Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8142
Peer-review started: April 10, 2021
First decision: July 5, 2021
Revised: July 15, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: September 26, 2021
An immediate hypersensitive immune response to Aspergillus fumigatus antigens is one of the main characteristic features of allergic bronchopulmonary asper
We present a case of ABPA in which the eosinophil count in peripheral blood was not increased, whereas the eosinophil percentage in BALF reached 60%. After antifungal and hormone therapy, imaging revealed very good resolution of lung infiltration.
The value of the eosinophil count in BALF for the diagnosis of ABPA is worthy of the clinician's attention, especially when the patient’s clinical features lack specificity and the diagnostic parameters are negative.
Core Tip: Peripheral blood eosinophilia rather than eosinophilia in bronchoalveolar lavage fluid (BALF) has been used as a diagnostic indicator for allergic bronchopulmonary aspergillosis (ABPA). However, in our case, the eosinophil count in peripheral blood was not increased, whereas the eosinophil percentage in BALF reached 60%. We present this case report to explain the value of the eosinophil count in BALF for the diagnosis of ABPA.
- Citation: Wang WY, Wan SH, Zheng YL, Zhou LM, Zhang H, Jiang LB. Value of eosinophil count in bronchoalveolar lavage fluid for diagnosis of allergic bronchopulmonary aspergillosis: A case report. World J Clin Cases 2021; 9(27): 8142-8146
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8142.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8142
Allergic bronchopulmonary aspergillosis (ABPA) is an allergic respiratory disease caused by an exaggerated response to Aspergillus fumigatus (A. fumigatus) exposure. Early diagnosis of ABPA is crucial for appropriate treatment and for preventing widespread bronchiectasis and pulmonary fibrosis[1].
Eosinophilia in the peripheral circulation is an important parameter in the diagnosis of eosinophilic lung diseases. However, some disorders are not accompanied by eosinophilia in peripheral blood[2]. Instead, the eosinophil count in bronchoalveolar lavage fluid (BALF) could provide the only (or first) indication. As ABPA is an eosinophilic lung disease, peripheral blood eosinophilia has been considered in the diagnostic criteria, but eosinophilia in BALF has not (Table 1). The use of eosinophilia in BALF for the diagnosis of ABPA has rarely been reported.
| Rosenberg ’s criteria (1977) [6] | |
| Major criteria | (1) Asthma; (2) Presence of fleeting or fixed pulmonary opacities on chest radiograph; (3) Immediate cutaneous hypersensitivity reaction to A. fumigatus; (4) Total serum IgE more than 1000 IU/mL; (5) Precipitating antibodies against A. fumigatus; (6) Peripheral-blood eosinophilia; and (7) Central or proximal bronchiectasis with normal tapering of distal bronchi |
| Minor criteria | (1) Golden-brown sputum plugs in expectorant; (2) Positive sputum culture for Aspergillus species; and (3) Late (arthus-type) skin reactivity to A. fumigatus |
| The ISHAM Working Group’s criteria (2013) [8] | |
| Predisposing conditions | (1) Asthma; and (2) Cystic fibrosis |
| Obligatory criteria (both criteria must be fulfilled) | (1) Positive immediate (type I) cutaneous hypersensitivity to Aspergillus antigen or elevated IgE levels against A. fumigatus; and (2) Total IgE levels > 1000 IU/mL |
| Other criteria (at least 2 out of 3 must be fulfilled) | (1) Presence of precipitating or IgG antibodies against A. fumigatus in serum; (2) Radiographic pulmonary opacities consistent with ABPA; and (3) Total eosinophil count > 500 cells/μL in steroid-naïve patients |
| New Clinical Diagnostic Criteria in Japan (2020) [9] | |
| (1) History of asthma or symptoms associated with asthma; (2) Blood eosinophils ≥ 500/μL; (3) Blood total IgE ≥ 417 IU/mL; (4) Positive findings in the type 1 mycosis skin test or specific IgE; (5) Positive findings of mycosis-specific precipitating antibodies or specific IgG; (6) Detection of mycosis in sputum or bronchoscopy specimen; (7) Positive findings of mycotic mycelium by Grocott staining of the mucus plug; (8) Dilatation of the central bronchus on chest CT; (9) Current or historical mucus plug existence on chest CT or bronchoscopy; and (10) Presence of high-attenuation mucus on chest CT (ABPA is diagnosed if more than 6 criteria are satisfied in a patient without cystic fibrosis). | |
A 39-year-old woman with a 2-d history of cough was admitted to hospital on September 4, 2020.
The patient developed cough with white sputum after catching cold 2 d prior to admission. According to the computed tomography (CT) of the chest and routine blood examination, antibiotic therapy (piperacillin–tazobactam, 1.8 g, intravenous drip every 8 h) was initiated. However, she developed the symptoms of respiratory distress and dyspnoea 3 d after hospital admission, and simultaneously, wheezing could be heard in both lungs.
The patient had no noteworthy previous illness history.
The patient had no remarkable personal or family history.
Physical examination showed a respiratory rate of 17/min, heart rate of 111 bpm, body temperature of 37 °C, and blood pressure of 138/88 mmHg.
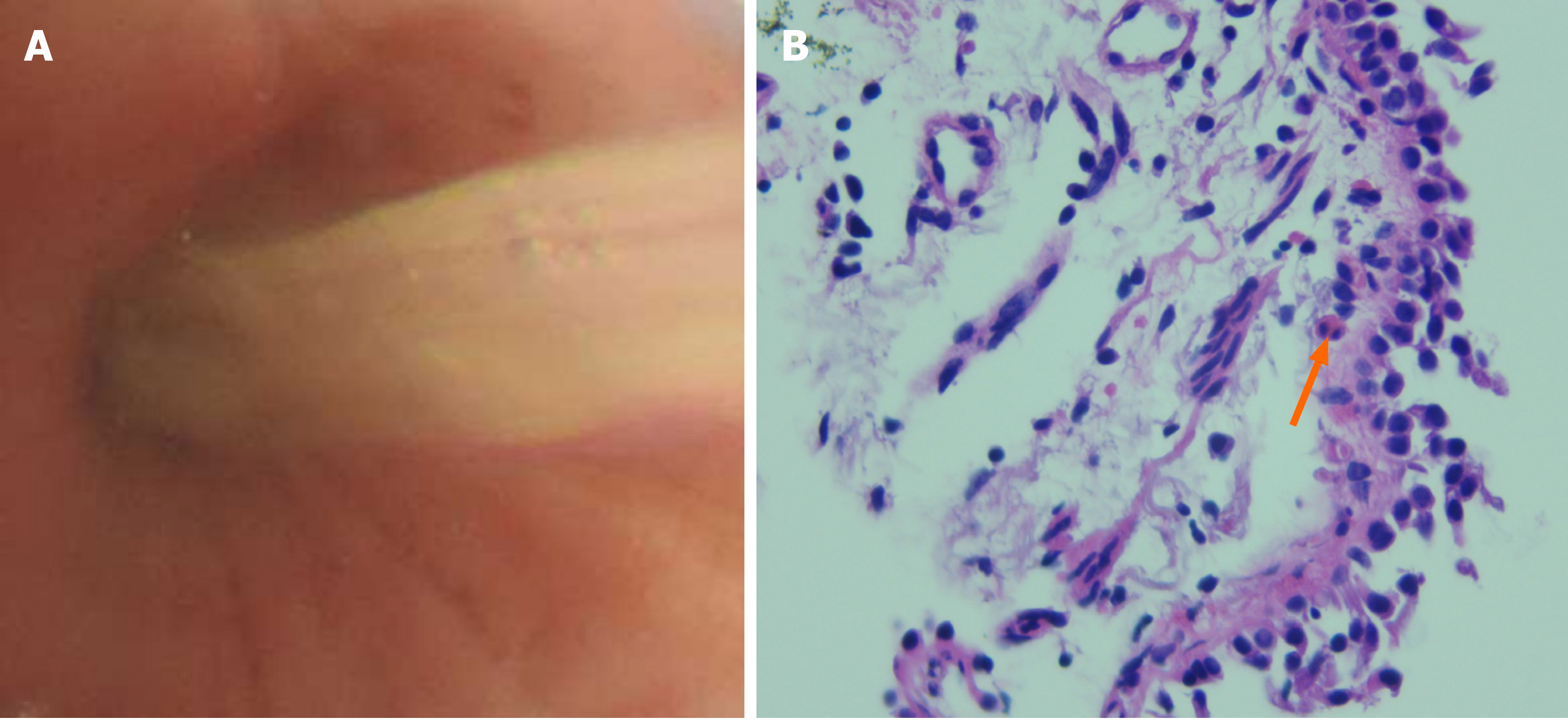
The white blood cell (WBC) count in serum was 10.8 × 109/L, the absolute neutrophil count was 8.6 × 109/L, and the eosinophil count was 0.27 × 109/L. Sputum culture revealed the presence of Klebsiella pneumoniae. A bronchial provocation test was positive. Total immunoglobulin (Ig) E) in serum was 1967.00 IU/mL. Aspergillus-specific IgE and IgG were positive. Bronchoscopy revealed yellowish mucus in the upper lobe of the right lung (Figure 1A). Aspergillus galactomannan antigen was negative in BALF. The pathological examination of bronchial biopsy showed scattered eosinophil infiltration (Figure 1B). A WBC differential count in BALF showed the eosinophil percentage to be 60%.
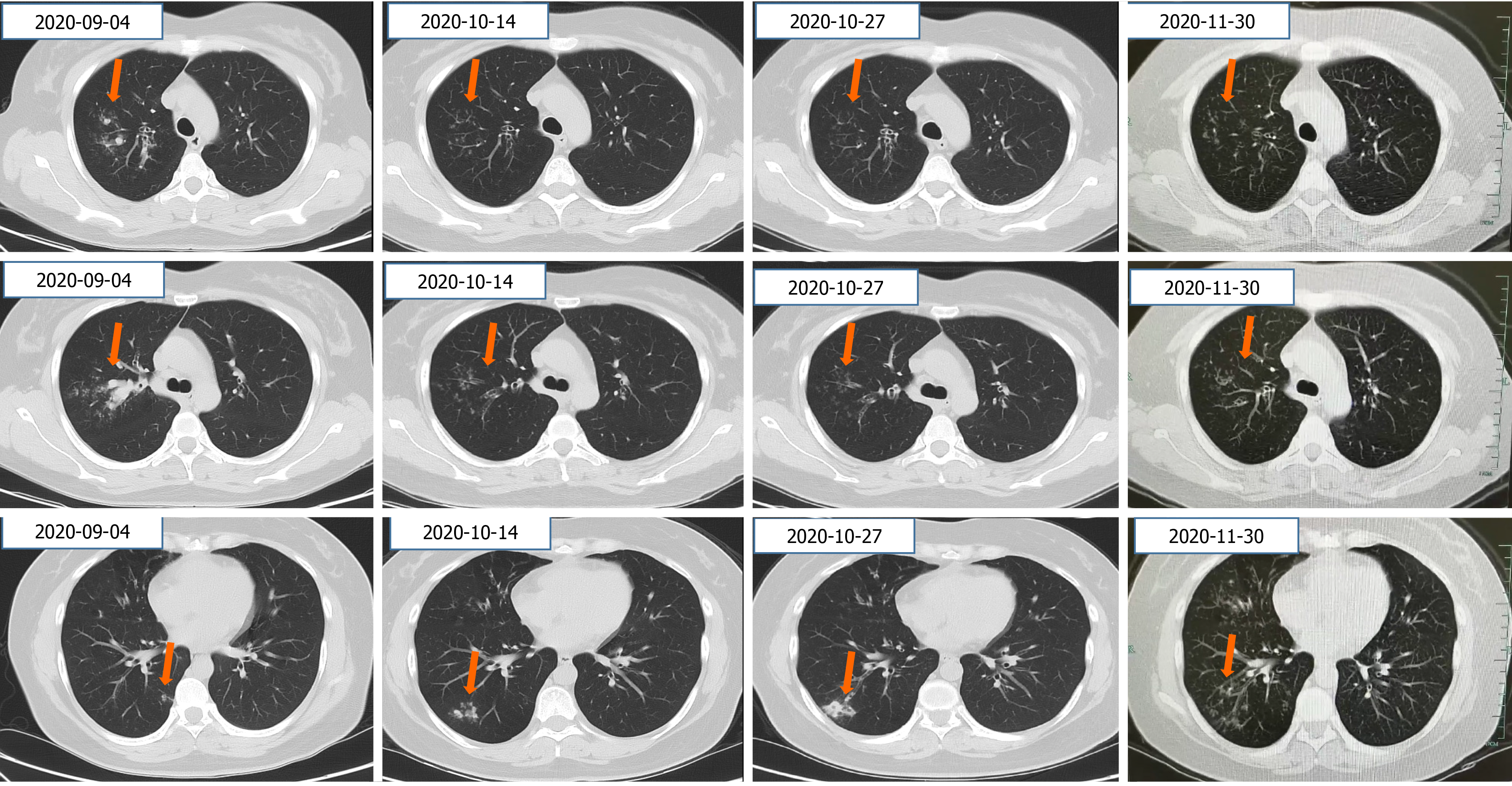
CT of the chest showed lung infiltration and mild bronchiectasis (Figure 2).
ABPA.
Antifungal therapy (voriconazole 200 mg, b.i.d.) and hormone therapy (methylprednisolone, 32 mg/d) were started. Her clinical symptoms improved gradually, the infiltration in the upper lobe of the right lung was improved, but new nodules appeared in the lower lobe of the right lung after 1 mo. The nodules continued to increase in size for 2 wk after antifungal therapy (voriconazole, 200 mg, intravenous drip every 12 h) and hormone therapy (methylprednisolone, 16 mg/d). She refused invasive efforts to exclude other diseases.
The patient’s clinical symptoms disappeared. Imaging follow-up after 1 mo on a new antifungal (posaconazole, 400 mg, b.d.) and the same hormone (methylprednisolone, 8 mg/d) revealed very good resolution of lung infiltration (Figure 2).
Our case reveals that eosinophilia in BALF can be helpful for the diagnosis of ABPA, and the curative effect varies according to the antifungal agent employed.
ABPA is easily misdiagnosed due to the diversity of disease manifestations and physicians’ incomplete knowledge of ABPA[3]. Misdiagnosis or delayed diagnosis of ABPA can lead to unnecessary treatments (e.g., intravenous antibiotics) and irreversible destruction to the airways (e.g., fibrosis and bronchiectasis)[1].
Conventionally, the diagnosis of ABPA is based on a combination of clinical, imaging, and immunological characteristics[4]. The most well-acknowledged diagnostic criteria for ABPA were proposed in 1977 and comprise eight major criteria and three minor criteria (Table 1)[5]. However, the criteria have several limitations, such as the lack of consensus on the number of components needed to make a diagnosis and the lack of a standardized methodology for immunologic tests[6]. The diagnostic criteria for ABPA will evolve as our understanding improves, and several research teams, such as the ISHAM Working Group and a group of Japanese scholars, have proposed a series of revised criteria (Table 1)[7,8]. Even so, a consensus on the minimum number of criteria required to make the diagnosis of ABPA is still lacking.
The main laboratory screening tests for the diagnosis of ABPA are an Aspergillus skin test, increased total IgE in serum, increased levels of Aspergillus-specific IgE and IgG, peripheral blood eosinophilia, and positive serum precipitins to A. fumigatus (Table 1). Eosinophilic lung diseases are a diverse group of pulmonary disorders characterized by eosinophilic infiltration of the pulmonary parenchyma. They are associated with eosinophilia in tissues or peripheral blood. An increased eosinophil count in peripheral blood is considered to be an important parameter for the diagnosis of ABPA. However, in one series, 25% of patients had an eosinophil count < 500 cells/μL[2].
Bronchoscopy and BALF are helpful for the diagnosis and evaluation of patients with eosinophilic lung diseases, especially those with atypical manifestations[9,10]. In healthy individuals, the eosinophil percentage in BALF is < 1%. In our case, the eosinophil percentage in BALF reached 60%, revealing an allergic disease.
In conclusion, we present this case to highlight the value of the eosinophil count in BALF for the diagnosis of ABPA. Of course, further studies are needed to confirm and explore these findings. As we learn more about ABPA, its diagnostic criteria will be refined further.
We acknowledge the contributions of Ms. Zeng XY for the research assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haque N S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Patel AR, Patel AR, Singh S, Khawaja I. Treating Allergic Bronchopulmonary Aspergillosis: A Review. Cureus. 2019;11:e4538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Agarwal R, Khan A, Aggarwal AN, Varma N, Garg M, Saikia B, Gupta D, Chakrabarti A. Clinical relevance of peripheral blood eosinophil count in allergic bronchopulmonary aspergillosis. J Infect Public Health. 2011;4:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Zhang C, Jiang Z, Shao C. Clinical characteristics of allergic bronchopulmonary aspergillosis. Clin Respir J. 2020;14:440-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Russo A, Tiseo G, Falcone M, Menichetti F. Pulmonary Aspergillosis: An Evolving Challenge for Diagnosis and Treatment. Infect Dis Ther. 2020;9:511-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 448] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Agarwal R, Sehgal IS, Dhooria S, Aggarwal AN. Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev Respir Med. 2016;10:1317-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, Moss R, Denning DW; ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 8. | Asano K, Hebisawa A, Ishiguro T, Takayanagi N, Nakamura Y, Suzuki J, Okada N, Tanaka J, Fukutomi Y, Ueki S, Fukunaga K, Konno S, Matsuse H, Kamei K, Taniguchi M, Shimoda T, Oguma T; Japan ABPM Research Program. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147:1261-1268.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Jeong YJ, Kim KI, Seo IJ, Lee CH, Lee KN, Kim KN, Kim JS, Kwon WJ. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617-37; discussion 637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Tamura A, Hebisawa A, Kurashima A, Kawabe Y, Machida K, Yotsumoto H, Mori M. The use of bronchofiberscopy for diagnosis of allergic bronchopulmonary aspergillosis. Intern Med. 1997;36:865-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |