Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4303
Peer-review started: January 9, 2021
First decision: January 24, 2021
Revised: February 3, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 16, 2021
Herpes zoster is a painful infectious disease caused by the varicella zoster virus. Herpes zoster radiculopathy, which is a type of segmental zoster paresis, can complicate the disease and cause motor weakness. This complication should be considered when a patient with a rash complains of acute-onset motor weakness, and the diagnosis can be verified via electrodiagnostic study.
A 64-year-old female with a history of asthma presented to the emergency department with stabbing pain, an itching sensation, and a rash on the right anterior shoulder that had begun 5 d prior. Physical examination revealed multiple erythematous grouped vesicles in the right C4-5 and T1 dermatome regions. Because herpes zoster was suspected, the patient immediately received intravenous acyclovir. On the third hospital day, she complained of motor weakness in the right upper extremity. Magnetic resonance imaging of the cervical spine revealed mild intervertebral disc herniation at C4-C5 without evidence of nerve root compression. On the 12th hospital day, electrodiagnostic study revealed right cervical radiculopathy, mainly in the C5/6 roots. Six months later, monoparesis resolved, and follow-up electrodiagnostic study was normal.
This case emphasizes that clinicians should consider the possibility of post-herpetic paresis, such as herpes zoster radiculopathy, and that electrodiagnostic study is useful for diagnosis and follow-up.
Core Tip: Herpes zoster is caused by reactivation of latent varicella zoster virus and characterized by clustered vesicles with neuralgic pain. We present a rare case of herpes zoster radiculopathy and accompanying severe motor weakness. The patient was diagnosed via electrodiagnostic study, and complete recovery was confirmed through follow-up electrodiagnostic study. This case emphasizes that clinicians should consider the possibility of post-herpetic paresis, such as herpes zoster radiculopathy, and that electrodiagnostic study is a helpful tool for diagnosis and follow-up.
- Citation: Kim HS, Jung JW, Jung YJ, Ro YS, Park SB, Lee KH. Complete recovery of herpes zoster radiculopathy based on electrodiagnostic study: A case report. World J Clin Cases 2021; 9(17): 4303-4309
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4303.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4303
Herpes zoster is a painful infectious disease caused by the varicella zoster virus (VZV). The incidence of herpes zoster infection varies with age and immune status, ranging from 0.4 to 11 cases per 1000 people[1]. Symptoms of herpes zoster infection include stabbing pain, numbness, and an erythematous vesicular rash. Several neurological complications can accompany herpes zoster infection, such as post-herpetic neuralgia, cranial nerve palsy, meningoencephalitis, myelopathy, and segmental zoster paresis[2,3]. Herpes zoster radiculopathy, which is a type of segmental zoster paresis, can complicate the disease and cause motor weakness. This complication should be considered when a patient with a rash complains of acute-onset motor weakness; the diagnosis can be verified via electrodiagnostic study. We present a case of herpes zoster radiculopathy with full recovery of motor weakness based on electrodiagnostic examination.
A 64-year-old female presented with stabbing pain, itching, and a rash on her right anterior shoulder. Moreover, she complained of motor weakness in the right upper extremity on the third hospital day.
The patient reported that, 5 d prior, a rash had emerged on her right shoulder and was accompanied by stabbing pain. Her skin symptoms and pain became worse over time, and she was hospitalized through the emergency department. Her initial visual analog scale pain score was 6/10.
The patient had a medical history of asthma.
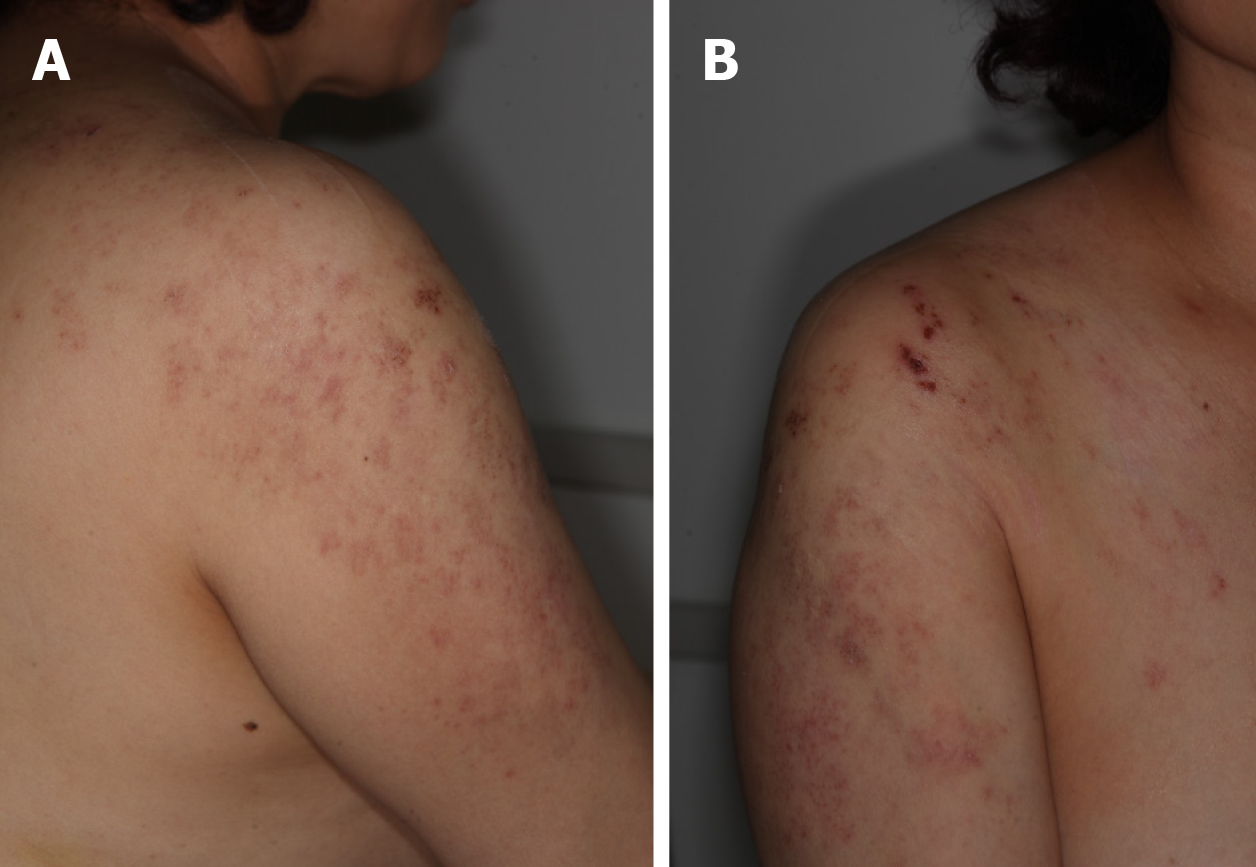
On initial physical examination, there were multiple erythematous grouped vesicles in the right C4-5 and T1 dermatome regions (Figure 1). Hypesthesia was noted in the right C5 dermatome. There were no findings of muscle weakness at initial evaluation.
However, on the third hospital day, the patient complained of monoparesis in the right upper extremity as follows: Shoulder flexor 2-/5, shoulder extensor 3/5, shoulder abductor 2-/5, elbow flexor 2/5, elbow extensor 4/5, wrist flexor 5/5, wrist extensor 4/5, and finger flexor 5/5. There was no limitation of passive range of motion in the right upper extremity, and the muscle stretch reflex was normal in the right biceps and triceps.
Laboratory assessment indicated that the C-reactive protein level was 1.59 mg/dL (normal range: 0-0.5 mg/dL), and polymerase chain reaction for VZV was positive. White blood cell, creatine kinase, lactate dehydrogenase, creatinine, blood urea nitrogen, and serum electrolyte levels were all within normal range.
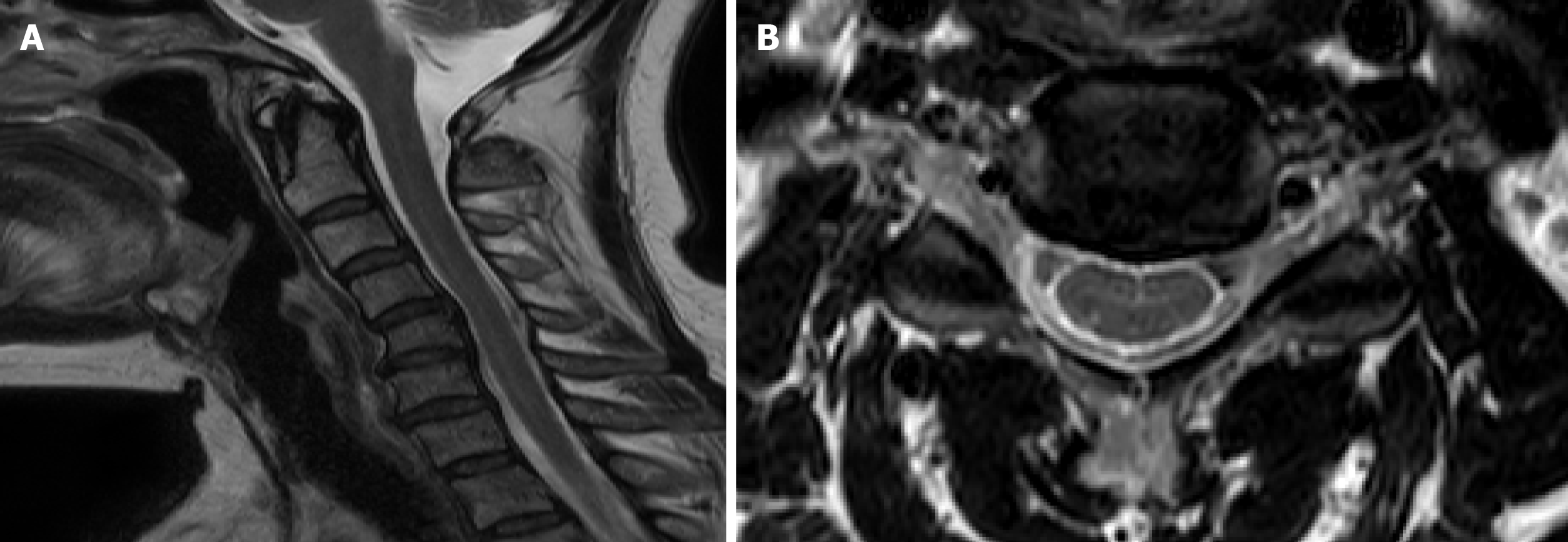
Computed tomography of the brain revealed no evidence of abnormalities in the brain parenchyma. Magnetic resonance imaging (MRI) of the cervical spine revealed cervical kyphosis and spondylosis with spur formation at C4-C6 (Figure 2A). Mild intervertebral disc herniation at C4-C5 without evidence of nerve root compression was also noted on MRI (Figure 2B).
On the 12th hospital day, electrodiagnostic study was performed to determine the cause of monoparesis in the right upper extremity. On needle electromyography, abnormal spontaneous activity (ASA) was observed in the right deltoid, teres major, and infraspinatus muscles, which are innervated by the C5 root. The right flexor carpi radialis, biceps, brachialis, and C5/6 paraspinalis muscles showed increased insertional activity. Long duration polyphasic motor units were found in the right deltoid, and the motor unit action potential recruitment pattern was reduced in the right biceps, deltoid, brachialis, and infraspinatus (Table 1).
| Muscle right | IA | Spontaneous activity | MUAP | Recruitment pattern | |||
| Fibs | Psw | Amp | Dur | Poly | |||
| Abductor pollicis brevis | N | - | - | N | N | - | N |
| Flexor carpi radialis | Incr | - | - | N | N | - | N |
| Flexor carpi ulnaris | N | - | - | N | N | - | N |
| Extensor carpi radialis longus | N | - | - | N | N | - | N |
| Biceps | Incr | - | - | N | N | - | R |
| Triceps | N | - | - | N | N | - | N |
| Deltoid | N | 1 + | 2 + | N | > 12 ms | 1 + | R |
| Brachialis | Incr | - | - | N | N | - | R |
| Teres minor | N | 2 + | 2 + | N | N | - | N |
| Infraspinatus | N | 3 + | 3 + | N | N | - | R |
| 1st dorsal interosseous | N | - | - | N | N | - | N |
| Trapezius | N | - | - | N | N | - | N |
| Cervical paraspinalis (C5/6) | Incr | - | - | N | N | - | N |
The motor nerve conduction study indicated that latent onset of right axillary compound muscle action potential (CMAP) was delayed to 5.2 msec compared to 3.6 msec of the left axillary nerve, and amplitude of the right axillary CMAP was slightly decreased to 4.9 mV compared to 6.4 mV of the left axillary nerve. Sensory nerve conduction study showed normal values, except for delayed conduction velocity in bilateral median sensory nerve action potentials around the wrists, which is sub-clinically compatible with carpal tunnel syndrome (Table 2). F-waves in the bilateral upper extremities were normal. These electrodiagnostic findings revealed right cervical radiculopathy, mainly from the C5/6 roots. Based on the laboratory, radiologic, and electrodiagnostic findings, the patient was diagnosed with herpes zoster radiculopathy of the right C5/6 roots.
| Nerve | Stimulation | Recording site | Onset latency, ms | Peak latency, ms | Peak amplitude, mV | ||
| Motor | |||||||
| Right | Axillary | Erb’s | Deltoid | 5.21 | 8.3 | 4.91 | |
| Suprascapular | Erb’s | Infraspinatus | 2.3 | 9.4 | 3.2 | ||
| Musculocutaneous | Erb’s | Biceps | 4.2 | 7.1 | 5.1 | ||
| Median | Wrist/elbow | APB | 4.0/7.4 | 6.8/10.6 | 6.7/6.6 | ||
| Radial | Elbow/axilla | EIP | 3.2/4.6 | 5.4/7.2 | 7.3/7.4 | ||
| Left | Axillary | Erb’s | Deltoid | 3.6 | 5.4 | 6.4 | |
| Suprascapular | Erb’s | Infraspinatus | 2.2 | 8.3 | 3.4 | ||
| Musculocutaneous | Erb’s | Biceps | 3.8 | 6.3 | 5.7 | ||
| Sensory | Peak amplitude (μV) | ||||||
| Right | LABC | Elbow | Forearm | 1.7 | 2.5 | 25.8 | |
| MABC | Elbow | Forearm | 2.0 | 2.7 | 15.1 | ||
| Median | Palm/wrist | 3rd digit | 1.0/3.2 | 1.8/4.11 | 68.1/45.4 | ||
| Left | Median | Palm/wrist | 3rd digit | 1.1/3.2 | 1.8/4.11 | 101/54.8 |
The final diagnosis of the presented case was herpes zoster radiculopathy of the right C5/6 roots based on electrodiagnostic findings.
Based on an initial suspected diagnosis of herpes zoster infection, intravenous acyclovir (250 mg every 8 h) was immediately administered and continued until the 10th hospital day. Tramadol (37.5 mg daily), acetaminophen (325 mg daily), and gabapentin (600 mg daily) were used for pain control.
Under the impression of herpes zoster radiculopathy, the patient received comprehensive rehabilitation including occupational therapy and electrical stimulation therapy on the right upper extremity during her hospital course. The rehabilitation program consisted of maintaining range of motion, strengthening exercises, and enhancing functional skills of daily living. Electrical stimulation therapy was performed to the deltoid and infraspinatus muscles. These rehabilitation treatments were performed for approximately 2 wk and discontinued after discharge.
On the 16th hospital day, the strength of the right shoulder flexor was slightly improved to 2/5, and the right elbow flexor was improved to 3/5. Symptoms of skin rash and stabbing pain resolved, and the patient was discharged from the hospital.
Six months after presentation, follow-up physical examination showed that the patient’s monoparesis had resolved, with upper extremity muscle strength values of 5/5. A follow-up nerve conduction study showed normal values including right axillary CMAP, which had a latent onset of 4.4 msec and an amplitude of 6.0 mV. Follow-up needle electromyography revealed no spontaneous activity or decreased motor unit action potential recruitment patterns in any examined muscles (Table 3).
| Muscle right | IA | Spontaneous activity | MUAP | Recruitment pattern | |||
| Fibs | Psw | Amp | Dur | Poly | |||
| Flexor carpi radialis | N | 0 | 0 | N | N | N | N |
| Biceps | N | 0 | 0 | N | N | N | N |
| Triceps | N | 0 | 0 | N | N | N | N |
| Deltoid | N | 0 | 0 | N | N | N | N |
| Brachialis | N | 0 | 0 | N | N | N | N |
| Teres minor | N | 0 | 0 | N | N | N | N |
| Infraspinatus | N | 0 | 0 | N | N | N | N |
| Cervical paraspinalis (C5/6) | N | 0 | 0 | N | N | N | N |
In the present case, dermatologic symptoms and electrodiagnostic findings were consistent with herpes zoster radiculopathy. According to a previous study, approximately 3%-5% of herpes zoster patients also experienced segmental limb paresis[4]. Motor weakness is frequent in the C5-C7 segments, affecting the upper limbs, and in the L1-L4, affecting the lower limbs[4]. Generally, motor weakness occurs several days after the onset of skin lesions[5].
Although the pathology of herpes zoster radiculopathy remains unclear, it is presumed that the virus causes local neuritis in the spinal nerve and gains access to motor axons[6]. Direct viral spread from the dorsal root ganglion to the anterior horn cell is thought to be the most likely pathophysiology[7]. Gadolinium-enhanced MRI findings from one report demonstrated that localized enhancement of the spinal nerve roots showed hypervascularity and disruption of the blood-nerve barrier caused by virus-induced inflammation[7]. Moreover, another study postulated that cell-to-cell contact is the major route of viral spread rather than intra-axonal spread from a dorsal root ganglion[8].
Electrodiagnostic study is helpful in determining the cause of motor weakness following herpes zoster infection. VZV-induced neurological complications such as meningoencephalitis, myelopathy, and segmental zoster paresis all can cause motor weakness after shingles, and they can be distinguished through MRI and electromyography. According to Zubair et al[9], gadolinium-enhanced MRI revealed abnormalities such as nerve signal increment or enlargement in seven of 10 patients with segmental zoster paresis[9]. Zubair et al[9] also reported that all patients with segmental zoster paresis had abnormal findings on electrodiagnostic study[9]. Moreover, in the case of segmental zoster paresis, electrodiagnostic study can be used to distinguish whether the affected site is the nerve root or the plexus. In the present study, electrodiagnostic results demonstrated ASA in muscles on the right C5/6 distributions with decreased amplitude of axillary CMAP, suggesting right cervical radiculopathy.
Previous reports suggested that appropriate antiviral therapy could be effective in reducing the incidence of segmental zoster paresis and the severity of electrophysiologic alterations[10]. Intravenous acyclovir and steroids are recommended for suppressing infections[10]. However, according to Kawajiri et al[4], initial antiviral treatment was not sufficient to prevent motor weakness in patients who developed segmental zoster paresis[4]. Likewise, the patient in this case report presented with motor weakness despite immediate administration of intravenous acyclovir. Early comprehensive rehabilitation is recommended for all patients with motor weakness secondary to herpes zoster radiculopathy[11]. Rehabilitation programs prevent disuse atrophy and range of motion limitation, playing an important role in recovery from motor weakness. Further clinical trials are needed to identify the effects of antiviral treatment or rehabilitation in herpes zoster radiculopathy compared with spontaneous improvement.
The prognosis of motor weakness caused by herpes zoster is relatively good, with 66% of patients with zoster paralysis recovering completely within 12 mo and 17% reported to have permanent paralysis[12]. Despite the good prognosis of segmental zoster paresis, permanent paresis can occur due to necrosis in anterior horn cells[12]. Therefore, administering antiviral treatment in the early stages might be beneficial to patients with segmental zoster paresis and could improve prognosis.
There are limitations to predicting the prognosis of herpes zoster radiculopathy through electrodiagnostic results. However, evaluating the course of recovery using electrodiagnostic follow-up can provide useful prognostic information. If there is significant recovery on follow-up electrodiagnostic results, a relatively good prognosis can be expected. The severity of radiculopathy could be estimated through CMAP amplitude or the amount of ASA in the involved muscles. Several previous studies diagnosed segmental zoster radiculopathy via electrodiagnostic study[13,14]. Chen et al[13] reported a case of segmental zoster paresis with electrodiagnostic findings of decreased amplitude in several nerves and ASA in clinically involved muscles[13]. According to Liu et al[14], electrodiagnostic study of 8 segmental zoster paresis patients revealed low amplitude of CMAPs or sensory nerve action potentials with various ASA in all patients[14]. Among 8 patients with segmental zoster paresis, 2 were diagnosed with preganglionic lesions (radiculopathy), while 6 patients were confirmed to have postganglionic lesions[14]. However, there have not been studies on the correlation between prognosis and the amount of ASA or the decrement in CMAP amplitude, and further clinical trials are warranted.
There are a few limitations to consider in this case report. First, MRI of the cervical spine was performed without gadolinium enhancement. Therefore, we could not identify virus-induced inflammatory signs in cervical nerve roots. Second, there were MRI findings of cervical spondylosis and mild disc herniation C4 to C5. Thus, we could not completely rule out the possibility of cervical root irritation caused by cervical disc herniation. However, there was no definite evidence of nerve root compression on cervical MRI. Moreover, considering that acute motor weakness only appeared after skin rash and the patient did not complain of posterior neck pain, it is likely that herpes zoster radiculopathy caused her motor weakness, rather than cervical disc herniation. Third, a follow-up electrodiagnostic study was performed at 6 mo from onset and was not repeated. If electrodiagnostic study had been performed several more times during the follow-up period, the course of motor nerve axonotmesis or changes in the amount of ASA could have been reported in greater detail.
This case reports a patient who demonstrated complete recovery as documented on follow-up electrodiagnostic study. The findings emphasize that clinicians should consider the possibility of segmental zoster paresis, such as herpes zoster radiculopathy, in the differential diagnosis of acute motor weakness. In addition, electrodiagnostic study is a helpful tool for diagnosis of herpes zoster radiculopathy and for determining the severity and extent of lesions. The course of recovery could be evaluated via follow-up electrodiagnostic study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fu TL, Li XM, Zeng YQ S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 385] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Cohen JI. VZV: molecular basis of persistence (latency and reactivation). In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [Cited in This Article: ] |
| 3. | Nagel MA, Gilden D. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol. 2013;15:439-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kawajiri S, Tani M, Noda K, Fujishima K, Hattori N, Okuma Y. Segmental zoster paresis of limbs: report of three cases and review of literature. Neurologist. 2007;13:313-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Teo HK, Chawla M, Kaushik M. A Rare Complication of Herpes Zoster: Segmental Zoster Paresis. Case Rep Med. 2016;2016:7827140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Dumitru D. Acquired Neuropathies. In: Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic Medicine, 2nd ed. Philadelphia: Hanley and Belfus, 2002: 937-1019. [Cited in This Article: ] |
| 7. | Hanakawa T, Hashimoto S, Kawamura J, Nakamura M, Suenaga T, Matsuo M. Magnetic resonance imaging in a patient with segmental zoster paresis. Neurology. 1997;49:631-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Devinsky O, Cho ES, Petito CK, Price RW. Herpes zoster myelitis. Brain. 1991;114 (Pt 3):1181-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 121] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Zubair AS, Hunt C, Watson J, Nelson A, Jones LK Jr. Imaging Findings in Patients with Zoster-Associated Plexopathy. AJNR Am J Neuroradiol. 2017;38:1248-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Mondelli M, Romano C, Passero S, Porta PD, Rossi A. Effects of acyclovir on sensory axonal neuropathy, segmental motor paresis and postherpetic neuralgia in herpes zoster patients. Eur Neurol. 1996;36:288-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Braverman DL, Ku A, Nagler W. Herpes zoster polyradiculopathy. Arch Phys Med Rehabil. 1997;78:880-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Gupta SK, Helal BH, Kiely P. The prognosis in zoster paralysis. J Bone Joint Surg Br. 1969;51:593-603. [PubMed] [Cited in This Article: ] |
| 13. | Chen GB, Tuan SH, Liou IH, Huang HY, Hu YC, Wu SY. Segmental zoster paresis of unilateral upper extremity: A case report and literature review. Medicine (Baltimore). 2020;99:e20466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Wu BY, Ma ZS, Xu JJ, Yang B, Li H, Duan RS. A retrospective case series of segmental zoster paresis of limbs: clinical, electrophysiological and imaging characteristics. BMC Neurol. 2018;18:121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |