Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4844
Peer-review started: February 6, 2021
First decision: March 14, 2021
Revised: March 27, 2021
Accepted: April 9, 2021
Article in press: April 9, 2021
Published online: June 26, 2021
Transduodenal local excision is an alternative treatment approach for benign ampullary tumors. However, this procedure has technical difficulties, especially during reconstruction of the pancreaticobiliary ducts. An operating microscope has been widely used by surgeons for delicate surgery due to its major advantages of magnification, illumination, and stereoscopic view. The application of an operating microscope in transduodenal excision of ampullary tumors has not been reported.
A 55-year-old woman was admitted for investigation of recurrent upper abdominal pain. Physical examination and laboratory tests found no abnormalities. Imaging identified a large mass in the descending part of the duodenum. Esophagogastroduodenoscopy revealed a 3.5-cm-sized villous growth over the major duodenal papilla. Pathology of the endoscopic biopsy indicated a villous adenoma with low-grade dysplasia. Microscopic transduodenal excision of the ampullary tumor was performed. The final pathological diagnosis was villous-tubular adenoma with low-grade dysplasia. The patient was discharged on postoperative day 12 after an uneventful recovery. Endoscopic retrograde cholangiopancreatography was performed 3 mo postoperatively and showed no bile duct or pancreatic duct strictures and no tumor recurrence. The patient is continuing follow-up at our clinic and remains well.
Operating microscope-assisted transduodenal local excision is a feasible and effective option for benign ampullary tumors.
Core Tip: Operating microscope-assisted transduodenal excision of ampullary tumors has not been reported. We present our experience of microscopic transduodenal local excision in a case of ampullary adenoma. The application of an operating microscope provides significant technical advantages, particularly in the reconstruction of pancreaticobiliary ducts. The successful outcome in this case suggests that microscopic transduodenal excision is feasible and effective for patients with ampullary tumors.
- Citation: Zheng X, Sun QJ, Zhou B, Jin M, Yan S. Microscopic transduodenal excision of an ampullary adenoma: A case report and review of the literature. World J Clin Cases 2021; 9(18): 4844-4851
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4844
Ampullary tumors have been increasingly encountered as esophagogastroduodenoscopy (EGD) is widely used in the clinic, of which adenomas are the most common tumors[1]. Complete resection of ampullary adenomas is necessary as they are potentially malignant[2]. Currently, there are three approaches to manage ampullary neoplasms: Endoscopic papillectomy (EP), pancreaticoduodenectomy (PD), and transduodenal excision. Although EP is globally recognized as the first choice for treatment of benign ampullary tumors, it can only be carried out on small tumors confined to the papilla and not involving the common bile duct or pancreatic duct[3,4]. Traditional PD is a standard surgical approach for malignant ampullary tumors. However, the application of PD in patients with benign lesions remains controversial given the significant postoperative morbidity and mortality. Compared to PD, transduodenal excision of ampullary tumors offers significantly lower morbidity and mortality. Several reports have indicated that transduodenal excision can be used as an alternative treatment approach for benign or ampulla of Vater neoplasms[5-7]. However, widespread use of this procedure has failed as it is technically demanding. Suboptimal dissection and reconstruction of the pancreaticobiliary duct system can lead to tumor residues and life-threatening complications.
An operating microscope has the major advantages of magnification, illumination, and stereoscopic view, and is now widely used in various surgical sub-specialties such as neurosurgery, ophthalmology, plastic surgery, and orthopedics[8,9]. The app
A 55-year-old woman was admitted to our hospital with a history of recurrent upper abdominal pain for 1 year. The patient had no symptoms of fever, nausea, vomiting, or weight loss.
The patient’s symptoms started 1 year ago, with recurrent episodes of upper abdominal pain that were relieved spontaneously.
The patient had no previous medical history.
The personal and family history was unremarkable.
The patient’s physical examination was unremarkable.
The laboratory findings, including liver function tests and tumor markers (carcinoembryonic antigen, alpha fetoprotein, and carbohydrate antigen 19-9), were all within normal limits.
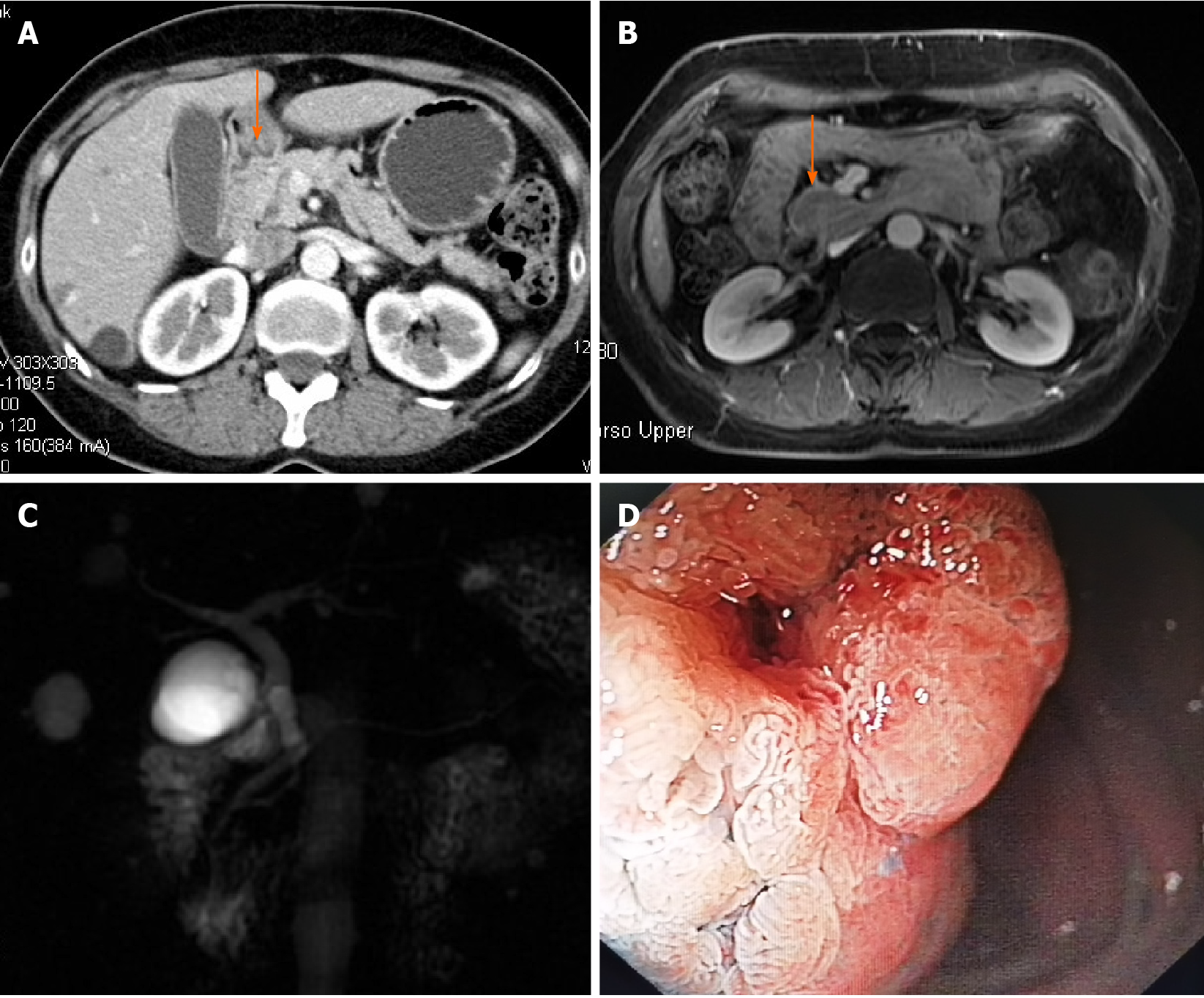
On computed tomography (CT) (Figure 1A) and magnetic resonance imaging (Figure 1B), a large mass with contrast enhancement was observed in the descending part of the duodenum. On magnetic resonance cholangiography (Figure 1C), no cut-off sign or stricture was found on either the bile duct or pancreatic duct except for a mild dilation of the common bile duct.
EGD revealed a 3.5-cm-sized villous growth over the major duodenal papilla (Figure 1D). Pathology of the endoscopic biopsy indicated a villous adenoma with low-grade dysplasia.
Based on the radiographic and pathological findings, an ampullary adenoma was diagnosed.
Microscopic transduodenal excision of the ampullary tumor was performed (Figure 2). The patient was placed in the supine position under general anesthesia. After a midline laparotomy, the duodenum was mobilized with the Kocher maneuver. The ampullary lesion was identified by palpation of the descending part of the duodenum. A 4-cm longitudinal duodenotomy was performed over the anterolateral wall and stay sutures were then placed to keep the duodenotomy open (Figure 2A). A Zeiss operating microscope (OPM2 VARIO 700, Carl Zeiss Meditec AG, Jena, Germany) was used. The ampullary tumor was excised using monopolar cautery and microscopic scissors under the operating microscope (Figure 2B). The pancreaticobiliary duct was identified by high magnification, and dissected carefully to ensure an adequate margin (Figure 2C and D). The specimen was sent for intraoperative pathological examination with the tumor orientation details. Intraoperative frozen section confirmed a villous adenoma with a negative margin. The pancreaticobiliary duct was sutured to the surrounding duodenal mucosa with an interrupted 6/0 prolene suture to reconstruct the ampulla (Figure 2E). A gauge 12 silicone catheter was inserted through the orifice into the pancreatic duct for stenting, and was anchored with 5/0 PDS sutures. The duodenotomy incision was closed with a one-layer transverse suture with interrupted 5/0 prolene stitches (Figure 2F). Anterior and posterior silicone drains were placed near the duodenotomy. The operative time was 160 min. The blood loss was 30 mL.
Postoperative pathology showed a villous-tubular adenoma with low-grade dysplasia. The patient started to take a semi-fluid diet 6 d post-operation. An abdominal CT scan was performed on postoperative day 7, and showed no leakage or passage disturbance. The patient was discharged on postoperative day 12 after an uneventful recovery. An endoscopy was performed 3 mo after surgery to remove the pancreatic duct stent, and endoscopic retrograde cholangiopancreatography was performed, which showed no recurrence of the ampullary lesion and no bile duct or pancreatic duct stricture. She is continuing follow-up at our clinic and remains well.
Adenomas are the most common ampullary tumors[10]. Complete resection is required to treat ampullary adenomas as they are premalignant[11]. Currently, the management strategies include EP, PD, and transduodenal excision. Although EP is an attractive option as it is minimally invasive, it is limited to patients with smaller lesions confined to the papilla and without involvement of the duodenal muscularis or pancreaticobiliary ducts. PD is a standard procedure for malignant ampullary tumors. However, the treatment of benign tumors by PD is still debated. It could be considered as overtreatment given the high postoperative morbidity (32.6%-57.6%)[12-15] and mortality (2%-5%)[16-19] rates associated with PD. Therefore, transduodenal excision has emerged as an alternative treatment option for ampullary adenomas.
Transduodenal excision has advantages over PD in terms of less invasiveness and organ preservation. Its safety and efficacy have been investigated in several case series[7,20,21]. The largest series of transduodenal excision was reported by the Heidelberg group[22]. Eighty-three patients were included in this study, of which 44 patients had adenomas. The postoperative morbidity and mortality rate were 24% and 1.2%, respectively, and were much lower than those following PD. A recent study reported by the Milan group confirmed the safety of transduodenal excision, with an overall morbidity rate of 44.4% and no mortality[6]. With regard to long-term outcomes, the local recurrence rate of adenomas after transduodenal excision was 4.5% in the Heidelberg study and 11.1% in the Milan study, lower than that following excision by EP (17%-45%) reported in a series including more than 20 cases[23-25]. These results suggest that transduodenal excision of ampullary tumors is an alternative treatment option in patients who are unsuitable for EP but too extensively treated by PD.
However, to date, there are limited reports on transduodenal excision in the English-language literature, and wide use of this procedure as standard has failed. This is due to the complex anatomy of the ampulla and the location of the tumor which is deep within the duodenum. In addition, the size of the pancreatic and biliary duct might be too small to be identified and delicately sutured to the duodenal mucosa. These features make the surgical procedure complex and technically demanding. Risks can arise from suboptimal dissection and reconstruction of the ampulla. In Lee et al[26], one (16.7%) of six patients developed biliary stricture 3 mo after the operation[26]. Operation-associated ductal strictures can be complicated by pancreatitis and cholangitis. The postoperative pancreatitis and cholangitis rate was 2.3% and 4.5%, respectively, in the Heidelberg study. In the Milan study, two (5.6%) of 36 patients developed acute pancreatitis postoperatively. In other studies, similar complications have been reported. Grobmyer et al[27] reported that three (10.3%) of 29 patients undergoing ampullectomy had postoperative pancreatitis[27]. Hong et al reported that one (3.8%) patient was readmitted with cholangitis 28 mo after the operation[21]. The difficulty in pancreaticobiliary duct reconstruction and the operation-associated complications prevent the widespread use of transduodenal excision.
Optical magnifying tools, such as laparoscopes, robot surgical systems, or operating microscopes, might be helpful when rebuilding the pancreaticobiliary duct system. Laparoscopic and robotic transduodenal excision of ampullary tumors is minimally invasive and facilitates recovery; however, only a few cases have been reported [26,28-31]. Transduodenal excision has been mainly performed by open laparotomy to date. This is probably due to the difficulty in proper exposure of the ampulla and the complicated procedures involved in delicate resection and reconstruction of fine pancreaticobiliary structures. Microsurgery has been widely used in clinical surgery, and is particularly favorable in dealing with delicate tissues. The application of operating microscopes provides surgeons with a more magnified and clearer view of the anatomy. Thus, operating microscopes are favorable in the field of neurosurgery, ophthalmology, plastic surgery, and orthopedics. Theoretically, the high magnification and illumination can help in the most complex and difficult part of the ampulla operation, namely, dissection of the ampulla and reconstruction of the pancreatic and biliary duct. However, to date, there have been no publications in the English-language literature to demonstrate the potential advantages of the operating microscope in local resection of ampullary tumors.
We report the first case of transduodenal excision of an ampullary tumor with the assistance of an operating microscope. The optimal exposure and magnification with the microscope facilitated identification of the tumor margin; therefore, an adequate margin was obtained. Most importantly, the magnified ductal structures are easier to identify and reconstruct, thus optimal suturing can be achieved when performing choledochoduodenostomy and pancreaticoduodenostomy. In the present case, the patient had an uneventful postoperative recovery and was discharged. Compared to the existing data, the application of an operating microscope neither increased the operation time nor the complications. No signs of tumor recurrence and ductal stricture were found during the follow-up period. These results suggest that our proposal of using an operating microscope during transduodenal excision is advantageous in this group of patients. However, microsurgical applications also have limitations. For example, surgeons require precise technical skills and continuous training to complete the resection. The use of an operating microscope may increase the operation time and operation-associated contamination[32,33]. Thus, more cases are needed for analysis to demonstrate the potential benefits.
We demonstrated that transduodenal excision is an ideal approach for treating patients with benign ampullary tumors who are not amenable to EP. The use of an operating microscope provides significant technical advantages, particularly in dissecting and rebuilding the pancreaticobiliary ducts.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87:37-42. [PubMed] [Cited in This Article: ] |
| 3. | De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol. 2014;20:1537-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 56] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 4. | Kang SH, Kim KH, Kim TN, Jung MK, Cho CM, Cho KB, Han JM, Kim HG, Kim HS. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol. 2017;17:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery. 2000;128:694-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Nappo G, Gentile D, Galvanin J, Capretti G, Ridolfi C, Petitti T, Spaggiari P, Carrara S, Gavazzi F, Repici A, Zerbi A. Trans-duodenal ampullectomy for ampullary neoplasms: early and long-term outcomes in 36 consecutive patients. Surg Endosc. 2020;34:4358-4368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Gao Y, Zhu Y, Huang X, Wang H, Yuan Z. Transduodenal ampullectomy provides a less invasive technique to cure early ampullary cancer. BMC Surg. 2016;16:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Uluç K, Kujoth GC, Başkaya MK. Operating microscopes: past, present, and future. Neurosurg Focus. 2009;27:E4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Damodaran O, Lee J, Lee G. Microscope in modern spinal surgery: advantages, ergonomics and limitations. ANZ J Surg. 2013;83:211-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Espinel J, Pinedo E, Ojeda V, Del Rio MG. Endoscopic management of adenomatous ampullary lesions. World J Methodol. 2015;5:127-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 56] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Fong ZV, Ferrone CR, Thayer SP, Wargo JA, Sahora K, Seefeld KJ, Warshaw AL, Lillemoe KD, Hutter MM, Fernández-Del Castillo C. Understanding hospital readmissions after pancreaticoduodenectomy: can we prevent them? J Gastrointest Surg. 2014;18:137-44; discussion 144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Newhook TE, LaPar DJ, Lindberg JM, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and mortality of pancreaticoduodenectomy for benign and premalignant pancreatic neoplasms. J Gastrointest Surg. 2015;19:1072-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Kang JS, Kim H, Kim JR, Han Y, Kim E, Byun Y, Choi YJ, Kwon W, Jang JY, Kim SW. Short- and long-term outcomes of pancreaticoduodenectomy in elderly patients with periampullary cancer. Ann Surg Treat Res. 2020;98:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Partelli S, Tamburrino D, Cherif R, Muffatti F, Moggia E, Gaujoux S, Sauvanet A, Falconi M, Fusai G. Risk and Predictors of Postoperative Morbidity and Mortality After Pancreaticoduodenectomy for Pancreatic Neuroendocrine Neoplasms: A Comparative Study With Pancreatic Ductal Adenocarcinoma. Pancreas. 2019;48:504-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Narayanan S, Martin AN, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Mortality after pancreaticoduodenectomy: assessing early and late causes of patient death. J Surg Res. 2018;231:304-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Stevens CL, Watters DAK. Short-term outcomes of pancreaticoduodenectomy in the state of Victoria: hospital resources are more important than volume. ANZ J Surg. 2019;89:1577-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Torphy RJ, Friedman C, Halpern A, Chapman BC, Ahrendt SS, McCarter MM, Edil BH, Schulick RD, Gleisner A. Comparing Short-term and Oncologic Outcomes of Minimally Invasive Versus Open Pancreaticoduodenectomy Across Low and High Volume Centers. Ann Surg. 2019;270:1147-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 19. | Yoshioka R, Yasunaga H, Hasegawa K, Horiguchi H, Fushimi K, Aoki T, Sakamoto Y, Sugawara Y, Kokudo N. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg. 2014;101:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Mathur A, Paul H, Ross S, Luberice K, Hernandez J, Vice M, Rosemurgy AS. Transduodenal ampullectomy for ampullary adenomas: a safe and effective procedure with long-term salutary outcomes. Am Surg. 2014;80:185-190. [PubMed] [Cited in This Article: ] |
| 21. | Hong S, Song KB, Lee YJ, Park KM, Kim SC, Hwang DW, Lee JH, Shin SH, Kwon J, Ma CH, Hwang S, Park G, Park Y, Lee SJ, Kim YW. Transduodenal ampullectomy for ampullary tumors - single center experience of consecutive 26 patients. Ann Surg Treat Res. 2018;95:22-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Schneider L, Contin P, Fritz S, Strobel O, Büchler MW, Hackert T. Surgical ampullectomy: an underestimated operation in the era of endoscopy. HPB (Oxford). 2016;18:65-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Jung MK, Cho CM, Park SY, Jeon SW, Tak WY, Kweon YO, Kim SK, Choi YH. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568-2574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Jeanniard-Malet O, Caillol F, Pesenti C, Bories E, Monges G, Giovannini M. Short-term results of 42 endoscopic ampullectomies: a single-center experience. Scand J Gastroenterol. 2011;46:1014-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Ceppa EP, Burbridge RA, Rialon KL, Omotosho PA, Emick D, Jowell PS, Branch MS, Pappas TN. Endoscopic vs surgical ampullectomy: an algorithm to treat disease of the ampulla of Vater. Ann Surg. 2013;257:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Lee JW, Choi SH, Chon HJ, Kim DJ, Kim G, Kwon CI, Ko KH. Robotic transduodenal ampullectomy: A novel minimally invasive approach for ampullary neoplasms. Int J Med Robot. 2019;15:e1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, Hochwald SN. Contemporary results with ampullectomy for 29 "benign" neoplasms of the ampulla. J Am Coll Surg. 2008;206:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Rosen M, Zuccaro G, Brody F. Laparoscopic resection of a periampullary villous adenoma. Surg Endosc. 2003;17:1322-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Ahn KS, Han HS, Yoon YS, Cho JY, Khalikulov K. Laparoscopic transduodenal ampullectomy for benign ampullary tumors. J Laparoendosc Adv Surg Tech A. 2010;20:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Zhang RC, Xu XW, Wu D, Zhou YC, Ajoodhea H, Chen K, Mou YP. Laparoscopic transduodenal local resection of periampullary neuroendocrine tumor: a case report. World J Gastroenterol. 2013;19:6693-6698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Wong FCH, Lai ECH, Chung DTM, Tang CN. Robotic transduodenal excision of ampullary tumour. Hepatobiliary Surg Nutr. 2017;6:312-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Weiner BK, Kilgore WB. Bacterial shedding in common spine surgical procedures: headlamp/Loupes and the operative microscope. Spine (Phila Pa 1976). 2007;32:918-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Bible JE, O'Neill KR, Crosby CG, Schoenecker JG, McGirt MJ, Devin CJ. Microscope sterility during spine surgery. Spine (Phila Pa 1976). 2012;37:623-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |