Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3403
Peer-review started: November 19, 2020
First decision: February 12, 2021
Revised: February 22, 2021
Accepted: March 5, 2021
Article in press: March 5, 2021
Published online: May 16, 2021
Primary bone lymphoma (PBL) is an uncommon extranodal disease that represents approximately 1%-3% of lymphomas. Anaplastic lymphoma kinase (ALK) positive anaplastic large-cell lymphoma (ALCL) is an extremely rare type of PBL. The aim of this report is describe the symptoms, diagnosis, and treatment of primary bone ALK-positive ALCL.
A 66-year-old man presented to our hospital with neck and shoulder pain and intermittent fever that lasted for 1 mo. After extensive evaluation, positron emission tomography-computed tomography (CT) examination showed multiple osteolytic bone lesions without other sites lesions. CT-guided biopsy of the T10 vertebral body was performed, and the pathology results showed that neoplastic cells were positive for ALK-1, CD30, and CD3. A diagnosis of primary bone ALK positive ALCL was ultimately made. The patient was in partial response after four cycle soft cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, and we planned to repeat the biopsy and radiological examination after completion of the fifth cycle of therapy.
Primary bone ALK positive ALCL is a rare disease and physicians should keep in mind that ALCL can present with isolated osseous involvement without nodal involvement, and lymphoma should be considered in the differential diagnosis of primary bone lesions.
Core Tip: Primary bone lymphoma (PBL) is an uncommon extranodal disease that represents approximately 1%-3% of lymphomas. Among PBLs, diffuse large B-cell lymphoma is the most common pathological type, accounting for approximately 70%-80% of all PBLs. The anaplastic large-cell lymphoma (ALCL) subtype of PBL is extremely rare, and it therefore remains unclear whether it is similar to ALCL in general or whether it is a subtype with unique clinical biological characteristics. Furthermore, the prognostic impact of anaplastic lymphoma kinase (ALK) expression in ALCL with primary bone lesions is still under debate. Herein, we report one rare case of primary bone ALK positive ALCL in a 66-year-old man. Our case suggests that physicians should keep in mind that ALCL can present with isolated osseous involvement without nodal involvement, and lymphoma should be considered in the differential diagnosis of primary bone lesions.
- Citation: Zheng W, Yin QQ, Hui TC, Wu WH, Wu QQ, Huang HJ, Chen MJ, Yan R, Huang YC, Pan HY. Primary bone anaplastic lymphoma kinase positive anaplastic large-cell lymphoma: A case report and review of the literature . World J Clin Cases 2021; 9(14): 3403-3410
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3403.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3403
Anaplastic large-cell lymphomas (ALCLs) are a subgroup of peripheral T-cell lymphomas thought to be derived from cytotoxic T cells. Most cases are characterized by the t(2;5)(p23;q35), which results in a fusion between the anaplastic lymphoma kinase (ALK) gene at chromosome band 2p23 and nucleophosmin (NPM) at chromosome band 5q35[1]. The 2016 revised World Health Organization (WHO) lymphoma classification recognizes four different entities: Systemic ALK-positive ALCL (ALK+ALCL), systemic ALK-negative ALCL (ALK-ALCL), primary cutaneous ALCL, and breast implant-associated ALCL. ALK expression has been considered an important favorable prognostic factor for ALCL. ALK positive ALCL represents approximately 3% of adult non-Hodgkin’s lymphomas and 10%-15% of childhood lymphomas[2]. ALCL mostly affects lymph nodes, while the involvement of extranodal sites, including the soft tissue, bone, lung, and liver, is uncommon[3].
Primary bone lymphoma (PBL) is a subtype of lymphoma that exclusively affects skeletal tissue. The prevalence of PBL is estimated to be 3%-7% among primary bone tumors and less than 2% among all lymphomas in adults[4,5]. Among PBLs, diffuse large B-cell lymphoma (DLBCL) is the most common pathological type, accounting for approximately70%-80% of all PBLs[6-9]. The ALCL subtype of PBL is extremely rare (3%-5% of all PBLs)[10-13], and it therefore remains unclear whether it is similar to ALCL in general or whether it is a subtype with unique clinical biological characteristics. Furthermore, the prognostic impact of ALK expression in ALCL with primary bone lesions is still under debate. Due to the rarity of this disease, more relevant studies and case reports are needed. Herein, we report one rare case of primary bone ALK positive ALCL in a 66-year-old man.
A 66-year-old man presented with a 1 mo history of neck and shoulder pain and intermittent fevers.
His fevers had no clear pattern in timing or duration. His neck and shoulder pain was not sharp, with no neck and shoulder stiffness or limited movement, and was relieved by nonsteroidal anti-inflammatory drugs. However, intermittent fevers of 37.7-38.9 °C persisted. Therefore, he was given empirical antimicrobial therapy with moxifloxacin for 5 d in his native hospital. However, 2 d after leaving the hospital, the fever (up to 38.8 °C) returned, and his neck and shoulder pain got worse.
His past medical history included diabetes and cervical spondylosis.
Physical examination showed no palpable lymph nodes, organomegaly, or cutaneous lesions.
Blood laboratory results were as follows: Leukocytosis (14.39 × 109/L, 84.4% neutrophils), elevated C-reactive protein (126.3 mg/L), elevated transaminases (alanine aminotransferase: 71 U/L; aspartate aminotransferase: 51 U/L), elevated alkaline phosphatase (208 U/L), elevated lactate dehydrogenase (LDH) (313 U/L), and hypoproteinemia (32.5 g/L). The anti-Epstein-Barr virus capsid antigen immunoglobulin G test was positive, and the tuberculosis antibody tests and tubercle-specific immune responses were negative. Urinalysis indicated 1 + protein, moderate blood (61 red blood cells), and trace leukocyte esterase (10 white blood cells/ hibernation-promoting factor). Blood and urine cultures were negative. Electrocardiogram, serum protein electrophoresis, and tumor markers, as well as abdomen and heart ultrasonography were normal. The trephine bone marrow biopsy showed hypocellularity, and the aspirate revealed granulocyte hyperplasia but no cellular atypia. Flow cytometry was negative for any atypical lymphocytes.
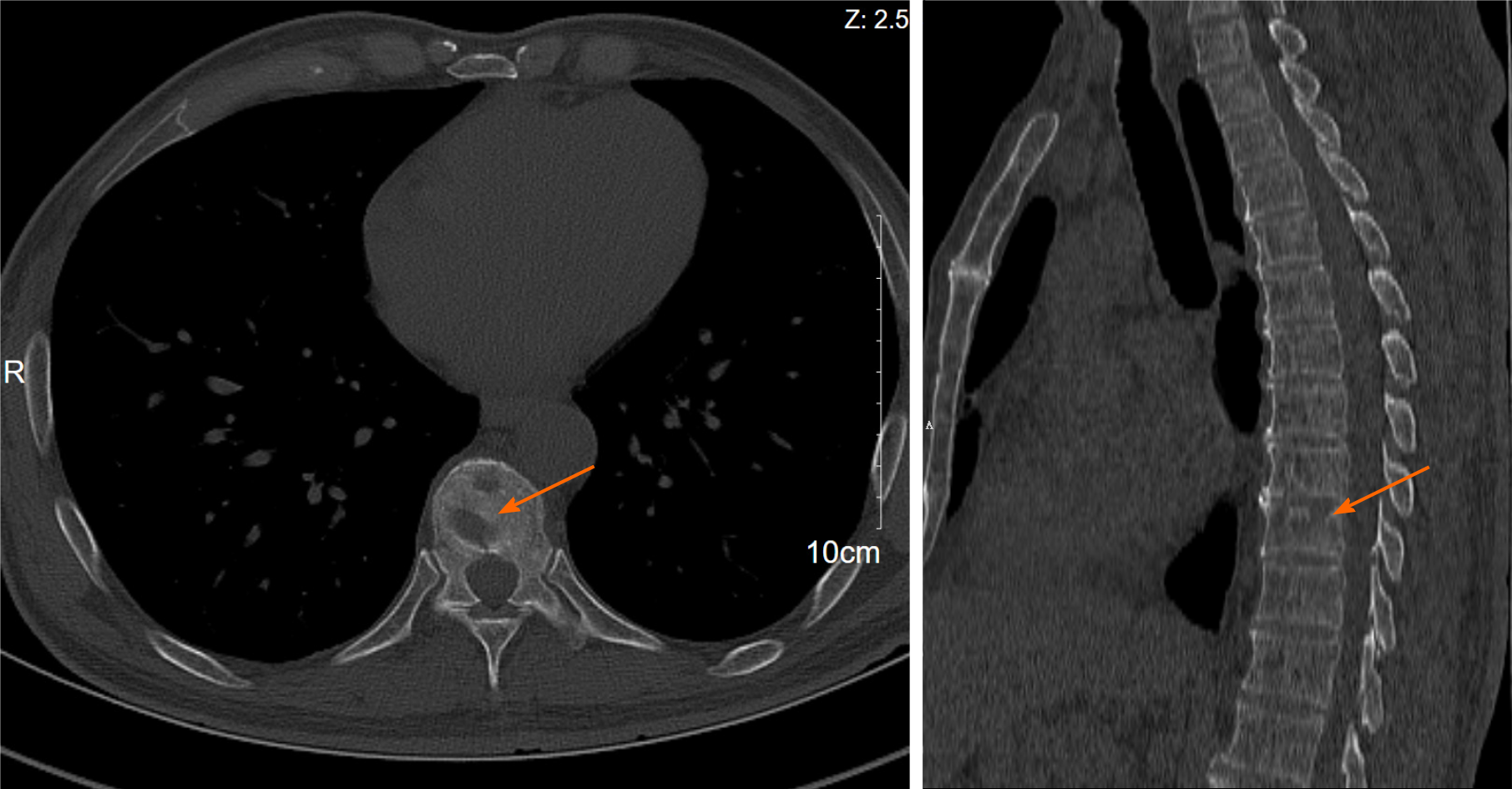
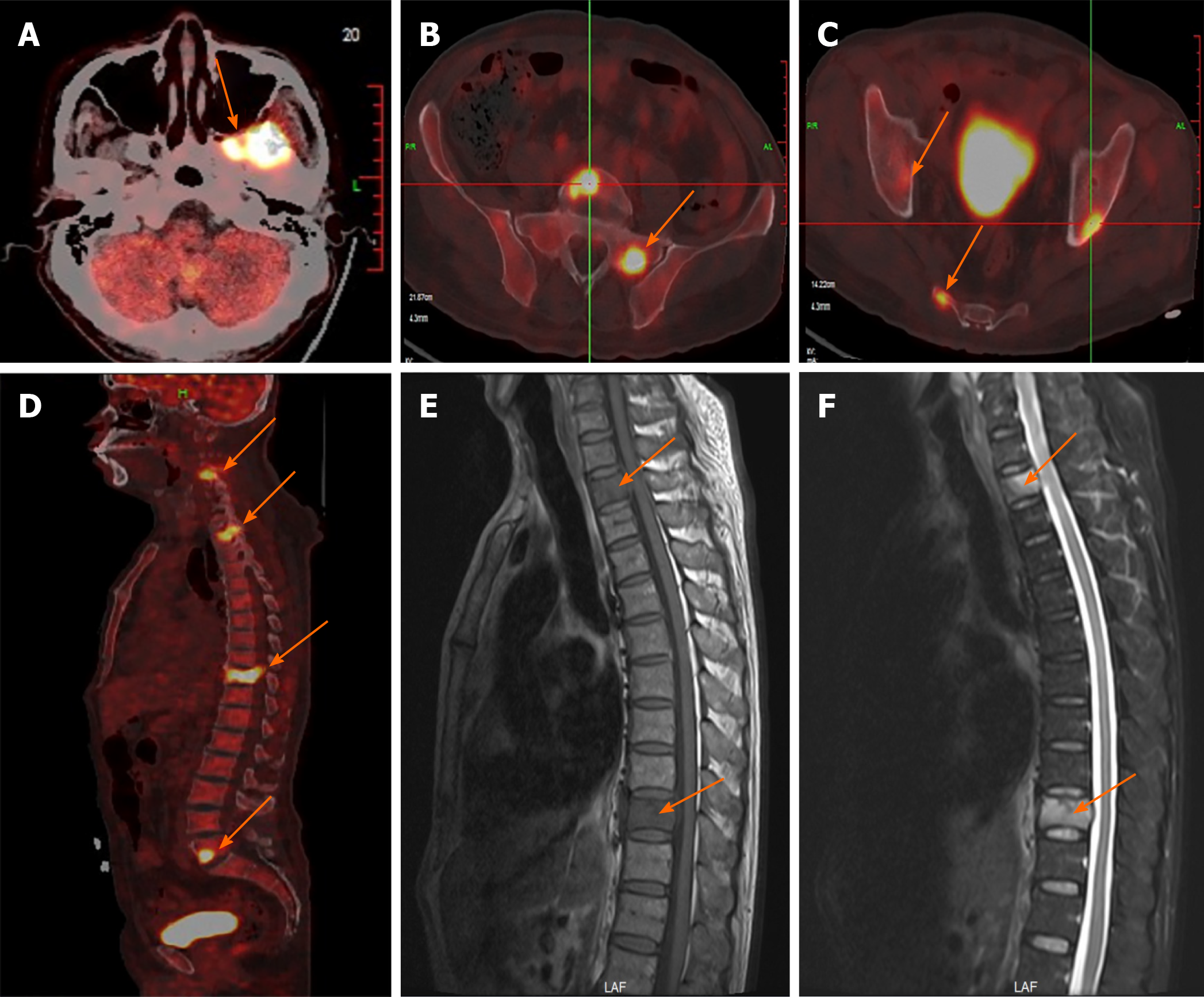
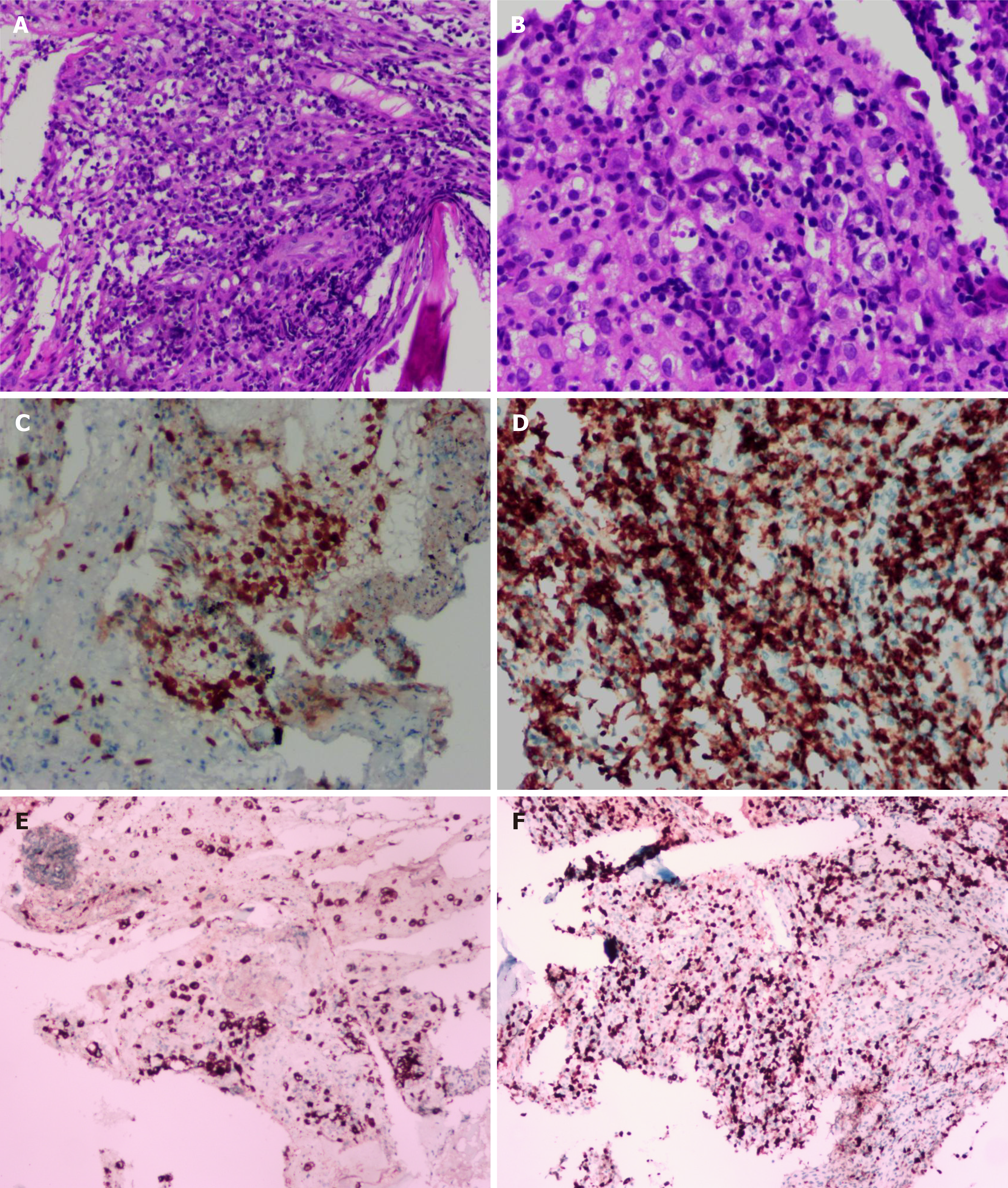
A chest computed tomography (CT) scan revealed local bronchiectasis in the upper left and right middle lobes, furthermore, an obviously osteolytic lesion in T10 vertebral body was also noted (Figure 1). Because of neck and shoulder pain, a thoracic enhanced magnetic resonance imaging (MRI) scan was performed. It indicated T2 and T10 vertebrae bone destruction, suggesting evident malignancy (Figure 2A and B). Based on these findings, the patient underwent a positron emission tomography (PET)-CT examination for further evaluation. On PET-CT, increased 18F-fluorodeoxyglucose (FDG) avidity involved the left sphenoid wing, the C4-5, T2, T10, L5, S1, and S5 vertebrae, the right humeral head, both sides of the humerus, and the right proximal femur. These lesions were identified as hypermetabolic lesions with a maximum standard uptake value of 18.64. Different degrees of bone destruction could be observed in corresponding sites, indicating lymphoma or multiple myeloma involvement (Figure 2C-F). No lymph node or extranodal site (such as the lung, liver, spleen, etc.) lesion was identified. CT-guided biopsy of the T10 vertebral body was performed and the pathological diagnosis was ALCL. Microscopic examination showed that the lesions of the vertebral body were infiltrated by pleomorphic tumor cells that have scanty cytoplasm and hyperchromatic nuclei. The neoplastic cells exhibited small- to medium-sized, irregular nuclei and abundant clear cytoplasm. Hallmark cells (horseshoe-shaped or doughnut cells) were present and Reed-Sternberg cell-like cell were also noted (Figure 3A and B). Immunohistochemistry showed that the large atypical cells were positive for ALK (Figure 3C), CD3 (Figure 3D), and CD30 (Figure 3E), but negative for CD2, CD5, CD7, CD4, CD8, CD10, CD19, CD79a, B-cell lymphoma-2, multiple myeloma-1, epithelial membrane antigen, and pan-cytokeratin. The proliferative index (Ki-67) was approximately 60% (Figure 3F). Moreover, we indagated the specific fusion partner using a two color, two fusion translocation probe, designed to detect the translocation between the ALK gene located at 2p23 and the NPM gene located at 5q35, and real-time reverse transcription polymerase chain reaction analysis demonstrated that the NPM/ALK fusion product was positive in the bone marrow.
Based on the above findings, a final diagnosis of primary bone ALCL, ALK-positive, stage IVB was made.
The patient was given chemotherapy with the cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen.
The patient was in partial response (PR) after four cycles of CHOP chemotherapy, and we planned to repeat the biopsy and radiological examination after completion of the fifth cycle of therapy.
PBL is an uncommon extranodal disease that represents approximately 1%-3% of lymphoma cases and is more common in males than in females (8:1). In 1928, Oberling[14] first described it as reticulum cell sarcoma. Based on their series in 1939, Parker and Jackson[15] established PBL as a distinct clinical entity. According to the last version of the WHO classification of tumors of soft tissue and bone, PBL is defined as a neoplasm composed of malignant lymphoid cells, producing one or more masses within the bone, without regional lymph node or distant extranodal involvement[16].
It is difficult to diagnose PBL by clinical manifestations and common laboratory examination. Pain (82%-92%) and swelling (34%-45%) of the involved site are two of the most common clinical manifestations of PBL. Other less common presentations include pathological fractures and systemic “B-type” symptoms such as fevers, weight loss, and night sweats. It can involve any skeletal site, and the axial skeleton is the most commonly involved site. PBL most commonly presents as osteolytic or osteoblastic lesions with disease involvement of the cortex and reactive periosteal changes[17]. Plain X-ray films are the initial diagnostic test of choice, but they often underestimate the extent of the lesion. CT scans are useful for disease staging and delineating spinal lesions. MRI is helpful in demonstrating bone marrow and soft tissue involvement. In addition, the functional assessment of bone lesions using FDG-PET imaging plays an important role. Studies have shown that FDG-PET displays a higher specificity and sensitivity than conventional bone scintigraphy in identifying lymphomatous infiltration of skeletal tissue[18].
Among the multiple testing modalities, bone biopsy and immunohistochemical studies remain essential for confirmation of PBL and for differential diagnosis. CT-guided percutaneous biopsy has proven to be a safe and reliable way to obtain sufficient samples[19,20]. Microscopically, DLBCL is the most common histological subtype of lymphoma with primary or secondary skeletal involvement. It accounts for 70%-80% of all bone lymphomas[6-9], with rare to anecdotal occurrences of follicular, marginal zone, lymphoplasmacytic, anaplastic large-cell, natural killer/T-cell, Burkitt, and Hodgkin lymphomas[21,22]. Available data on primary bone ALCLs are currently rare. ALCLs with primary bone involvement fulfill the previously mentioned PBL definition and show the typical immunohistochemical and molecular findings noted in a few case reports in the literature. The main differential considerations of PBL include secondary osseous lymphoma, other subtypes of lymphoma (DLBCL, NK/T-cell lymphoma, Burkitt’s, follicular, and lymphoplasmacytic)[23], osteosarcoma, metastases, Ewing sarcoma[24], chronic osteomyelitis, and granulomatous infection such as tuberculosis[25].
The prognosis of patients with primary bone DLBCL is directly correlated with the stage of disease. The 5-year overall survival (OS) varies from 82% for patients with stage IE disease to 38% for patients with disseminated DLBCL with skeletal involvement. However, the prognosis of ALCL-type PBLs is controversial. Noh et al[26] collected 22 cases of ALCL with primary bone involvement and found that the ALCL type of PBL showed poor biological behavior compared with PBL (5-year OS was 43.1% and 62%-76%, respectively). In addition, the expression of ALK-1 protein has been reported to be a favorable prognostic factor in conventional nodal ALCL, but Nagasaka et al[27] showed that ALK-1 positivity is not a favorable prognostic feature for patients with primary bone ALCL. Therefore, further studies investigating the clinical behavior and pathogenesis of primary bone ALCL are warranted.
Strategies such as chemotherapy, immunotherapy, surgery, and radiotherapy have been used to treat primary bone ALCL, yet CHOP remains the most commonly used initial therapy. The addition of etoposide to CHOP (also known as CHOEP) improved outcomes for younger patients with ALK+ ALCL (especially those with normal LDH levels at diagnosis) in a large retrospective meta-analysis (P < 0.04); however, the regimen was too toxic for older patients[28]. The infusional dose-adjusted etoposide, cyclophosphamide, doxorubicin, vincristine, and prednisone (EPOCH) protocol produced very encouraging outcomes in a single institution long-term prospective study that enrolled ALCL patients with high-risk features. After a median follow-up of more than 12 years, median survival for both ALK+ and ALK- patients was not reached, with a 10-year OS rate of 75%[29]. On the basis of these results, CHOEP should be considered in younger patients with ALK+ ALCL for initial therapy, while CHOP and da-EPOCH should be reserved for older or less fit patients.
Recently, novel agents have emerged in the treatment of ALCL. Brentuximab vedotin (BV) is an anti-CD30 antibody-drug conjugate that selectively delivers an antimicrotubule agent, monomethyl auristatin E, into CD30-expressing cells. The initial phase 1 study conducted in patients with CD30-positive lymphomas, including ALCL, showed that BV treatment led to a response rate of 38%, including 11 cases of complete remission. The two patients with ALCL in the study achieved complete remission[30]. Subsequently, a phase 2 study in relapsed/refractory ALCL demonstr
In summary, a rare case of primary bone ALK-positive ALCL is reported in this study. Physicians should keep in mind that ALCL can present with isolated osseous involvement without nodal involvement, and lymphoma should be considered in the differential diagnosis of primary bone lesions. For an accurate and prompt diagnosis, clinical features, PET-CT images, pathological histology, and immunophenotype should all be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mologni L S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1698] [Cited by in F6Publishing: 1650] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 2. | Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1369] [Cited by in F6Publishing: 1468] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 3. | Yang Z, Liu Y, Guo F, Chen W, Yin Y, Chen Z, Li H, Luo Y, Zhang Y. Anaplastic large cell lymphoma with primary involvement of the skeletal muscle: A case report. Oncol Lett. 2015;9:2815-2818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Hayase E, Kurosawa M, Suzuki H, Kasahara K, Yamakawa T, Yonezumi M, Suzuki S, Teshima T. Primary Bone Lymphoma: A Clinical Analysis of 17 Patients in a Single Institution. Acta Haematol. 2015;134:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Pilorge S, Harel S, Ribrag V, Larousserie F, Willems L, Franchi P, Legoff M, Biau D, Anract P, Roux C, Blanc-Autran E, Delarue R, Gisselbrecht C, Ketterer N, Recher C, Bonnet C, Peyrade F, Haioun C, Tilly H, Salles G, Brice P, Bouscary D, Deau B, Tamburini J. Primary bone diffuse large B-cell lymphoma: a retrospective evaluation on 76 cases from French institutional and LYSA studies. Leuk Lymphoma. 2016;57:2820-2826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer. 2006;106:2652-2656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Fletcher C, Bridge J, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon: IARC Press, 2013: 15-18. [Cited in This Article: ] |
| 8. | Messina C, Christie D, Zucca E, Gospodarowicz M, Ferreri AJ. Primary and secondary bone lymphomas. Cancer Treat Rev. 2015;41:235-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Glotzbecker MP, Kersun LS, Choi JK, Wills BP, Schaffer AA, Dormans JP. Primary non-Hodgkin's lymphoma of bone in children. J Bone Joint Surg Am. 2006;88:583-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zinzani PL, Carrillo G, Ascani S, Barbieri E, Tani M, Paulli M, Stefoni V, Sabattini E, Alinari L, Binazzi R, Tura S, Baccarani M, Pileri SA. Primary bone lymphoma: experience with 52 patients. Haematologica. 2003;88:280-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Ramadan KM, Shenkier T, Sehn LH, Gascoyne RD, Connors JM. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol. 2007;18:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Alencar A, Pitcher D, Byrne G, Lossos IS. Primary bone lymphoma--the University of Miami experience. Leuk Lymphoma. 2010;51:39-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Cai L, Stauder MC, Zhang YJ, Poortmans P, Li YX, Constantinou N, Thariat J, Kadish SP, Nguyen TD, Kirova YM, Ghadjar P, Weber DC, Bertran VT, Ozsahin M, Mirimanoff RO. Early-stage primary bone lymphoma: a retrospective, multicenter Rare Cancer Network (RCN) Study. Int J Radiat Oncol Biol Phys. 2012;83:284-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Oberling C. Les Rèticulosarcomes et Les Reticuloendothèliosarcomes de la Moelle Osseuse (Sarcomes d'Ewing). Bull Assoc Fr Etude Cancer. 1928;17:259-296. [Cited in This Article: ] |
| 15. | Parker FJ, Jackson HJ. Primary reticulum cell sarcoma of bone. Surg Gynecol Obstet. 1939;68:45-53. [Cited in This Article: ] |
| 16. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4245] [Cited by in F6Publishing: 4848] [Article Influence: 606.0] [Reference Citation Analysis (0)] |
| 17. | Shoji H, Miller TR. Primary reticulum cell sarcoma of bone. Significance of clinical features upon the prognosis. Cancer. 1971;28:1234-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Moog F, Kotzerke J, Reske SN. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. J Nucl Med. 1999;40:1407-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Maciel MJ, Tyng CJ, Barbosa PN, Bitencourt AG, Matushita Junior JP, Zurstrassen CE, Chung WT, Chojniak R. Computed tomography-guided percutaneous biopsy of bone lesions: rate of diagnostic success and complications. Radiol Bras. 2014;47:269-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Espinosa LA, Jamadar DA, Jacobson JA, DeMaeseneer MO, Ebrahim FS, Sabb BJ, Kretschmer MT, Biermann JS, Kim SM. CT-guided biopsy of bone: a radiologist's perspective. AJR Am J Roentgenol. 2008;190:W283-W289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Pettit CK, Zukerberg LR, Gray MH, Ferry JA, Rosenberg AE, Harmon DC, Harris NL. Primary lymphoma of bone. A B-cell neoplasm with a high frequency of multilobated cells. Am J Surg Pathol. 1990;14:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Huebner-Chan D, Fernandes B, Yang G, Lim MS. An immunophenotypic and molecular study of primary large B-cell lymphoma of bone. Mod Pathol. 2001;14:1000-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Mikhaeel NG. Primary bone lymphoma. Clin Oncol (R Coll Radiol). 2012;24:366-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Krishnan A, Shirkhoda A, Tehranzadeh J, Armin AR, Irwin R, Les K. Primary bone lymphoma: radiographic-MR imaging correlation. Radiographics. 2003;23:1371-83; discussion 1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Khor LK, Wang S, Lu SJ. Anaplastic large cell lymphoma of the vertebra masquerading as tuberculous spondylitis: potential pitfalls of conventional imaging. Intern Emerg Med. 2012;7:573-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Noh BJ, Han CS, Park JS, Lee J, Kim YW, Park YK. ALK-positive anaplastic large-cell lymphoma with primary bone involvement: A rare case and review of the literature. Malays J Pathol. 2018;40:161-167. [PubMed] [Cited in This Article: ] |
| 27. | Nagasaka T, Nakamura S, Medeiros LJ, Juco J, Lai R. Anaplastic large cell lymphomas presented as bone lesions: a clinicopathologic study of six cases and review of the literature. Mod Pathol. 2000;13:1143-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Sibon D, Fournier M, Brière J, Lamant L, Haioun C, Coiffier B, Bologna S, Morel P, Gabarre J, Hermine O, Sonet A, Gisselbrecht C, Delsol G, Gaulard P, Tilly H. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol. 2012;30:3939-3946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 30. | Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812-1821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1052] [Cited by in F6Publishing: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 31. | Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Yang Y, Sievers EL, Kennedy DA, Shustov A. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190-2196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 707] [Cited by in F6Publishing: 709] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 32. | Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364:775-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 33. | Gambacorti Passerini C, Farina F, Stasia A, Redaelli S, Ceccon M, Mologni L, Messa C, Guerra L, Giudici G, Sala E, Mussolin L, Deeren D, King MH, Steurer M, Ordemann R, Cohen AM, Grube M, Bernard L, Chiriano G, Antolini L, Piazza R. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst. 2014;106:djt378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |