy (PTX), the required EPO dose in patients with CKD-related anemia significantly declines[33]. Together, these findings suggest that surgical or medical treatments directed toward SHPT and associated abnormal bone metabolism can potentially improve symptoms related to anemia (Figure 1).
Abnormal bone metabolism can be exacerbated by anemia: Due to the complications of abnormal calcium and phosphorus metabolism, patients with CKD frequently have osteodystrophy. Anemia will further attenuate the normal bone growth and affect the formation of bone marrow as well as the generation of hematopoietic stem cells. This constitutes a vicious circle.
Patients with CKD invariably suffer from a status of low tissue oxygen tension. Hypoxia is a common precipitator of abnormal bone metabolism and anemia. Because HIF-PHD inhibitors (HIF-PHI) have been used to treat renal anemia and abnormal bone metabolism interacts with anemia, it is possible that HIF-PHIs exert similar therapeutic efficacy against bone disease in patients with CKD. In the following sections, we will provide several unifying theories to support this therapeutic plau
Hypoxic environment and anemia: Hypoxia may occur during episodes of microcirculatory insufficiency and hypoperfusion involving different tissues, including the kidney[34,35]. Studies have shown that the pathogenesis of CKD might include the loss of coherence within the microvascular network, resulting in an aberrantly heterogeneous pattern of focal microvascular rarefaction; this abnormality could diminish local blood flow velocity, relax vessel tone, and impair the oxygen uptake of tissues. From this perspective, tissue hypoxia is not uncommon during CKD[36,37]. Further
Hypoxic environment and bone development: Hypoxia exhibits complex effects on bone metabolism. Heterotopic ossification (HO) refers to the formation of bone-like tissues outside the skeletal system, and the process of adaptation to a hypoxic microenvironment is a powerful driver for the development of HO. The hypoxic microenvironment increases the stability of HIF-1α, which regulates a coordinated network consisting of bone morphogenetic proteins, vascular endothelial growth factor, and neuropilin-1, all of which are implicated in the formation of ectopic bone-like tissues[45]. Existing studies have found that the severity and duration of hypoxia to which tissues are exposed and the stage of osteoblast differentiation during which hypoxia occurs may influence bone growth and reconstruction.
In an environment of low oxygen level, pathways involved in bone metabolism are altered, which affect the maturation and differentiation of osteoblasts/osteoclasts. For osteoblasts, hypoxia predominantly occurs during their early stage of differentiation, and hypoxia facilitates premature osteoblast differentiation with incorrect signals produced for stimulating matrix maturation and mineralization[46,47]. Through up-regulating HIF-1α, short-term hypoxia enhances matrix mineralization, promotes osteoblast differentiation and maturation, and accelerates osteogenesis[48-51]. For osteoclasts, hypoxia increases osteoclast production irrespective of the differentiation stage during which hypoxia occurs, but the duration and severity of hypoxia may influence osteoclast differentiation. During hypoxia, anaerobic metabolism becomes predominant with acidic metabolites accumulation, causing mild acidosis of the local microenvironment and driving the activation of osteoclasts[52]. The regulatory relationship between HIF and adenosine A2B receptors in the hypoxic microenvironment can also enhance glycolysis and alter mitochondrial metabolism within osteoclasts, increasing the likelihood of bone absorption[53].
CKD patients with abnormal bone metabolism, especially those who are older, are at a higher risk of developing pathological fractures due to aberrant bone metabolism and the co-existing osteoporosis. Prior studies have demonstrated that hypoxic preconditioning of MSCs can enhance their paracrine effects by increasing the pro
FGF23 is mainly secreted by osteocytes. The bone-derived FGF23 acts in concert with PTH and active vitamin D calcitriol to regulate calcium and phosphate home
Current guidelines for treating bone diseases fail to consider the control of hypoxia as a therapeutic option. Sustained and intermittent hypoxia may inhibit osteogenic differentiation and promote osteoclast function, and cyclic hypoxia has been proposed as a promising strategy for favorably affecting bone metabolism. Exposure to mo
Delaying CKD progression reduces complications: Li et al[58] studied the stress response of renal tubules to hypoxia and found that during the transition from acute kidney injury to CKD, the absence of forkhead box O3 in renal tubules led to the deterioration of tubular structure and function, manifesting as a more severe CKD phenotype. In hypoxic kidneys, transcription factors associated with stress responses can be activated to ameliorate hypoxic injury and reduce the risk of progression to CKD.
Previous studies have shown that HIF-1 restricts the anabolic actions of PTH[59]. In the bidirectional relationship between anemia and abnormal bone metabolism (Figure 1), lowering PTH can improve anemia and abnormal bone metabolism through multiple pathways. Although there is no clear evidence that HIF enhances vitamin D metabolism, HIF can act separately on several downstream pathways including calcitriol transformation, osteoblasts and osteoclasts growth and development, EPO production, and iron transport. Unfortunately, due to the limited evidence available, currently there is no therapeutic approach related to hypoxia for promoting bone metabolism. It is expected that potential HIF subtypes and pathways involved in the hematopoietic system and bone metabolisms will be discovered in the future.
This review summarizes findings from recent studies on renal anemia and abnormal bone metabolism in patients with CKD. Mounting evidence supports the notion that there is a connection between both CKD complications, ranging from their patho
Abnormal bone metabolism and renal anemia seriously affect the prognosis of patients with chronic kidney disease (CKD). Currently, there are few studies on the evaluation of their mutual connection. With the availability of hypoxia-inducible factor (HIF) stabilizers, more therapeutic choices for renal anemia are expected in the future. However, the effects posed by the hypoxic environment on abnormal bone metabolism remain incompletely understood. If we can find evidence that HIF could improve both complications, it will be a great advantage to improve the prognosis of patients with CKD.
The purpose of this article is to summarize the relationship between renal anemia and abnormal bone metabolism, and to discuss the influence of hypoxia on bone meta
To clarify the bidirectional relationship between anemia and abnormal bone meta
We searched relevant articles published in multiple languages and fields, summarized important information, and drew our conclusions.
Anemia and bone metabolism interact. The hypoxic environment could affect osteoblast/osteoclast maturation and differentiation, enhance the paracrine effect of mesenchymal stem cells, and reduce the inhibitory effect of fibroblast growth factor 23 on osteocyte differentiation. Hypoxia potentially improves bone metabolism, but the optimal concentration and duration of hypoxia remain unclear and need further study.
There is a bidirectional relationship between renal anemia and abnormal bone metabolism. The relationship has rarely been studied. Hypoxia may improve bone metabolism, but the concentration and duration of hypoxia remain unclear and need further study. To improve the quality of life of patients with CKD, future studies should address the effect of HIF on bone metabolism while treating anemia, and HIF may be a useful treatment for improving the prognosis of patients with CKD.
In future studies, we can focus more on the exact degree of hypoxia concentration and duration required for improving bone metabolism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vela D S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Geddes CC. Pathophysiology of renal anaemia. Nephrol Dial Transplant. 2019;34:921-922. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011;25:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Galassi A, Bellasi A, Auricchio S, Papagni S, Cozzolino M. Which vitamin D in CKD-MBD? Biomed Res Int. 2013;2013:864012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Tanaka M, Komaba H, Fukagawa M. Emerging Association Between Parathyroid Hormone and Anemia in Hemodialysis Patients. Ther Apher Dial. 2018;22:242-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting Hypoxia-Inducible Factors for the Treatment of Anemia in Chronic Kidney Disease Patients. Am J Nephrol. 2017;45:187-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Haase VH. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int. 2017;21 Suppl 1:S110-S124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Dhillon S. Roxadustat: First Global Approval. Drugs. 2019;79:563-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 9. | Paredes R, Arriagada G, Cruzat F, Olate J, Van Wijnen A, Lian J, Stein G, Stein J, Montecino M. The Runx2 transcription factor plays a key role in the 1alpha,25-dihydroxy Vitamin D3-dependent upregulation of the rat osteocalcin (OC) gene expression in osteoblastic cells. J Steroid Biochem Mol Biol. 2004;89-90:269-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem. 2005;280:40589-40598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Takeda S, Yoshizawa T, Nagai Y, Yamato H, Fukumoto S, Sekine K, Kato S, Matsumoto T, Fujita T. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology. 1999;140:1005-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, Schoonjans K, Verstuyf A. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2338] [Cited by in F6Publishing: 2300] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 14. | Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2203] [Cited by in F6Publishing: 2096] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 15. | Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1629] [Cited by in F6Publishing: 1708] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 16. | Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol. 2000;7:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Patel NM, Gutiérrez OM, Andress DL, Coyne DW, Levin A, Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. 2010;77:715-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117:2800-2806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204-3209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 662] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 21. | Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 22. | Icardi A, Paoletti E, De Nicola L, Mazzaferro S, Russo R, Cozzolino M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transplant. 2013;28:1672-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med. 1993;328:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 250] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Bogin E, Massry SG, Levi J, Djaldeti M, Bristol G, Smith J. Effect of parathyroid hormone on osmotic fragility of human erythrocytes. J Clin Invest. 1982;69:1017-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Dunn CD, Trent D. The effect of parathyroid hormone on erythropoiesis in serum-free cultures of fetal mouse liver cells. Proc Soc Exp Biol Med. 1981;166:556-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Boxer M, Ellman L, Geller R, Wang CA. Anemia in primary hyperparathyroidism. Arch Intern Med. 1977;137:588-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Hanudel MR, Rappaport M, Chua K, Gabayan V, Qiao B, Jung G, Salusky IB, Ganz T, Nemeth E. Levels of the erythropoietin-responsive hormone erythroferrone in mice and humans with chronic kidney disease. Haematologica. 2018;103:e141-e142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Koizumi M, Komaba H, Nakanishi S, Fujimori A, Fukagawa M. Cinacalcet treatment and serum FGF23 Levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, Drüeke TB, Herzog CA, London GM, Mahaffey KW, Wheeler DC, Stolina M, Dehmel B, Goodman WG, Floege J; Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial Investigators*. Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation. 2015;132:27-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 30. | Torun D, Yildiz I, Micozkadioglu H, Nursal GN, Yigit F, Ozelsancak R. The effects of cinacalcet treatment on bone mineral metabolism, anemia parameters, left ventricular mass index and parathyroid gland volume in hemodialysis patients with severe secondary hyperparathyroidism. Saudi J Kidney Dis Transpl. 2016;27:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Behets GJ, Spasovski G, Sterling LR, Goodman WG, Spiegel DM, De Broe ME, D'Haese PC. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;87:846-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 501] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 33. | Trunzo JA, McHenry CR, Schulak JA, Wilhelm SM. Effect of parathyroidectomy on anemia and erythropoietin dosing in end-stage renal disease patients with hyperparathyroidism. Surgery. 2008;144:915-918; discussion 919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 693] [Cited by in F6Publishing: 681] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 35. | Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol. 2002;13:1795-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Prommer HU, Maurer J, von Websky K, Freise C, Sommer K, Nasser H, Samapati R, Reglin B, Guimarães P, Pries AR, Querfeld U. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci Rep. 2018;8:5317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, Dong Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 38. | Urban ML, Manenti L, Vaglio A. Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med. 2015;373:95-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 39. | Fu Q, Colgan SP, Shelley CS. Hypoxia: The Force that Drives Chronic Kidney Disease. Clin Med Res. 2016;14:15-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 40. | Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 41. | Shima H, Takubo K, Iwasaki H, Yoshihara H, Gomei Y, Hosokawa K, Arai F, Takahashi T, Suda T. Reconstitution activity of hypoxic cultured human cord blood CD34-positive cells in NOG mice. Biochem Biophys Res Commun. 2009;378:467-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 365] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 43. | Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis. 2017;69:815-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 44. | Sakashita M, Tanaka T, Nangaku M. Hypoxia-Inducible Factor-Prolyl Hydroxylase Domain Inhibitors to Treat Anemia in Chronic Kidney Disease. Contrib Nephrol. 2019;198:112-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Huang Y, Wang X, Lin H. The hypoxic microenvironment: a driving force for heterotopic ossification progression. Cell Commun Signal. 2020;18:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 47. | Lechler P, Klein SM, Prantl L, Englert C, Renkawitz T, Grifka J. Hypoxic downregulation of cellular proliferation and loss of phenotype stability in human osteoblasts is mediated by HIF-1α. Clin Hemorheol Microcirc. 2011;49:279-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645-9652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 593] [Cited by in F6Publishing: 610] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 49. | Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2013;154:623-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402-6412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14:1853-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Fukuoka H, Aoyama M, Miyazawa K, Asai K, Goto S. Hypoxic stress enhances osteoclast differentiation via increasing IGF2 production by non-osteoclastic cells. Biochem Biophys Res Commun. 2005;328:885-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Knowles HJ. The Adenosine A2B Receptor Drives Osteoclast-Mediated Bone Resorption in Hypoxic Microenvironments. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 196] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 55. | Mace ML, Olgaard K, Lewin E. New Aspects of the Kidney in the Regulation of Fibroblast Growth Factor 23 (FGF23) and Mineral Homeostasis. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Egli-Spichtig D, Imenez Silva PH, Glaudemans B, Gehring N, Bettoni C, Zhang MYH, Pastor-Arroyo EM, Schönenberger D, Rajski M, Hoogewijs D, Knauf F, Misselwitz B, Frey-Wagner I, Rogler G, Ackermann D, Ponte B, Pruijm M, Leichtle A, Fiedler GM, Bochud M, Ballotta V, Hofmann S, Perwad F, Föller M, Lang F, Wenger RH, Frew I, Wagner CA. Tumor necrosis factor stimulates fibroblast growth factor 23 Levels in chronic kidney disease and non-renal inflammation. Kidney Int. 2019;96:890-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Camacho-Cardenosa M, Camacho-Cardenosa A, Timón R, Olcina G, Tomas-Carus P, Brazo-Sayavera J. Can Hypoxic Conditioning Improve Bone Metabolism? Int J Environ Res Public Health. 2019;16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Li L, Kang H, Zhang Q, D'Agati VD, Al-Awqati Q, Lin F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest. 2019;129:2374-2389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 59. | Frey JL, Stonko DP, Faugere MC, Riddle RC. Hypoxia-inducible factor-1α restricts the anabolic actions of parathyroid hormone. Bone Res. 2014;2:14005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |