Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.476
Peer-review started: August 27, 2020
First decision: November 3, 2020
Revised: November 9, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: January 16, 2021
Fat embolism syndrome (FES) is a rare disease characterized by pulmonary distress, neurologic symptoms, and petechial rash and seriously threatens human life and health. It is still neglected clinically because of the lack of verifiable diagnostic criteria and atypical clinical symptoms. No studies on FES with pulmonary embolism (PE) and tympanic membrane perforation have been reported to date. Here, we report a rare case of concomitant FES, PE and tympanic membrane perforation after surgery in a patient with a tibiofibular fracture.
A 39-year-old man presented with right lower extremity pain due to a car accident while driving a motorbike on the road. X-ray and computed tomography scans revealed a fracture of the right mid-shaft tibia and proximal fibula categorized as a type A2 fracture according to the AO classification. A successful minimally invasive operation was performed 3 d after the injury. Postoperatively, the patient developed sudden symptoms of respiratory distress and hearing loss. Early diagnosis was made, and supportive treatments were used at the early stage of FES. Seven days after surgery, he presented a clear recovery from respiratory symptoms. The outcome of fracture healing was excellent, and his hearing of the left ear was mildly impaired at the last follow-up of 4 mo.
Concomitant FES, PE and tympanic membrane perforation are very rare but represent potentially fatal complications of trauma or orthopedic surgery and present with predominantly pulmonary symptoms. Early diagnosis and treatment can reduce the mortality of FES, and prevention is better than a cure.
Core Tip: Fat embolism syndrome (FES) is a rare complication after internal fixation. This case suggests that any clinical manifestations of patients should be identified after internal fixation to avoid delays in treatment. Even with a lack of verifiable diagnostic criteria for FES, it should be highly suspected for patients with sudden hypoxemia and atypical neurological symptoms. Early diagnosis and supportive treatment are still recommended.
- Citation: Shao J, Kong DC, Zheng XH, Chen TN, Yang TY. Postoperative complications of concomitant fat embolism syndrome, pulmonary embolism and tympanic membrane perforation after tibiofibular fracture: A case report. World J Clin Cases 2021; 9(2): 476-481
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/476.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.476
Fat embolism syndrome (FES) is a rare disease characterized by pulmonary distress, neurologic symptoms, and petechial rash and seriously threatens human life and health. Due to the lack of verifiable diagnostic criteria, the actual incidence of FES varies in different reports[1-3]. Despite extensive reports of the morbidity and mortality of FES[4-6], this condition may still be neglected clinically. FES refers to the clinical symptoms that follow a clear history of trauma, particularly long bone fractures (e.g., femur or tibia). Although the exact mechanisms that result in FES are still unknown, it is likely that both mechanical and biochemical components contribute to the pathologic and physical manifestations of FES. Here, we report a rare case of concomitant FES, pulmonary embolism (PE), and tympanic membrane perforation after surgery in a patient with a tibiofibular fracture.
Severe pain and swelling on his right calf.
A 39-year-old man was admitted to the Department of Emergency Orthopedics in our hospital due to right lower extremity trauma from a car accident 2 h prior.
The patient had a free previous medical history.
The patient had no prior surgeries and did not smoke tobacco or drink alcohol. Family history was negative.
After admission, the patient’s temperature was 36.8 °C, heart rate was 89 bpm, respiratory rate was 19 breaths per minute, and blood pressure was 112/89 mmHg. Severe pain and swelling were found on the patient’s right calf, while no numbness was found. Peripheral circulation was in good condition, with the dorsalis pedis artery pulse detected. There was limitation of the right ankle joint during flexion and extension. Calcaneal traction was used to relieve pain and reduce swelling after admission.
There are no specific laboratory tests for FES; however, anemia, hypoxia, decreases in red blood cell count and hematocrit, and fluctuations in the inflammatory indices (e.g., white blood cell (WBC), C-reactive protein (CRP) were found at the early stage of FES (Table 1).
| Variable | Reference range | Preoperation | 30 h after surgery | 48 h after surgery | 72 h after surgery |
| WBC, 109/L | 3.50-9.50 | 13.63 | 11.25 | 8.73 | 14.8 |
| HG, g/L | 130-175 | 146 | 104 | 91 | 93 |
| RBC, 1012/L | 4.30-5.80 | 4.85 | 3.27 | 2.98 | 2.97 |
| Hematocrit, % | 40.0-50.0 | 44.8 | 30.5 | 28.0 | 28.1 |
| Platelet count, 109/L | 125-350 | 245 | 198 | 207 | 233 |
| CRP, mg/L | 0-10.0 | 8.6 | 85.4 | 116.0 | 44.7 |
| Prothrombin time, s | 11.0-15.0 | 14.0 | 14.9 | 14.5 | 14.5 |
| Prothrombin time/international normalized ratio | 0.85-1.50 | 1.10 | 1.19 | 1.15 | 1.15 |
| Activated partial-thromboplastin time, s | 25.0-45.0 | 37.1 | 38.1 | 52.9 | 39.2 |
| Potassium, mmol/L | 3.50-5.10 | 4.50 | 3.65 | 3.76 | 3.50 |
| Sodium, mmol/L | 137-147 | 141 | 140 | 143 | 141 |
| Chloride, mmol/L | 99.0-110.0 | 104.0 | 108.6 | 109.3 | 108.9 |
| Calcium, mmol/L | 2.11-2.52 | 2.19 | 1.89 | 1.95 | 1.85 |
| Carbon dioxide, mmol/L | 22.0-29.0 | 30.0 | 27.9 | 28.9 | 27.4 |
| Alanine aminotransferase, U/L | 9.0-60.0 | 24.3 | 70.0 | 64.0 | 47.8 |
| Aspartate aminotransferase, U/L | 15.0-45.0 | 21.0 | 48.0 | 28.0 | 29.9 |
| Alkaline phosphatase, U/L | 45.0-125.0 | 49.1 | 104.0 | 91.0 | 58.3 |
| Albumin, g/L | 40.0-55.0 | 42.4 | 27.8 | 29.6 | 31.1 |
| Total protein, g/L | 65.0-85.0 | 68.8 | 54.5 | 56.4 | 51.4 |
| Urea, mmol/L | 3.10-8.00 | 6.19 | 3.25 | 5.64 | 7.63 |
| Creatinine, μmol/L | 57-97 | 62 | 44 | 49 | 60 |
| Uric acid, μmol/L | 238-416 | 311 | 183 | 178 | 163 |
| Arterial blood gas, fraction of inspired oxygen | |||||
| Partial pressure of CO2, mmHg | 35-48 | 43 | 38 | 37 | |
| Partial pressure of O2, mmHg | 83-108 | 58 | 135 | 130 | |
| Base excess, % | -2.0-3.0 | 1.3 | 3.9 | 2.6 |
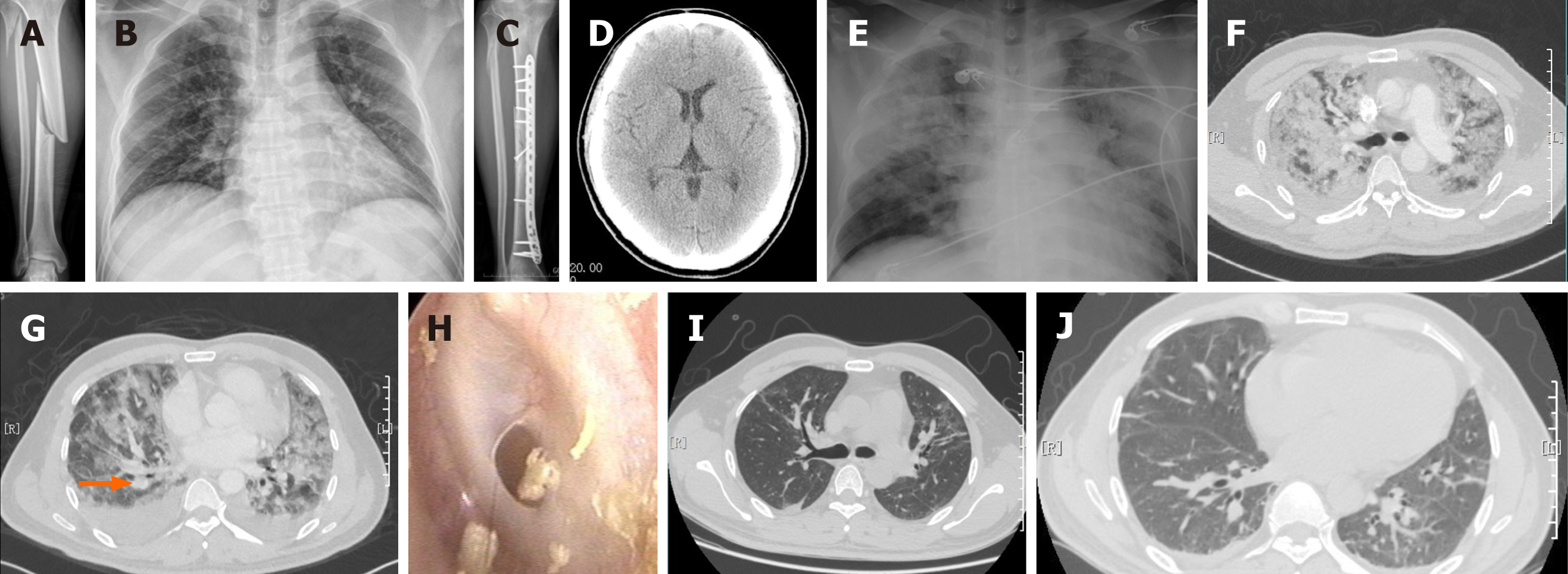
After X-ray and computed tomography (CT) examinations, he was diagnosed with fractures of the right midshaft tibia and proximal fibula (type A2 according to the AO classification) (Figure 1A). Preoperative routine examinations, including chest radiography (Figure 1B) and color Doppler ultrasound of the lower extremity blood vessels, were performed, and the results showed no significant abnormalities.
The patient was diagnosed with fracture of the right midshaft tibia and proximal fibula.
The patient was scheduled to undergo internal fixation of the tibia 3 d after the injury. A successful operation (Figure 1C) was performed via a minimally invasive anteromedial approach with an operation time of 90 min and intraoperative bleeding volume of 100 mL.
Unfortunately, approximately 30 h after surgery, the patient developed sudden symptoms of respiratory distress. He became confused and developed tachypnea (respiratory rate of 36 breaths/min) and tachycardia (heart rate: 120 bpm), and his oxygen saturation dropped below 90% on 4 L/min of oxygen via nasal cannula. No obvious petechial rash was present. A medical consultation was requested immediately for persistent respiratory distress symptoms, and the patient became weaker and more unresponsive. Decreased breath sounds and a small amount of scattered moist rales could be heard. However, his pupils were equal and round, and his reaction to light was normal. Computed tomography angiography (CTA) of the chest and CT of the head were performed. Moreover, the patient was transferred to the intensive care unit for close monitoring and supportive care. Then, he developed a fever of 38.4 °C 4 h later and required 50 L/min of oxygen to maintain a saturation > 95%. The CT scan of the head did not show any lesions (Figure 1D); however, the chest radiograph (Figure 1E) and CTA (Figure 1F) demonstrated diffuse ground-glass opacities in both lungs, and a limited mural thrombus was found in one distal pulmonary artery (Figure 1G, orange arrow). There are no specific laboratory tests for FES; however, anemia, hypoxia, decreases in red blood cell count and hematocrit, and fluctuations in the inflammatory indices (e.g., WBC, CRP) are found at the early stage of FES (Table 1). Given that the patient’s dyspnea was caused by a fat embolism (FE) and PE, high-flow oxygen inhalation (50 L/min), corticosteroids, anticoagulants, and other supportive treatments were used. He experienced hearing loss in his left ear 4 d after surgery, and an otoscopic examination demonstrated that the left tympanic membrane had a small circular perforation (Figure 1H). He was treated conservatively with analgesia and empiric antibiotics.
The patient gradually became more alert, his breathing became smooth, and his hearing in the left ear improved. Seven days after surgery, he presented a clear recovery from respiratory symptoms, showed improvements, and moved all his limbs against gravity without problems. His oxygen requirements decreased from 50 L/min to 4 L/min to maintain a saturation of > 95%. On the 10th d after the operation, the repeat chest CT demonstrated remarkable absorption of the diffuse ground-glass opacities and mural thrombus (Figure 1I and J). The patient returned to the orthopedic ward and was discharged on the 18th d after the operation. The outcome of fracture healing was excellent, and his hearing of the left ear was mildly impaired at the last follow-up of 4 mo. He was satisfied with the treatments he received.
FE is defined as the presence of fat droplets in the systemic circulation after trauma, especially after long bone fracture fixation[7]. FE is not similar to FES; only a minority of patients develop FES clinically, and most patients present as asymptomatic. Numerous studies have found that early surgical stabilization of the fracture (external or internal fixation) before 24 h dramatically reduced the incidence of FES and pulmonary complications[9-11]. However, the timing of fracture fixation remains controversial, especially in patients with polytraumatic injuries, in terms of the advantages of early fixation and the risk of serious complications of early definitive fixation (a potential second source of trauma)[10].
Both mechanical and biochemical components contribute to the physical manifestations and pathology of FES. Mechanically, fat droplets as emboli are forced into the circulation during orthopedic procedures, especially intramedullary fixation. The emboli can affect different organs and cause symptoms, especially in the lungs and brain. Biochemically, free fatty acids are released when lipase produced by the lungs acts on embolic fat. Pulmonary endothelial cells and pneumocytes are damaged, leading to arterial hypoxemia[12].
FES is a potentially devastating cause of morbidity and mortality in polytrauma patients. The clinical features of FES vary. FES usually has an asymptomatic interval for approximately 12 to 72 h after the initial injury, followed by a classical triad of findings: pulmonary distress, neurologic symptoms, and petechial rash[9]. This condition is often asymptomatic until pulmonary manifestations occur, which is when we first considered FES in this case. A massive pulmonary FE is often found at autopsy. Pulmonary manifestations, including dyspnea, tachypnea, and hypoxemia to acute respiratory distress syndrome (ARDS), are the earliest symptoms and can be seen in 75% of patients[13]. Hypoxia is the most common finding and is present in 95% of patients[7]. Neurologic manifestations may include ischemic/hemorrhagic strokes, retinal ischemia, seizures, autonomic dysfunction, and diffuse brain injury[14], which are present in 80% of patients[9]. FES is not necessarily accompanied by organic changes in brain tissue; CT scans of the brain may be normal, but brain magnetic resonance imaging is more sensitive in detecting FES[9,15]. However, isolated cerebral FES without any pulmonary symptoms was reported in a patient with multiple injuries[16]. This patient initially presented with hypoxia and atypical neurological symptoms 30 h after surgery. The delay in presentation is consistent with the biochemical theory.
A number of respiratory parameters, vital signs, and laboratory results can provide additional information according to Gurd, Schonfeld, and Lindeque’s criteria[9]. Overall, in this patient, we diagnosed FES with PE and conductive deafness based on typical hypoxia (PaO2: 58 mmHg in the arterial blood), mild neurological symptoms, fever (temperature: 38.4 °C > 38 °C), changes in vital signs (respiratory rate: 36/min > 30/min; heart rate: 120 bpm), and some laboratory tests combined with pulmonary CT findings. Nonetheless, there is no benchmark diagnostic test for FES. Many imaging modalities can facilitate the diagnosis of FES, but none of the findings are specific. Chest radiographic findings may show diffuse bilateral patchy infiltrates, consistent with acute ARDS, although this must be differentiated from pulmonary hemorrhage or pulmonary edema. Computed tomography angiography of the chest can demonstrate diffuse interlobular septal thickening with a superimposed ground-glass abnormality as well as numerous discrete small nodules, which are consistent with FES.
The most important treatment for FES is supportive care for the lung and cardiovascular system to correct hypoxia and maintain hemodynamic stability. Unnecessary transfers between wards and movement should be avoided to reduce the risk of the embolus breaking off and causing cardiopulmonary collapse. If patients exhibit neurologic involvement, frequent neurological examinations and intracranial pressure monitoring should be considered. The use of corticosteroids and heparin has been suggested as a possible treatment but remains controversial and has not been shown to reduce morbidity or mortality[17].
Concomitant FES, PE, and tympanic membrane perforation are very rare but represent potentially fatal complications of trauma or orthopedic surgery and present with predominantly pulmonary symptoms. This case reminds us to carefully consider and examine similar patients who are encountered in clinical practice. Early diagnosis and treatment can reduce the mortality of FES, and prevention is better than a cure.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ampollini L S-Editor: Huang P L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336:472-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Milroy CM, Parai JL. Fat Embolism, Fat Embolism Syndrome and the Autopsy. Acad Forensic Pathol. 2019;9:136-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Cvetković D, Živković V, Nikolić S. An unusual case of pulmonary fat embolism following blunt trauma. Forensic Sci Med Pathol. 2019;15:292-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Li S, Zou D, Qin Z, Liu N, Zhang J, Li Z, Shao Y, Deng K, Chen Y, Huang P. Nonfracture-associated pulmonary fat embolism after blunt force fatality: case report and review of the literature. Am J Forensic Med Pathol. 2015;36:61-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Takada M, Chiba S, Nagai T, Takeshita H, Kanno S, Ikawa T, Sakamoto K, Sagi M, Ichiba K, Mukai T. Inflammatory responses to neutral fat and fatty acids in multiple organs in a rat model of fat embolism syndrome. Forensic Sci Int. 2015;254:126-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Newbigin K, Souza CA, Torres C, Marchiori E, Gupta A, Inacio J, Armstrong M, Peña E. Fat embolism syndrome: State-of-the-art review focused on pulmonary imaging findings. Respir Med. 2016;113:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Porpodis K, Karanikas M, Zarogoulidis P, Konoglou M, Domvri K, Mitrakas A, Boglou P, Bakali S, Iordanidis A, Zervas V, Courcoutsakis N, Katsikogiannis N, Zarogoulidis K. Fat embolism due to bilateral femoral fracture: a case report. Int J Gen Med. 2012;5:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Rothberg DL, Makarewich CA. Fat Embolism and Fat Embolism Syndrome. J Am Acad Orthop Surg. 2019;27:e346-e355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 10. | Blokhuis TJ, Pape HC, Frolke JP. Timing of definitive fixation of major long bone fractures: Can fat embolism syndrome be prevented. Injury. 2017;48:Suppl 1: S3-S6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Dunn RH, Jackson T, Burlew CC, Pieracci FM, Fox C, Cohen M, Campion EM, Lawless R, Mauffrey C. Fat emboli syndrome and the orthopaedic trauma surgeon: lessons learned and clinical recommendations. Int Orthop. 2017;41:1729-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Paredes JC, Syquia JF, Chang AM, Zamuco JT. Fat embolism syndrome after shoulder hemiarthroplasty. J Shoulder Elbow Surg. 2011;20:e1-e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Powers KA, Talbot LA. Fat embolism syndrome after femur fracture with intramedullary nailing: case report. Am J Crit Care. 2011;20:267, 264-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Morales-Vidal SG. Neurologic Complications of Fat Embolism Syndrome. Curr Neurol Neurosci Rep. 2019;19:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Citerio G, Bianchini E, Beretta L. Magnetic resonance imaging of cerebral fat embolism: a case report. Intensive Care Med. 1995;21:679-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Huang CK, Huang CY, Li CL, Yang JM, Wu CH, Chen CH, Wu PT. Isolated and early-onset cerebral fat embolism syndrome in a multiply injured patient: a rare case. BMC Musculoskelet Disord. 2019;20:377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37 Suppl 4:S68-S73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |