Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4859
Peer-review started: February 8, 2021
First decision: April 4, 2021
Revised: April 13, 2021
Accepted: May 6, 2021
Article in press: May 6, 2021
Published online: June 26, 2021
Follicular lymphoma is an indolent lymphoma that may progress to a highly aggressive form requiring immunochemotherapy. Most regimens utilize rituximab, an anti-CD20 monoclonal antibody, which may affect the clinical course of novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections [coronavirus disease 2019 (COVID-19)]. Here we describe the first case of mild COVID-19 during ongoing oncological treatment without significant deterioration after rituximab administration.
A 74-year-old female with an enlargement of her right palatine tonsil was diagnosed with follicular lymphoma following tonsillectomy and started immunochemotherapy according to the rituximab, cyclophosphamide, vincristine, prednisone regimen. At home before the fourth cycle, she developed nonspecific symptoms (excessive fatigue, loss of appetite and nausea), misdiagnosed as adverse effects of chemotherapy. Unexpectedly, interim positron emission tomography-computed tomography scan, performed shortly before rituximab administration, revealed previously nonexistent pulmonary changes, potentially of infectious etiology. SARS-CoV-2 infection was confirmed by a nasopharyngeal swab (with reverse transcriptase polymerase chain reaction test) performed the following day. Despite rituximab infusion, the patient remained oligosymptomatic and was discharged home for self-isolation. Having reached a negative SARS-CoV-2 status before the subsequently scheduled regimen, the patient successfully received six cycles of rituximab, cyclophosphamide, vincristine, prednisone and obtained complete remission by positron emission tomography-computed tomography.
Our case shows that rituximab-based immunotherapy due to follicular lymphoma may have no evident negative effect on the COVID-19 clinical course.
Core Tip: Follicular lymphoma is an indolent lymphoma requiring immunochemotherapy with rituximab. This anti-CD20 monoclonal antibody depletes malignant and normal B-cells, resulting in a significantly increased risk of infectious complications, including the novel coronavirus, severe acute respiratory syndrome coronavirus 2 infection [coronavirus disease 2019 (COVID-19)]. We present the first case of mild COVID-19 in an elderly patient during ongoing follicular lymphoma treatment, without significant deterioration after rituximab administration. This case highlights that rituximab-based therapy may have no evident negative effect on the clinical course of COVID-19 in patients with follicular lymphoma, which may be significant during the current pandemic.
- Citation: Łącki S, Wyżgolik K, Nicze M, Georgiew-Nadziakiewicz S, Chudek J, Wdowiak K. Low symptomatic COVID-19 in an elderly patient with follicular lymphoma treated with rituximab-based immunotherapy: A case report. World J Clin Cases 2021; 9(18): 4859-4865
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4859.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4859
Follicular lymphoma (FL) is an incurable, indolent form of non-Hodgkin lymphomas, deriving from follicular center B-lymphocytes, mostly connected with asymptomatic lymphadenopathy. In a few recent decades, the occurrence of this neoplasm has significantly increased, amounting to 5/100000 cases, making it the second most frequent nodal lymphoid malignancy in Western Europe[1]. Diagnosis is made based on a surgical specimen or excisional lymph node biopsy, followed with the pathological and immunohistological tissue examination. Therapeutic management may differ depending on the FL clinical stage, presented symptoms, comorbidities and life expectancy, but the front-line treatment is immunochemotherapy with rituximab-based regimens[2]. This chimeric anti-CD20 monoclonal antibody causes rapid and prolonged (up to 12 mo) depletion of both malignant and normal B-cells[3], resulting in a significantly increased risk of infectious complications, particularly in immunocompromised patients[4], which seems to be crucial especially during the novel coronavirus disease 2019 (COVID-19) ongoing pandemic.
Although most human coronavirus infections are mild, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel strain of coronavirus causing COVID-19, contributed to numerous severe pneumonia cases, which started in December 2019 in Wuhan (China) and has spread worldwide since then[5].
On March 11, 2020, the World Health Organization announced COVID-19 a pandemic[6]. To date, COVID-19 has affected almost 100 million patients and caused more than 2 million deaths all over the world[7], posing a major global healthcare threat. As reported by Yang et al[8], COVID-19 infection in patients under oncological therapy is associated with an increase in mortality. However, during the present pandemic, the benefit/risk ratio of cancer treatment may need to be reconsidered individually[9]. The European Society for Medical Oncology has released new guidelines recommending physicians to watch their patients carefully, and while developing COVID-19 symptoms ongoing treatment should be stopped[10]. Here we report the case of a 74-year-old female patient with FL treated with rituximab-based immunochemotherapy regimen, unexpectedly diagnosed with oligosymptomatic COVID-19 during the fourth cycle of treatment, which was paused after the administration of rituximab solely. After recovery from COVID-19, she continued oncological therapy.
A 74-year-old female patient presented with an enlargement of her right palatine tonsil accompanied by difficulties with swallowing lasting for 2 mo.
In May 2020, the patient was referred to a laryngologist, and the right-side tonsillectomy was performed, obtaining a surgical specimen measuring 3.5 cm × 2.5 cm × 2.0 cm. This pathological mass with polypoid lesions was sent for pathological, immunohistochemical and genetic analyses, which confirmed Grade 2 FL with the following marker combinations: CD20 (+), CD23 (+), CD3 (-), CD5 (-), bcl-2 (+), bcl-6 (+), CD10 (+) and Ki-67 (80%). Afterwards, in July 2020, she was admitted to the Department of Internal Diseases and Oncological Chemotherapy of the Medical University of Silesia in Katowice to evaluate the advancement of the disease and implement the targeted therapy.
The patient reported suffering from arterial hypertension, hypothyroidism, glaucoma and hyperuricemia. She underwent a radical, right-side nephrectomy 25 years ago due to kidney tumor (the estimated glomerular filtration rate of the remaining kidney was 41 mL/min per 1.73 m2).
The family history was not relevant as for neoplasms, and there were not any known drug allergies nor use of any psychoactive substances.
On admission, the patient was alert, self-oriented and complained of progressive fatigue (without B symptoms). No additional symptoms on the part of other organs or systems were reported. Her general well-being and activities of daily life were assessed as ECOG 1 (symptomatic but completely ambulatory). General examination, apart from obesity (body mass index 30.5 kg/m2) and the nephrectomy scar, did not reveal any other abnormalities.
Conducted blood tests primarily did not indicate infectious background (lymphopenia 0.89 G/L, C-reactive protein 16.47 mg/L, procalcitonin < 0.05 ng/mL). Reported symptoms were connected to the adverse effects of immunochemotherapy. After infusion of multielectrolyte fluids, the patient felt better.
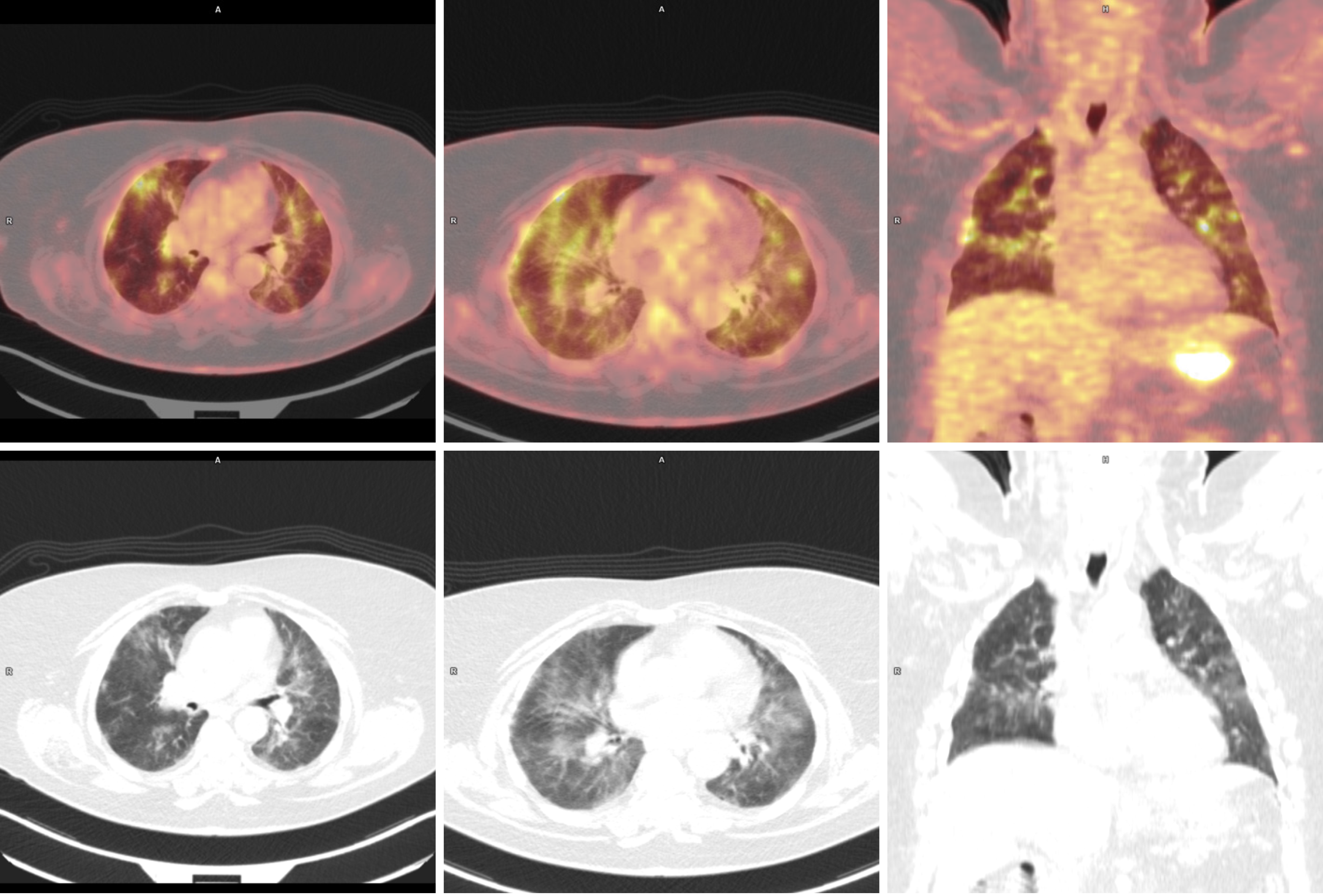
Interim positron emission tomography-computed tomography (PET-CT) scan was performed, as planned, before the fourth cycle, and then rituximab infusion was given (before PET-CT description). The following day, the results of imaging (Figure 1) demonstrated the regression in metabolism and measurements of affected lymph nodes and spleen.
Nonspecific, previously nonexistent pulmonary changes, predominant in the upper and middle lung areas, were described. The differential considerations in the first instance included ongoing inflammatory processes (especially the viral ones) but also drug toxicity reactions.
After the initial routine diagnostic process for the patient with FL, including a PET-CT scan of the neck, thorax, abdomen and pelvis (to stage nodal and extranodal site involvement) as well as laboratory tests such as complete blood count, lactate dehydrogenase, beta-2 microglobulin and uric acid, the clinical stage was evaluated as III according to the Ann Arbor classification system (involved nodes on both sides of the diaphragm and the spleen). Furthermore, Follicular Lymphoma International Prognostic Index was established 3 out of 5 (due to advanced age, anemia and clinical stage III), predicting a poor prognosis of overall life survival. Although European Society for Medical Oncology guidelines prefer rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone regimen as a front-line treatment of FL[11], patient was qualified for immunochemotherapy according to the R-CVP protocol (rituximab: 375 mg/m2, cyclophosphamide: 1200 mg, vincristine: 1 mg, prednisone: 10 mg)[12]. The prevention of herpes simplex virus and pneumocystis infections and emetic complications was applied. At home, after the third cycle of therapy, the patient developed excessive fatigue, loss of appetite and nausea deteriorating since the previous week. During the succeeding hospitalization, the patient’s physical condition weakened, but ECOG remained 1.
Having regard to the current global epidemiological situation, on October 1, 2020 we extended the diagnostics taking a nasopharyngeal swab to test for SARS-CoV-2, which led to a positive result.
SARS-CoV-2 infection during FL treatment with rituximab. Given the overall clinical picture and the virus incubation period, we could presume community-acquired infection.
Initiated therapy was limited to rituximab only, with the discontinuation of other regimen components. Despite implementing immunotherapy, the patient remained asymptomatic for fever and typical pulmonary symptoms (cough, dyspnea) accompanying COVID-19. Moreover, her general medical state improved, despite the lack of any other complementary drugs, and she was discharged home for self-isolation.
Having reached a seronegative status along with no auscultation abnormalities or other physical examination findings, the patient successfully received six cycles of R-CVP regimen. PET-CT scan was conducted and confirmed complete remission. Then maintenance treatment with rituximab monotherapy was implemented. At the time of this report, the patient received two injections of rituximab.
In this paper, we presented the case report of an elderly woman with FL treated with rituximab, who was diagnosed with COVID-19 during oncological therapy. The course of this infectious disease appeared to be oligosymptomatic with uncharacteristic and nonrelevant to respiratory system symptoms. Such a clinical manifestation mainly suggested the adverse effects of ongoing immunochemotherapy.
The recent scientific studies report mild to moderate natural history of SARS-CoV-2 infection as the most frequent one, typically with possible fever, dry cough and tiredness[13]. In COVID-19 positive people, there may also occur other symptoms, in the combination as presented by our patient, but they are not specific enough to test for the novel coronavirus in the first instance. Such a situation can especially be noted in hemato-oncological disorders, where similar manifestations can derive from the targeted treatment as its adverse effects as well as the progression of the neoplasm itself[14].
Furthermore, there is scientific evidence that elderliness and conditions such as hematological malignancies, obesity or arterial hypertension predispose to an increased vulnerability to COVID-19 and particularly high risk of serious events related to it[15-17]. According to observations from Yang et al[8], receiving chemotherapy 4 wk before the infection is an unfavorable risk factor. Fortunately, in our case, the course was mild and has not generated any complications so far. Nevertheless, oncologists should always bear in mind the current epidemiological status and have awareness of the possible new viral threat even among asymptomatic people. Similar to our patient, Albano et al[18] described five asymptomatic patients undergoing routine PET-CT scans who had radiologically found interstitial pneumonia indeterminate for COVID-19, subsequently proven with reverse transcriptase polymerase chain reaction.
The available literature refers to the treatment during detected COVID-19 in autoimmune and autoinflammatory diseases, such as granulomatosis with polyangiitis, systemic sclerosis, cryopyrin-associated periodic syndrome, spondyloarthritis and pemphigus vulgaris, in which there were severe viral pneumonia or even some fatalities in patients medicated with rituximab[19,20], whereas other drugs widely administered in the foregoing conditions (tumor necrosis factor-alpha antagonists, anakinra or tocilizumab) did not deteriorate the natural course of COVID-19[21-24].
Interestingly, Yasuda et al[25] reported persistent COVID-19 pneumonia and failure of seroconversion during rituximab maintenance therapy for FL, underlining that rituximab therapy should be avoided whenever possible during the ongoing pandemic.
Another important issue is how treatment with rituximab may affect the success of a possible future vaccine in the patient. According to Rubin et al[26], patients receiving rituximab generally should receive the vaccine ≥ 6 mo after therapy because of their poor immune response. Our patient should wait this long as well.
Given the abovementioned, it is worth emphasizing that we did not find any data concerning the course of COVID-19 while ongoing immunotherapy with monoclonal anti-CD20 antibodies due to oncological purposes. According to our knowledge, the presented patient is the first one ever described with such a favorable course of this infection and therefore becomes the unique case.
From our point of view, this case report may be important, especially regarding patients with an oligosymptomatic course of COVID-19 but requiring urgent lymphoma treatment.
The presented course of events was not a standard one, and the rituximab-containing regimen was administered to the SARS-CoV-2 positive patient before having possessed the knowledge about the viral status. Although the described case report shows that rituximab-based therapy may have no evident negative effect on the clinical course of COVID-19 in patients with FL, it should be remembered that the administration of cancer chemotherapy in patients with COVID-19 is associated with increased mortality. Certainly, further research is needed to learn the causes of distinct COVID-19 clinical courses in patients undergoing the same anti-CD20 treatment.
Manuscript source: Unsolicited manuscript
Corresponding Author’s Membership in Professional Societies: European Society for Medical Oncology, No. 445708.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nagaraju GP, Villalba R S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Mounier M, Bossard N, Remontet L, Belot A, Minicozzi P, De Angelis R, Capocaccia R, Iwaz J, Monnereau A, Troussard X, Sant M, Maynadié M, Giorgi R; EUROCARE-5 Working Group; CENSUR Working Survival Group. Changes in dynamics of excess mortality rates and net survival after diagnosis of follicular lymphoma or diffuse large B-cell lymphoma: comparison between European population-based data (EUROCARE-5). Lancet Haematol. 2015;2:e481-e491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Bargetzi M, Baumann R, Cogliatti S, Dietrich PY, Duchosal M, Goede J, Hitz F, Konermann C, Lohri A, Mey U, Novak U, Papachristofilou A, Stenner F, Taverna C, Zander T, Renner C. Diagnosis and treatment of follicular lymphoma: an update. Swiss Med Wkly. 2018;148:w14635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? Int J Infect Dis. 2011;15:e2-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28474] [Article Influence: 7118.5] [Reference Citation Analysis (3)] |
| 6. | World Health Organization. World Health Organization Regional Office for Europe: WHO announces COVID-19 outbreak a pandemic, 2019. [cited 8 November 2020]. In: World Health Organization [Internet]. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic. [Cited in This Article: ] |
| 7. | World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update, 2021. [cited 19 January 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports?fbclid=IwAR2Ls-e9P7a-xWYuxmRZQ0VhErC-VTJ80qQL8uU7FQObYh6hBXjFi_1Jpo8. [Cited in This Article: ] |
| 8. | Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y, Pan D, Shu C, Li J, Wei J, Huang Y, Peng L, Wu M, Zhang R, Wu B, Li Y, Cai L, Li G, Zhang T, Wu G. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 376] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 9. | European Society for Medical Oncology. Cancer patient management during the COVID-19 pandemic, 2020. [cited 9 November 2020]. In: European Society for Medical Oncology [Internet]. Available from: https://www.esmo.org/content/download/300571/5991421/1/ESMO-Recommendations-Covid-19-General-Slide-Set.pptx. [Cited in This Article: ] |
| 10. | European Society for Medical Oncology. ESMO Management and treatment adapted recommendations in the COVID-19 era: Indolent B-NHL (follicular lymphoma, marginal zone lymphoma, Waldenström’s macroglobulinemia, 2020. [cited 9 November 2020]. In: European Society for Medical Oncology [Internet]. Available from: https://www.esmo.org/content/download/301081/5998774/1/ESMO-Recommendations-Covid-19-Indolent-Lymphoma-Slide-Set.pptx. [Cited in This Article: ] |
| 11. | Dreyling M, Ghielmini M, Rule S, Salles G, Ladetto M, Tonino SH, Herfarth K, Seymour JF, Jerkeman M; ESMO Guidelines Committee. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:298-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 12. | Walewski J, Paszkiewicz-Kozik E, Michalski W, Rymkiewicz G, Szpila T, Butrym A, Giza A, Zaucha JM, Kalinka-Warzocha E, Wieczorkiewicz A, Zimowska-Curyło D, Knopińska-Posłuszny W, Tyczyńska A, Romejko-Jarosińska J, Dąbrowska-Iwanicka A, Gruszecka B, Jamrozek-Jedlińska M, Borawska A, Hołda W, Porowska A, Romanowicz A, Hellmann A, Stella-Hołowiecka B, Deptała A, Jurczak W. First-line R-CVP vs R-CHOP induction immunochemotherapy for indolent lymphoma with rituximab maintenance. A multicentre, phase III randomized study by the Polish Lymphoma Research Group PLRG4. Br J Haematol. 2020;188:898-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, Wade RG. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 367] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 14. | Carbone A, Roulland S, Gloghini A, Younes A, von Keudell G, López-Guillermo A, Fitzgibbon J. Follicular lymphoma. Nat Rev Dis Primers. 2019;5:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 15. | Yigenoglu TN, Bascı S, Dal MS, Korkmaz S, Turgut B, Altuntas F. The outcome ofancy. J Med Virol. 2021;93:1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | He W, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y, Li L, Wang H, Chen Z, Li Q, Gale RP. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 323] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 17. | Giovannoni G. Anti-CD20 immunosuppressive disease-modifying therapies and COVID-19. Mult Scler Relat Disord. 2020;41:102135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Albano D, Bertagna F, Bertoli M, Bosio G, Lucchini S, Motta F, Panarotto MB, Peli A, Camoni L, Bengel FM, Giubbini R. Incidental Findings Suggestive of COVID-19 in Asymptomatic Patients Undergoing Nuclear Medicine Procedures in a High-Prevalence Region. J Nucl Med. 2020;61:632-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 19. | Rondaan C, Furer V, Heijstek MW, Agmon-Levin N, Bijl M, Breedveld FC, D'Amelio R, Dougados M, Kapetanovic MC, van Laar JM, Ladefoged de Thurah A, Landewé R, Molto A, Müller-Ladner U, Schreiber K, Smolar L, Walker J, Warnatz K, Wulffraat NM, van Assen S, Elkayam O. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5:e001035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | Avouac J, Airó P, Carlier N, Matucci-Cerinic M, Allanore Y. Severe COVID-19-associated pneumonia in 3 patients with systemic sclerosis treated with rituximab. Ann Rheum Dis. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Duret PM, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis. 2020;79:1251-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Moutsopoulos HM. Anti-inflammatory therapy may ameliorate the clinical picture of COVID-19. Ann Rheum Dis. 2020;79:1253-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Mihai C, Dobrota R, Schröder M, Garaiman A, Jordan S, Becker MO, Maurer B, Distler O. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79:668-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 24. | Beyzaee AM, Rahmatpour Rokni G, Patil A, Goldust M. Rituximab as the treatment of pemphigus vulgaris in the COVID-19 pandemic era: A narrative review. Dermatol Ther. 2021;34:e14405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Yasuda H, Tsukune Y, Watanabe N, Sugimoto K, Uchimura A, Tateyama M, Miyashita Y, Ochi Y, Komatsu N. Persistent COVID-19 Pneumonia and Failure to Develop Anti-SARS-CoV-2 Antibodies During Rituximab Maintenance Therapy for Follicular Lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20:774-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, Kang I; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 645] [Cited by in F6Publishing: 620] [Article Influence: 62.0] [Reference Citation Analysis (0)] |