Published online Apr 16, 2021. doi: 10.12998/wjcc.v9.i11.2641
Peer-review started: December 12, 2020
First decision: December 17, 2020
Revised: January 1, 2021
Accepted: February 22, 2021
Article in press: February 22, 2021
Published online: April 16, 2021
Melanoma is uncommonly found in lymph nodes, subcutaneous tissue, or visceral organs without a primary lesion, where it is identified as metastatic melanoma with unknown primary (MUP). Hepatic MUP is extremely rare and has a poor prognosis. There is limited information on its pathogenesis, clinical and imaging features, and pathological findings. There are no guidelines for the use of immune checkpoint inhibitors (ICIs) in hepatic MUP, and the treatment outcome has rarely been reported.
A 42-year-old woman presented to our hospital with hepatic tumors found incidentally during a routine check-up. Contrast-enhanced abdominal com-puterized tomography showed multiple mass lesions in the liver. Pathological results revealed melanoma, which was confirmed by immunohistochemical staining for HMB-45(+), Melan-A(+), S-100(+), and SOX10(+). There was no evidence of primary cutaneous, ocular, gastrointestinal, or anal lesion on a comprehensive examination. The patient was diagnosed with hepatic MUP. She received combined antibodies against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4, ipilimumab) and programmed death protein-1 (PD-1, nivolumab). She died of hepatic failure 9 mo after hepatic MUP was diagnosed. This the first case of hepatic MUP treated with combined ipilimumab and nivolumab, who showed better outcome than previous cases.
Combined ICIs of PD-1 and CTLA-4 may be considered as the first-line therapy for patients with hepatic MUP.
Core Tip: Hepatic metastatic melanoma with an unknown primary site (MUP) is extremely rare and there is limited information on its pathogenesis, clinical and imaging features, pathological findings, and treatment outcome. There are no guidelines for the use of immune checkpoint inhibitors (ICIs) in hepatic MUP. We report the first case of hepatic MUP treated with combined ICIs of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death protein-1 (PD-1), describing the clinical features, imaging, pathological findings, and outcome. This case showed better treatment outcome than previous cases of hepatic MUP. Combined ICIs of PD-1 and CTLA-4 may be considered as the first-line therapy for patients with hepatic MUP.
- Citation: Cheng AC, Lin YJ, Chiu SH, Shih YL. Combined immune checkpoint inhibitors of CTLA4 and PD-1 for hepatic melanoma of unknown primary origin: A case report. World J Clin Cases 2021; 9(11): 2641-2648
- URL: https://www.wjgnet.com/2307-8960/full/v9/i11/2641.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i11.2641
Metastatic melanoma is a fatal disease that is difficult to treat. Most metastatic melanomas have known primary sites, including the skin, eyes, and mucous membranes. It is rarely found in lymph nodes (LNs), subcutaneous tissue, or visceral organs, without a cutaneous, mucosal, or ocular primary lesion, where it is identified as metastatic melanoma with unknown primary (MUP)[1]. Previous studies have suggested that patients with MUP have better clinical outcomes than those with stage-matched melanoma of known primary (MKP) and the consequence may be associated with the protective immune response of the host[2,3,4]. Therefore, it is considered that MUP may have a biology different from that of MKP. However, according to the National Comprehensive Cancer Network clinical practice guidelines[5], metastatic melanoma, including metastatic MKP and MUP, are treated using a similar approach, regardless of the subtype.
In the new era of immunotherapies, immune checkpoint inhibitors (ICIs) including antibodies against programmed death protein-1 (PD-1, nivolumab) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4, ipilimumab) are the drugs widely used for melanoma treatment. ICIs act by blocking the inhibitory receptors of immune system elements on T cells (PD-1, CTLA4), thereby leading to the activation of tumor-specific T cells to destroy tumor cells[6]. Recently, combined nivolumab and ipilimumab were reported to have survival benefits in patients with metastatic melanoma and became the standard of care in clinical practice[7,8]. However, there are no specific guidelines for the use of ICIs in hepatic MUP, and the treatment outcome has rarely been reported. We present the case of a 42-year-old female patient with hepatic MUP, who received combined ICIs of PD-1 and CTLA4. To the best of our knowledge, this is the first hepatic MUP treated with combined ICIs of CTLA-4 and PD-1 that showed better survival outcome than previous cases of hepatic MUP[9,10,11].
A 42-year-old Taiwanese woman was admitted to our hospital due to hepatic tumors found incidentally during a routine check-up.
The patient denied any symptoms including abdominal pain, loss of body weight, and anorexia.
The patient denied any history of liver disease, including chronic hepatitis B or C viral infection. She was not taking any medications such as oral contraceptives or estrogens. She had been smoking 2 to 5 cigarettes per day for ten years but denied alcohol use. Her family history was negative for hereditary disease or malignancy.
Physical examination revealed no yellowish discoloration of the skin or sclera. There was no superficial lymph node enlargement, including the neck and the supra-clavicular, axillary, and inguinal regions. The abdomen was soft with no shifting dullness or tenderness.
Routine blood tests revealed the following: White blood cell count, 9950/µL; hemoglobin, 15.0 g/dL; prothrombin time, 10.6 s; and international normalized ratio, 1.0. Biochemistry metrics included aspartate aminotransferase, 37 U/L; alanine aminotransferase, 82 U/L; total bilirubin, 0.6 mg/dL; albumin, 3.5 g/dL; alkaline phosphatase, 167 U/L; and lactate dehydrogenase (LDH), 180 U/L. Viral serology showed negativity for hepatitis B surface antigen and anti-hepatitis C virus antibodies. The tumor markers alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 199, and cancer antigen 125 were all in the normal range.
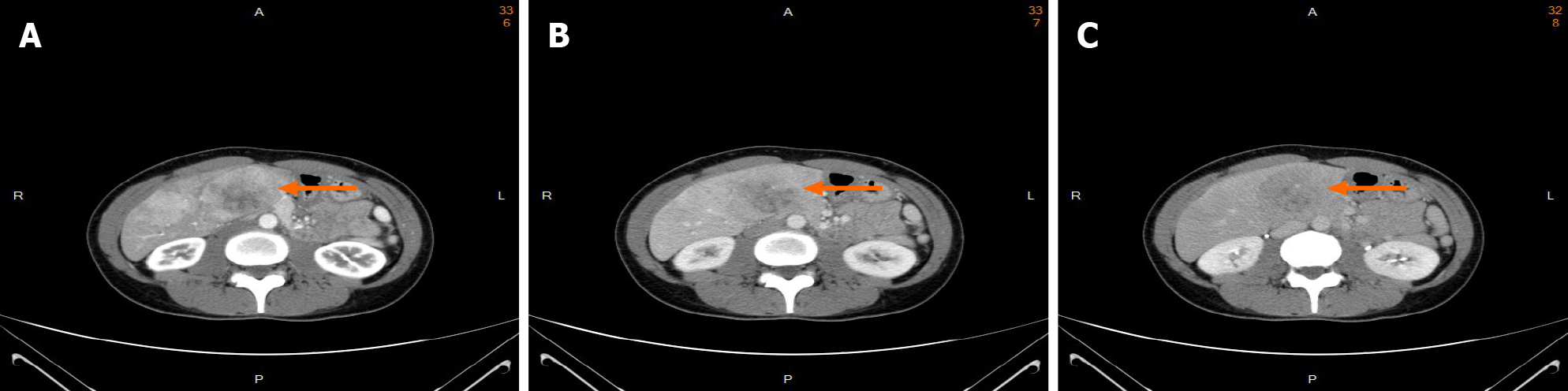
Tri-phasic, contrast-enhanced abdominal computer tomography (CT) revealed several heterogeneous masses and nodules in both lobes of the liver with a maximal size of 8.3 cm at segment 4. The masses showed uneven enhancement in the arterial phase (Figure 1A). The enhancement washout occurred in the portal venous phase (Figure 1B) and delayed phase (Figure 1C).
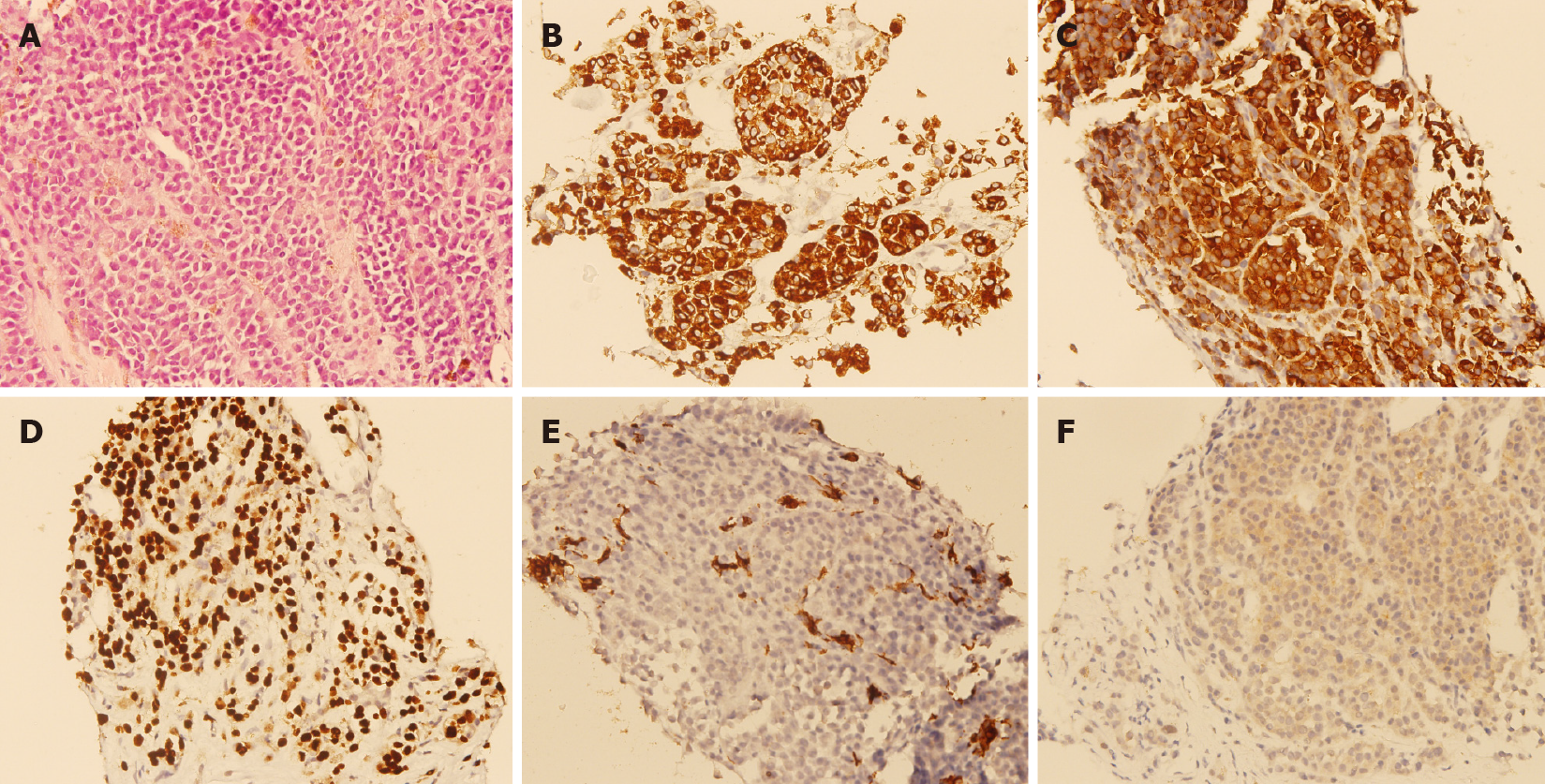
A percutaneous ultrasound-guided liver biopsy was performed on the patient. Microscopical examination showed epithelioid tumor cells with moderate nuclear atypia, as well as focal intracytoplasmic melanin pigments arranged in solid nests infiltrating in the liver parenchyma (Figure 2A). Immunohistochemical staining revealed that the specimen was HMB45 diffusely positive (Figure 2B), Melan-A diffusely positive (Figure 2C), SOX10 diffusely positive (Figure 2D), cytokeratin negative (Figure 2E), and BRAF-V600E positive (Figure 2F). Based on the findings of pathological and immunohistochemical staining, melanoma was diagnosed.
After the pathologic diagnosis was confirmed, a detailed medical history was recorded. The patient had no history of any excised melanocytic or pigmented lesion. Comprehensive and rigorous examinations were performed. No skin lesions were found on her skin surface, including the scalp, face, soles of the feet, genitalia, and anus. Otolaryngologic and ophthalmologic examinations were normal. Upper gastrointestinal panendoscopy and colonoscopy showed negative results. Positron emission tomography-computer tomography (PET-CT) was performed and showed multiple fluorodeoxyglucose (FDG)-avid mass lesions in the bilateral lobes of the liver, with the most prominent being about 8.2 cm in size at segment 4 with an maximum standardized uptake value of 11.9. No marked FDG-avid primary cutaneous melanoma lesion was found.
The final diagnosis of the presented case was hepatic MUP.
The patient initially received combined targeted therapy with BRAF and MEK inhibitors, which was terminated one month later because of the adverse effects. She underwent immunotherapy with combined nivolumab (1 mg/kg body weight) and ipilimumab (3 mg/kg body weight) every 3 wk for four cycles. After four cycles of nivolumab and ipilimumab treatment, enhanced abdominal computed tomography (CT) showed stable disease. Then, she received another six cycles of nivolumab at a dose of 3 mg/kg body weight every 2 wk.
After six cycles of nivolumab, progression of hepatic melanomas was noted. The patient died of hepatic failure 9 mo after hepatic MUP was diagnosed.
MUP is defined as melanoma discovered in subcutaneous tissue, LNs, or visceral organs without a cutaneous, ocular, or mucosal primary site[1]. The diagnostic criteria for MUP, proposed by Dasgupta et al[1], are as follows. First, metastatic melanoma was confirmed clinically, histologically, and immunohistochemically. Second, there was no history of melanocytic, pigmented lesions that were excised without a histological examination. Third, unusual primary sites, including oral, urogenital, otolaryngologic, ophthalmologic, and anal areas, were excluded. The diagnosis of MUP in our case was based on the patient’s clinical history, physical and laboratory examinations, imaging studies, and pathological findings. The findings of upper gastrointestinal panendoscopy and colonoscopy excluded primary lesions from the gastrointestinal tract. The findings of PET-CT supported the final diagnosis.
The incidence of MUP comprises approximately 3% of all melanoma cases[12,13]. MUP is most commonly diagnosed in LNs (approximately 60%), followed by subcutaneous sites (approximately 30%). It occurs least in visceral organs (approximately 10%)[12]. Hepatic MUP is extremely rare. Only five previous English studies in PubMed were related to hepatic MUP[9-11,14,15] (Table 1). The clinical symptoms and image findings of hepatic MUP are nonspecific. Previous case reports suggested that patients may experience abdominal pain, anorexia, or weight loss. However, our patient did not have these symptoms. Image findings in previous cases included single or multiple lesions. In our case, contrast-enhanced abdominal CT showed multiple masses with uneven enhancement in the arterial phase and washout in the portal venous and delayed phases. Hypervascular neoplasms, such as hepatocellular carcinoma, hepatic hemangioma, hepatic angiosarcoma, and metastases, were initially considered.
| Ref. | Clinical symptoms | Image findings (computed tomography) | Treatment | Prognosis |
| Tanaka et al[9] | Anorexia, abdominal distension | Disseminated liver infiltrations | Palliative therapy | Died 10 d after diagnosis |
| Shan et al[10] | Anorexia, abdominal distension | Enlarged liver without individual lesion | Traditional medicine | Died 31 d after diagnosis |
| Yenıova et al[15] | Abdominal distension, weight loss | Multiple masses in the liver | Palliative care | Not stated |
| Bostanci et al[14] | Right upper quadrant abdominal pain, weight loss | Cystic lesion in the left lobe of the liver | Surgical excision | Discharged 4 d post- surgery without compliance to follow-up |
| Tanaka et al[11] | Fever, multiple sites of erythema with crusting on thetrunk region | Multiple poorlydefined irregularly shaped masses in the liver | Palliative therapy | Died 47 d after diagnosis |
| Case in study | No significant symptoms | Multiple heterogeneous masses and nodules in both lobes of the liver | Immunotherapy with combined nivolumab and ipilimumab | Died 9 mo after diagnosis |
The pathological characteristics of melanoma can mimic epithelial, hematologic, mesenchymal, and neural tumors. Immunohistochemical staining can provide valuable information to distinguish melanomas. In our case, the specimen showed epithelioid-like tumor cells with moderate nuclear atypia, arranged in solid nests with low mitotic figures and no tumor necrosis. The differential diagnosis for these findings included hepatocellular carcinoma, metastatic carcinoma of breast origin, and metastatic neuroendocrine tumor. Immunohistochemical staining revealed that the specimen was HMB45(+), Melan-A(+), SOX10(+), S100(+), Hepa-1(-), Cytokeratin(-), and INSM-1(-). Malignant melanoma was diagnosed based on these results.
According to the American Joint Committee on Cancer eighth edition cancer staging manual[16], MUP in LNs indicates stage III disease, and MUP in visceral organs, soft tissue, including muscle, and non-regional lymph node, indicates stage IV disease. Our patient had multiple melanomas in the liver, confirming the diagnosis of stage IV disease. Recent studies have demonstrated that inhibition of BRAF and MEK improved the overall survival among patients with BRAF V600–mutated metastatic melanoma[17,18]. In addition, combined ICIs of CTLA-4 (ipilimumab) and PD-1 (nivolumab) were shown to have survival benefits in patients with metastatic melanoma[7,8]. Our patient initially received a combination of BRAF and MEK inhibitors because she possessed BRAF-V600E gene mutations and health insurance support. However, this was discontinued because she could not tolerate the adverse effect. She then received combined ICIs of CTLA-4 (ipilimumab) and PD-1 (nivolumab), but this was not effective. The patient died 9 mo after initial diagnosis. To the best of our knowledge, this was the first hepatic MUP case treated with ICIs, which improved survival time compared to previous cases[9-11].
The prognostic factors for patients with MUP include the number of involved LNs and visceral metastases, age, and serum lactate dehydrogenase (LDH) level[19,20]. Liver function impairment caused by hepatic metastatic melanoma is usually not severe. However, patients from previous reports initially presented with mild liver dysfunction, which rapidly developed to acute liver failure within days, subsequently leading to death[9-11]. All patients had significantly increased serum LDH levels while liver lesions were detected[9-11]. This may suggest that elevated serum LDH level was a risk factor for hepatic failure among patients with hepatic metastatic melanoma. Our case had normal serum LDH levels during the initial diagnosis, which may indicate a more favorable outcome than the other cases[9-11].
The biological phenomenon of MUP is still not well understood. Various theories have been proposed for MUP. A more commonly accepted theory is the spontaneous regression of a previously unrecognized primary melanoma after metastatic spread[21]. The spontaneous regression of melanoma occurs through an immune process. For example, studies have shown that regressing melanomas were characterized by an increased number of tumor-infiltrating T-lymphocytes and the expression of the interleukin 2 receptor, which is an activation marker for T-lymphocytes[22]. Cellular immune response to melanoma-associated antigens has been thought to induce spontaneous regression, mediated by cytotoxic lymphocytes[23]. The humoral immune response for melanoma tumor destruction caused by antibody attachment to melanoma cell membranes has been identified using immunofluorescence[22]. These host antitumor immune responses were associated with a better survival in patients with MUP than in patients with stage-matched MKP[2-4]. The association between antitumor immune response and clinical significance remains speculative. Further investigations are required to understand the regulatory network between melanoma and the immune system.
PD-1 is a cell surface receptor commonly found in activated T cells[6]. PD-1 signaling inhibits T cell-mediated immune responses[6]. In the microenvironment, PD-1 signaling, which is highly expressed in tumor-infiltrating lymphocytes in melanoma, promotes T cell dysfunction and apoptosis and confers resistance to tumor cells against cytotoxic T cell (CD8+)-mediated tumor cell killing, finally leading to tumor progression[6,24]. CTLA-4 is a receptor on the surface of activated T cells, and it mediates immunosuppression by inhibition at the T-cell receptor immune synapse and the signaling pathway of CD28, thereby reducing immune responses to tumor antigens[6]. Recently, preclinical studies and clinical trials have shown the synergistic effect of antitumor responses using combined anti-PD-1 and anti-CTLA-4 compared with that observed in single-agent blockade in patients with metastatic melanoma[7,8,25]. However, clinical trials of combination ICIs have not reported patients with hepatic MUP specifically. The patient in our case, who was treated with a combination of nivolumab plus ipilimumab, showed better outcome than patients in previous cases who did not receive immunotherapy. Based on the immune-mediated spontaneous regression as previously discussed and the present case, hepatic MUP patients may be good candidates for combined ICI therapy due to enhanced anti-tumor immune responses under combined ICI treatment. Further studies are needed in the future to investigate the subtype of melanoma under immunotherapy.
We report the first case of hepatic MUP treated with combined ICIs of CTLA-4 and PD-1, describing the clinical features, imaging, pathological findings, and treatment outcome. Clinical guidelines may consider combined ICIs of PD-1 and CTLA-4 as the first-line therapy for patients with hepatic MUP. Further studies should be conducted for gaining a better understanding of the immune regulation intersects and clinical outcomes in patients with hepatic MUP who are treated with ICIs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Uhlmann D, Xu ZL S-Editor: Liu M L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Dasgupta T, Bowden L, Berg JW. Malignant melanoma of unknown primary origin. Surg Gynecol Obstet. 1963;117:341-345. [PubMed] [Cited in This Article: ] |
| 2. | Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol. 2009;27:3489-3495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Bae JM, Choi YY, Kim DS, Lee JH, Jang HS, Kim H, Oh BH, Roh MR, Nam KA, Chung KY. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2015;72:59-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Verver D, van der Veldt A, van Akkooi A, Verhoef C, Grünhagen DJ, Louwman WJ. Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: A Dutch population-based study. Int J Cancer. 2020;146:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cutaneous Melanoma (Version 1.2021). Nov 25, 2020 [cited December 1, 2020] [Internet]. Available from: http://www.nccn.org. [Cited in This Article: ] |
| 6. | Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 803] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 7. | Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone vs ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 928] [Article Influence: 154.7] [Reference Citation Analysis (0)] |
| 8. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1826] [Cited by in F6Publishing: 2133] [Article Influence: 426.6] [Reference Citation Analysis (0)] |
| 9. | Tanaka M, Watanabe S, Masaki T, Kurokohchi K, Kinekawa F, Inoue H, Uchida N, Kuriyama S. Fulminant hepatic failure caused by malignant melanoma of unknown primary origin. J Gastroenterol. 2004;39:804-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Shan GD, Xu GQ, Chen LH, Wang ZM, Jin EY, Hu FL, Li YM. Diffuse liver infiltration by melanoma of unknown primary origin: one case report and literature review. Intern Med. 2009;48:2093-2096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Tanaka K, Tomita H, Hisamatsu K, Hatano Y, Yoshida K, Hara A. Acute Liver Failure Associated with Diffuse Hepatic Infiltration of Malignant Melanoma of Unknown Primary Origin. Intern Med. 2015;54:1361-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol. 2011;78:112-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Savoia P, Fava P, Osella-Abate S, Nardò T, Comessatti A, Quaglino P, Bernengo MG. Melanoma of unknown primary site: a 33-year experience at the Turin Melanoma Centre. Melanoma Res. 2010;20:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Bostanci O, Kartal K, Battal M. Liver metastases of unknown primary: malignant melanoma. Case Reports Hepatol. 2014;2014:131708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Yenıova Ö, Altinbaş A, Ersoy O, Aydinli M, Bayraktar Y. Metastatic liver malignant melanoma of unknown origin. Turk J Gastroenterol. 2012;23:420-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, Haydu LE, Eggermont AMM, Flaherty KT, Balch CM, Thompson JF; for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1294] [Cited by in F6Publishing: 1407] [Article Influence: 201.0] [Reference Citation Analysis (0)] |
| 17. | Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Chiarion-Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, Davies MA, Lane SR, Legos JJ, Mookerjee B, Grob JJ. Dabrafenib plus trametinib vs dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 18. | Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Sovak MA, Chang I, Choong N, Hack SP, McArthur GA, Ribas A. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1490] [Cited by in F6Publishing: 1473] [Article Influence: 147.3] [Reference Citation Analysis (0)] |
| 19. | Pfeil AF, Leiter U, Buettner PG, Eigentler TK, Weide B, Meier F, Garbe C. Melanoma of unknown primary is correctly classified by the AJCC melanoma classification from 2009. Melanoma Res. 2011;21:228-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18:1399-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, von Blomberg BM, Bloemena E, Scheper RJ, van Ham SM, Pinedo HM, van den Eertwegh AJ. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother. 2006;55:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Saleh FH, Crotty KA, Hersey P, Menzies SW. Primary melanoma tumour regression associated with an immune response to the tumour-associated antigen melan-A/MART-1. Int J Cancer. 2001;94:551-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Li F, Zhang Z, Xuan Y, Zhang D, Liu J, Li A, Wang S, Li T, Shi X, Zhang Y. PD-1 abrogates the prolonged persistence of CD8+ CAR-T cells with 4-1BB co-stimulation. Signal Transduct Target Ther. 2020;5:164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Gellrich FF, Schmitz M, Beissert S, Meier F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma-An Update. J Clin Med. 2020;9:223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |