Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5188
Peer-review started: May 12, 2020
First decision: August 21, 2020
Revised: August 31, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: November 6, 2020
Pneumonia of uncertain cause has been reported in Wuhan, China since the beginning of early December 2019. In early January 2020, a novel strain of β-coronavirus was identified by the Chinese Center for Disease Control and Prevention from the pharyngeal swab specimens of patients, which was recently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There is evidence of human-to-human transmission and familial cluster outbreak of SARS-CoV-2 infection. The World Health Organization(WHO) recently declared the SARS-CoV-2 epidemic a global health emergency. As of February 17, 2020, 71329 laboratory-confirmed cases (in 25 countries, including the United States and Germany) have been reported globally. Other than its rapid transmission, the epidemiological and clinical characteristics of coronavirus disease 2019 (COVID-19) remain unclear. In December 2019, coronavirus disease (named COVID-19 by the WHO) associated with the SARS-CoV-2 emerged in Wuhan, China and spread quickly across the country.
To analyze the epidemiological and clinical characteristics of confirmed cases of this disease in Liaoning province, a Chinese region about 1800 km north of Wuhan.
The clinical data of 56 laboratory-confirmed COVID-19 cases due to 2019-nCoV infection were analyzed. The cases originated from eight cities in Liaoning province.
The median age of the patients was 45 years, and 57.1% of them were male. No patient had been in direct contact with wild animals. Among them, 23 patients (41.1%) had resided in or traveled to Wuhan, 27 cases (48.2%) had been in contact with confirmed COVID-19 patients, 5 cases (8.9%) had been in contact with confirmed patients with a contact history to COVID-19 patients, and 1 case (1.8%) had no apparent history of exposure. Fever (75.0%) and cough (60.7%) were the most common symptoms. The typical manifestations in lung computed tomography (CT) included ground-glass opacity and patchy shadows, with 67.8% of them being bilateral. Among the patients in the cohort, 78.6% showed reduction in their lymphocyte counts, 57.1% showed increases in their C-reactive protein levels, and 50.0% showed decreases in their blood albumin levels. Eleven patients (19.6%) were admitted to intensive care unit, 2 patients (3.5%) progressed to acute respiratory distress syndrome, 4 patients (7.1%) were equipped with non-invasive mechanical ventilation, and 1 patient (1.8) received extracorporeal membrane oxygenation support. There were 5 mild cases (5/56, 8.9%), 40 moderate cases (40/56, 71.4%), 10 severe cases (10/56, 17.9%), and 1 critical case (1/56, 1.8%). No deaths were reported.
SARS-CoV-2 can be transmitted among humans. Most COVID-19 patients show symptoms of fever, cough, lymphocyte reduction, and typical lung CT manifestations. Most are moderate cases. The seriousness of the disease (as indicated by blood oxygen saturation, respiratory rate, oxygenation index, blood lymphocyte count, and lesions shown in lung CT) is related to history of living in or traveling to Wuhan, underlying diseases, admittance to intensive care unit, and mechanical ventilation.
Core Tip: This is a retrospective, single-center study on the clinical characteristics of laboratory-confirmed coronavirus disease 2019 (COVID-19) cases caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We summarized the clinical data of 56 patients with COVID-19 diagnosed and treated in Liaoning. The main conclusion is SARS-CoV-2 can be transmitted among humans, and cases were mainly imported and were moderate. Severe cases were characterized by severe hypoxemia, lymphocytopenia, pulmonary consolidation, admittance to intensive care unit, and mechanical ventilation. Infection of SARS-CoV-2 and seriousness of COVID-19 are related to old age and underlying diseases.
- Citation: Wang JB, Wang HT, Wang LS, Li LP, Xv J, Xv C, Li XH, Wu YH, Liu HY, Li BJ, Yu H, Tian X, Zhang ZY, Wang Y, Zhao R, Liu JY, Wang W, Gu Y. Epidemiological and clinical characteristics of fifty-six cases of COVID-19 in Liaoning Province, China. World J Clin Cases 2020; 8(21): 5188-5202
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5188.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5188
Pneumonia of uncertain cause has been reported in Wuhan, China since the beginning of early December 2019[1]. Most of the cases had a history of living in or working at the local Huanan Seafood Wholesale Market[2,3]. In early January 2020, a novel strain of β-coronavirus was identified by the Chinese Center for Disease Control and Prevention (CDC) from the pharyngeal swab specimens of patients, which was recently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[4,5]. This virus bears similarities to SARS-CoV. It has an enveloped virion that measures approximately 50-200 nm in diameter with positive-sense, single-stranded ribonucleic acid, belongs to family Coronaviridae of order Nidovirales, and is the seventh member of the coronavirus family[6]. Coronavirus can cause multiple-system infections in various animals. In humans, it mainly infects the respiratory system, similar to the infections caused by SARS and Middle East respiratory syndrome (MERS). Most patients show mild symptoms and have a good prognosis; however, some may progress to severe pneumonia and/or acute respiratory distress syndrome (ARDS) or even die as a result of multiple-organ failure[7-9].
There is evidence of human-to-human transmission and familial cluster outbreak of SARS-CoV-2 infection[10-14]. The World Health Organization (WHO) recently declared the SARS-CoV-2 epidemic a global health emergency[15]. As of February 17, 2020, 71329 laboratory-confirmed cases (in 25 countries, including the United States and Germany) have been reported globally[11,12,15,16]. As of February 17, 2020, 70635 laboratory-confirmed cases have been reported in China, with 1772 deaths[17]. Other than its rapid transmission, the epidemiological and clinical characteristics of COVID-19 remain unclear. In two recent studies, 41 and 99 laboratory-confirmed clinical cases admitted to hospitals in Wuhan were included, with some presenting severe symptoms similar to those caused by SARS-CoV[1,18]. Another study followed 1099 laboratory-confirmed cases, most of which came from southern provinces and cities around Hubei province, with some from the northern city of Beijing, and analyzed the epidemiological and clinical characteristics of COVID-19[19]. At present, there is an urgent need to update the analysis with a focus on imported cases, second-generation cases, and, in the future, third-generation cases, to compare epidemiologically and clinically the different manifestations of this disease in the climates of North China and Wuhan. Such knowledge would help the research community form a bigger picture of the epidemiological and clinical features of COVID-19 in China.
This is a retrospective, single-center study on the clinical characteristics of laboratory-confirmed COVID-19 cases caused by SARS-CoV-2 infection. COVID-19 patients admitted to The Sixth People’s Hospital of Shenyang from January 24, 2020 to February 17, 2020 were enrolled as the study subjects. The Sixth People’s Hospital of Shenyang is a specialized hospital for infectious diseases and a designated hospital for COVID-19 in Liaoning province by the provincial government. All the confirmed cases from the eight cities of the province were sent here for centralized treatment. Patients enrolled consisted exclusively of confirmed cases of SARS-CoV-2 infection as per the WHO interim guidelines. The study was approved by the Ethics Committee of the hospital, but written informed consent was not obtained due to the urgent need for clinical data.
Epidemiological, demographic, clinical, laboratory, and imaging data were obtained from progress notes and medical examination reports of patients during their hospitalization. The following laboratory examinations were performed: Complete blood count, coagulation function, liver and kidney function, electrolytes, C-reactive protein, procalcitonin, lactate dehydrogenase, and creatine kinase. Lung computed tomography (CT) scans were performed for imaging. The clinical outcomes were followed up until February 21, 2020. Data were confirmed as necessary by direct communication with the attending doctors and nurses. All data were checked by two clinicians.
The basis for the diagnosis of suspected cases was two-fold: (1) Cases with any one of the four epidemiological features: (a) Having traveled to or resided in Wuhan, its surrounding areas, or areas with confirmed cases during the past 2 wk; (b) Having been in contact with fever patients or patients with respiratory disease symptoms from Wuhan, its surrounding areas, or areas with confirmed cases during the past 2 wk; (c) Having been in contact with infected cases of SARS-CoV-2 during the past 2 wk; and (d) Having been to a location of cluster outbreak during the past 2 wk; and (2) Cases with any two of the three clinical manifestations: (a) Fever and/or respiratory symptoms; (b) Multiple patchy opacities or ground-glass opacities or infiltration; and (c) Normal or reduced white blood cell count or reduced lymphocyte count[20]. Confirmed cases were diagnosed following the specifications of the WHO interim guidelines[5] and tested positive for 2019-nCoV nucleic acid by real-time reverse transcription polymerase chain reaction (RT-PCR) of their respiratory tract specimens or blood specimens.
Mild cases presented light clinical symptoms and no signs of pneumonia in the lung CT images. Moderate cases had a fever, respiratory symptoms, and visible signs of pneumonia in lung CT images. Severe cases showed one of the following three conditions: respiratory distress (respiratory rate ≥ 30 times/min), resting-state oxygen saturation ≤ 93% (as measured by fingertip pulse oximeter), and arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa). Critical cases were those showing one of the following three conditions: respiratory failure with the need for mechanical ventilation; shock or complications by other types of organ failure; and the need for intensive care unit monitoring and treatment. Only laboratory-confirmed cases were analyzed. The incubation period was defined as the time between exposure to the infection source and the appearance of symptoms. A primary endpoint event is one marked by the occurrence of ARDS or the need for mechanical ventilation.
Laboratory diagnosis was performed in the SARS-CoV-2 laboratory of Shenyang CDC. Pharyngeal swabs and sputum samples were collected from all patients and kept in culture medium for the virus during transportation. RT-PCR was performed as specified by the WHO protocol[21]. Other viruses of the respiratory system, such as influenza A viruses (H1N1, H3N2, and H7N9), influenza B virus, respiratory syncytial virus, parainfluenza viruses, adenovirus, SARS-CoV, and MERS-CoV, were also tested using real-time RT-PCR.
SPSS (version 26.0, Armonk, NY, United States) was used for the statistical analysis. Continuous variables are presented as the mean and standard deviation if they were normally distributed and as the median if they were not. Categorical variables are presented as the number and percentage (%). Independent-sample Student’s t-test was applied to all the continuous variables, whereas Chi-squared test and Fisher’s exact test were used for the categorical variables. All laboratory results were assessed to determine whether the measurements fell outside the normal range. Potential risk factors included exposure history, old age, abnormal imaging and laboratory test results, and comorbidities. Differences were considered statistically significant if P < 0.05.
The demographic and clinical characteristics of our cohort are listed in Table 1. No medical staff was found among the 56 confirmed cases. Among them, 6 patients (10.7%) had a history of long-term residence in Wuhan, 17 (30.4%) had traveled to or resided in Wuhan for a short time, 27 (48.2%) had been in contact with confirmed COVID-19 patients, 5 (8.9%) were infected by contact with confirmed patients who had been in contact with COVID-19 patients, and 1 (1.8%) had no clear exposure history. The median incubation period was 5 d (range, 1-15 d). The median age of patients was 45 years, with 57.1% of them being male. Among all the age groups, most of the enrolled COVID-19 patients were in the 15-49 age group (35 cases, accounting for 62.5%), with no patients under 15 years. At least one underlying disease (such as hypertension, diabetes, chronic obstructive pulmonary disease, or chronic liver diseases) was found in 26 patients (46.4%). The most common symptoms were fever (75.0%) and cough (60.7%), followed by shortness of breath (26.7%) and expectoration (21.4%). By contrast, diarrhea (5.3%) and nausea and vomiting (5.3%) were uncommon. In serious cases (severe and critical cases), fever could last for 11.0 d, significantly longer than the 2.4 d in non-serious cases (mild and moderate cases) (P < 0.001). There were 45 non-serious cases and 11 serious cases. Exposure to Wuhan was the most common feature among the serious cases compared to that among the non-serious cases (63.6% vs 35.5% P < 0.001). The only patient (a critical case) receiving extracorporeal membrane oxygenation (ECMO) support lived 3 km away from the Huanan Seafood Market in Wuhan. The two groups showed significant differences (72.2% vs 46.4%, P < 0.001) in terms of underlying diseases but no differences in terms of age and smoking history. Cluster outbreak was observed in 10 families, involving 25 patients (44.6%). One family had a succession of 5 members contracting the disease.
| Clinical characteristics, symptoms or signs | Disease severity | Composite endpoint | |||||
| All patients, n = 56 | Non-severe, n = 45 | Severe, n = 11 | P value | Yes, n = 5 | No, n = 51 | P value | |
| Age, median (range), yr | 45.0 (21.0-80.0) | 45.4 (21.0-80.0) | 46.1 (26.0-70.0) | 0.689 | 43.8 (26.0-70.0) | 45.6 (21.0-80.0) | 0.601 |
| Age groups, n (%) | |||||||
| 0-14 yr | 0/56 (0.0) | 0/45 (0.0) | 0/11 (0.0) | 0.780 | 0/5 (0.0) | 0/51 (0.0) | 0.280 |
| 15-49 yr | 35/56 (21.0-49.0) | 29/45 (21.0-49.0) | 6/11 (26.0-45.0) | - | 4/5 (26.0-45.0) | 31/51 (21.0-49.0) | - |
| 50-64 yr | 15/56 (50.0-64.0) | 11/45 (50.0-64.0) | 4/11 (50.0-57.0) | - | 0/5 (0.0) | 15/51 (50.0-64.0) | - |
| ≥ 65 yr | 6/56 (66.0-80.0) | 5/45 (66.0-80.0) | 1/11 (70.0) | - | 1/5 (70.0) | 5/51 (66.0-80.0) | - |
| Female sex, n (%) | 32/56 (57.1) | 24/45 (53.3) | 8/11 (72.7) | 0.319 | 3/5 (60.0) | 29/51 (56.9) | 1.000 |
| Smoking history, n (%) | |||||||
| Never smokers | 45/56 (80.3) | 38/45 (84.4) | 7/11 (63.6) | 0.170 | 4/5 (80.0) | 41/51 (80.3) | 0.450 |
| Long-term smoking | 6/56 (10.7) | 4/45 (8.9) | 2/11 (18.2) | - | 0/5 (0.0) | 6/51 (11.8) | - |
| Occasionally smoking | 5/56 (8.9) | 3/45 (6.7) | 2/11 (18.2) | - | 1/5 (20.0) | 4/51 (7.8) | - |
| Exposure to source of transmission within 14 d, n (%) | |||||||
| Local residents of Wuhan | 6/56 (10.7) | 4/45 (8.9) | 2/11 (18.2) | 0.129 | 2/5 (40.0) | 4/51 (7.8) | 0.125 |
| Wuhan short-term tourism history | 17/56 (30.4) | 12/45 (26.7) | 5/11 (45.4) | 0.280 | 1/5 (20.0) | 16/51 (31.4) | 1.000 |
| NCP patient exposure history | 27/56 (48.2) | 24/45 (51.1) | 3/11 (27.3) | 0.181 | 1/5 (20.0) | 26/51 (50.9) | 0.353 |
| Contacted NCP patients and confirmed diagnosis | 5/56 (8.9) | 4/45 (8.9) | 1/11 (9.1) | 1.000 | 1/5 (20.0) | 4/51 (7.8) | 0.385 |
| No explicit exposure history | 1/56 (1.8) | 1/45 (2.2) | 0/11 (0.0) | 1.000 | 0/5 (0.0) | 1/51 (1.9) | 1.000 |
| Symptoms or signs, n (%) | |||||||
| Fever | 42/56 (75.0) | 31/45 (68.9) | 11/11 (100.0) | 0.049 | 5/5 (100.0) | 37/51 (72.5) | 0.316 |
| Temperature on admission, °C | 37.4 (36.1-39.8) | 37.3 (36.1-39.8) | 38.3 (36.7-39.7) | - | 38.3 (37.7-38.9) | 37.0 (36.1-39.8) | - |
| < 37.5 | 22/56 (39.3) | 21/45 (46.7) | 1/11 (9.1) | 0.000 | 0/5 (0.0) | 29/51 (56.8) | 0.007 |
| 37.5-38.0 | 22/56 (39.3) | 21/45 (46.7) | 1/11 (9.1) | - | 1/5 (20.0) | 13/51 (25.5) | - |
| 38.1-39.0 | 10/56 (17.8) | 2/45 (4.4) | 8/11 (72.7) | - | 4/5 (80.0) | 8/51 (15.7) | - |
| > 39.0 | 2/56 (2.5) | 1/45 (2.2) | 1/11 (9.1) | - | 0/5 (0.0) | 1/51 (2.0) | - |
| Highest temperature during hospital admission | 37.8 (36.4-39.8) | 38.1 (36.4-39.8) | 38.4 (37.7-39.8) | 0.025 | 38.5 (37.7-39.8) | 38.1 (36.4-39.8) | 0.391 |
| < 37.5 | 18/56 (32.1) | 17/45 (37.8) | 1/11 (9.1) | 0.017 | 0/5 (0.0) | 16/51 (31.4) | 0.008 |
| 37.5-38.0 | 12/56 (21.4) | 11/45 (24.4) | 1/11 (9.1) | - | 1/5 (20.0) | 11/51 (21.5) | - |
| 38.1-39.0 | 16/56 (28.6) | 9/45 (20.0) | 7/11 (63.6) | - | 3/5 (60.0) | 15/51 (29.4) | - |
| > 39.0 | 10/56 (17.8) | 8/45 (17.8) | 2/11 (18.2) | - | 1/5 (20.0) | 9/51 (17.6) | - |
| Fever duration, d | 7.0 (0-17) | 2.4 (0-17) | 11.0 (7-13) | - | 10.4 (7-13) | 2.8 (0-17) | - |
| Cough | 34/56 (60.7) | 29/45 (64.4) | 5/11 (45.4) | 0.310 | 4/5 (80.0) | 30/51 (58.8) | 0.638 |
| Sputum | 12/56 (21.4) | 6/45 (13.3) | 6/11 (54.5) | 0.008 | 4/5 (80.0) | 8/51 (15.7) | 0.006 |
| Breath shortness | 15/56 (26.7) | 5/45 (11.1) | 10/11 (90.9) | 0.000 | 5/5 (100.0) | 10/51 (19.6) | 0.001 |
| Sore throat | 8/56 (14.2) | 6/45 (13.3) | 2/11 (18.2) | 0.649 | 1/5 (20.0) | 7/51 (13.7) | 0.552 |
| Headache | 7/56 (12.5) | 4/45 (8.9) | 3/11 (27.3) | 0.128 | 3/5 (60.0) | 4/51 (7.8) | 0.011 |
| Myalgia | 6/56 (10.7) | 3/45 (6.7) | 3/11 (27.3) | 0.083 | 3/5 (60.0) | 3/51 (5.9) | 0.007 |
| Chest pain | 4/56 (7.1) | 2/45 (4.4) | 2/11 (18.2) | 0.169 | 2/5 (40.0) | 2/51 (3.9) | 0.036 |
| Fatigue | 10/56 (17.8) | 7/45 (15.5) | 3/11 (27.3) | 0.393 | 2/5 (40.0) | 8/51 (15.7) | 0.214 |
| Diarrhea | 3/56 (5.3) | 2/45 (4.4) | 1/11 (9.1) | 0.488 | 1/5 (20.0) | 2/51 (3.9) | 0.249 |
| Nausea or vomiting | 3/56 (5.3) | 2/45 (4.4) | 1/11 (9.1) | 0.488 | 1/5 (20.0) | 2/51 (3.9) | 0.249 |
| Throat congestion | 42/56 (75.0) | 31/45 (68.9) | 11/11 (100.0) | 0.049 | 5/5 (100.0) | 37/51 (72.5) | 0.316 |
| Thick lung sounds | 38/56 (67.8) | 27/45 (60.0) | 11/11 (100.0) | 0.011 | 5/5 (100.0) | 33/51 (64.7) | 0.164 |
| Wet and dry rales in the lungs | 9/56 (16.1) | 5/45 (11.1) | 4/11 (36.4) | 0.063 | 5/5 (100.0) | 4/51 (7.8) | 0.000 |
| Coexisting disorders, n (%) | |||||||
| Any | 26/56 (46.4) | 18/45 (40.0) | 8/11 (72.2) | 0.009 | 5/5 (100) | 11/51 (21.5) | 0.001 |
| Respiratory diseases | 2/56 (3.6) | 0/45 (0.0) | 2/11 (18.2) | 0.036 | 1/5 (20.0) | 1/51 (2.0) | 0.172 |
| Diabetes | 7/56 (12.5) | 6/45 (13.3) | 1/11 (9.1) | 1.000 | 0/5 (0.0) | 7/51 (13.7) | 1.000 |
| Thyroid disease (hyperthyroidism or hypothyroidism) | 3/56 (5.3) | 2/45 (4.4) | 1/11 (9.1) | 0.488 | 1/5 (20.0) | 2/51 (3.9) | 0.249 |
| Hypertension | 9/56 (16.1) | 9/45 (20.0) | 0/11 (0.0) | 0.180 | 0/5 (0.0) | 9/51 (17.6) | 0.580 |
| Cardiovascular and Cerebrovascular diseases | 4/56 (7.1) | 3/45 (6.7) | 1/11 (9.1) | 1.000 | 1/5 (20.0) | 3/51 (5.9) | 0.320 |
| Chronic liver diseases1 | 10/56 (17.8) | 9/45 (20.0) | 1/11 (9.1) | 0.667 | 1/5 (20.0) | 9/51 (17.6) | 1.000 |
| Nervous system disease | 3/56 (5.3) | 2/45 (4.4) | 1/11 (9.1) | 0.488 | 1/5 (20.0) | 2/51 (3.9) | 0.249 |
| Anal fistula | 1/56 (1.8) | 0/45 (0.0) | 1/11 (9.1) | 0.196 | 0/5 (0.0) | 1/51 (2.0) | 1.000 |
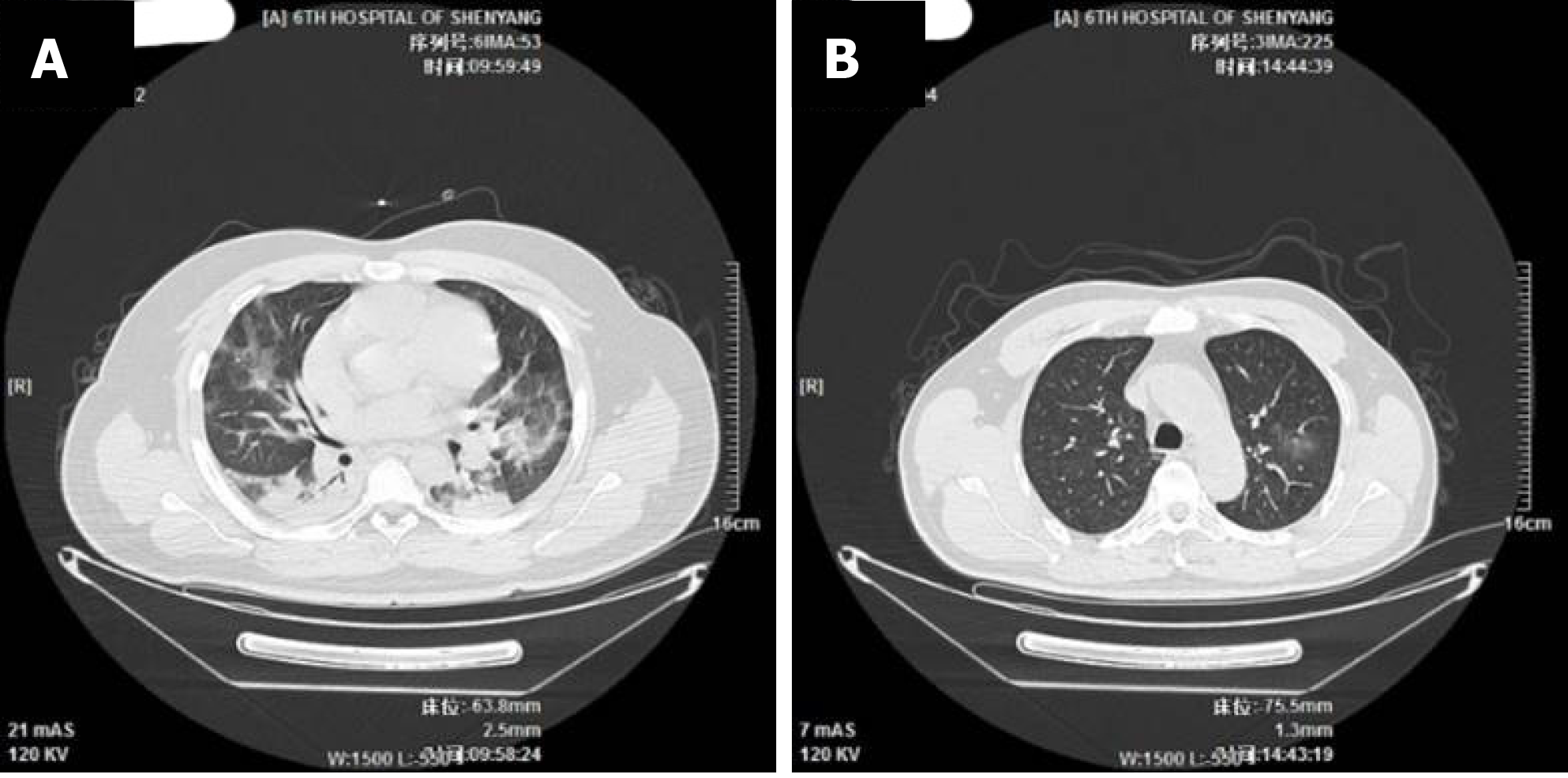
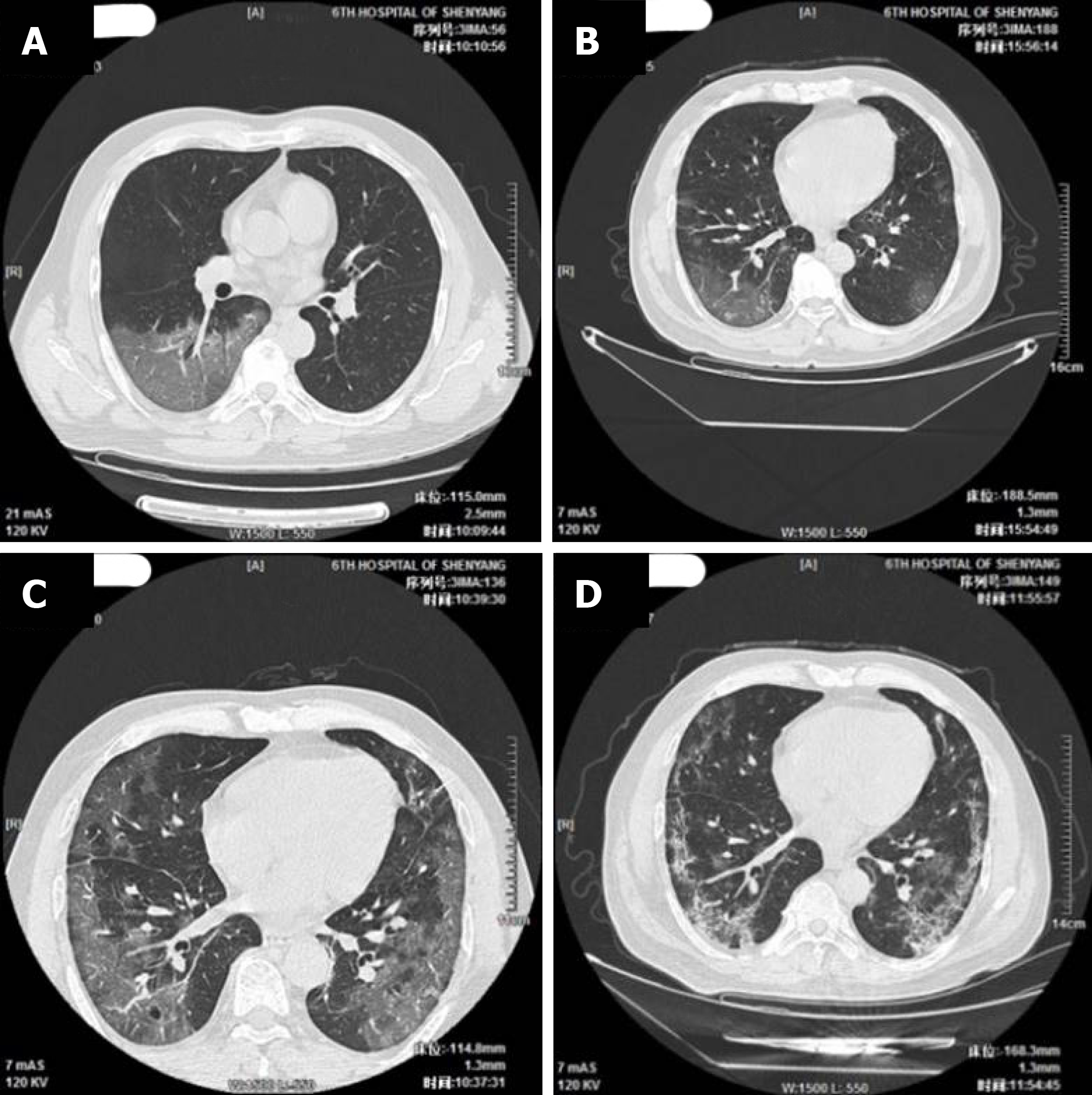
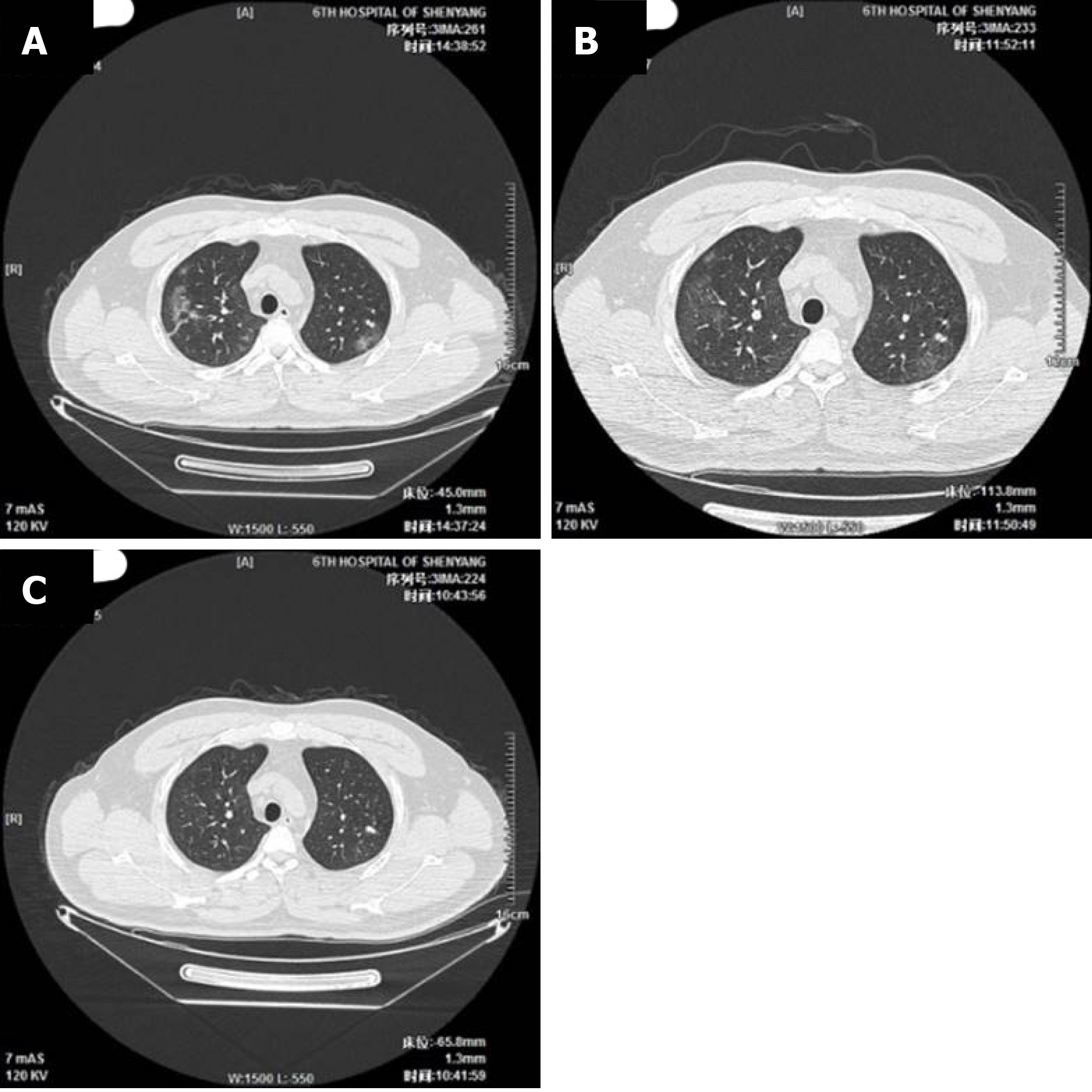
The imaging and laboratory test results are provided in Table 2. All 56 patients underwent lung CT examination upon admission to hospital. Among them, 51 (91.1%) showed signs of pneumonia. The typical manifestations of pneumonia in the lung CT images were ground-glass opacity (85.7%) and patchy opacity (78.6%), with bilateral lesions being the most common (67.8%). In Figure 1, the lung CT images of a serious case are compared to those of a mild case. In Figures 2 and 3, the development of pneumonia is shown, as observed in the lung CT images of the serious case and the mild case, respectively. The lesions shown in lung CT images are more serious and obvious and had progressed faster in the serious case than the non-serious case (P < 0.05).
| Radiographic and laboratory findings | Disease severity | Composite endpoint | |||||
| All patients, n = 56 | Non-severe, n = 45 | Severe, n = 11 | P value | Yes, n = 5 | No, n = 51 | P value | |
| Radiographic findings | |||||||
| Abnormalities on chest CT, n/total n (%) | 51/56 (91.1) | 40/45 (88.9) | 11/11 (100.0) | 0.571 | 5/5 (100.0) | 46/51 (90.2) | 0.504 |
| Ground-glass opacity | 48/56 (85.7) | 37/45 (82.2) | 11/11 (100.0) | 0.333 | 5/5 (100.0) | 43/51 (84.3) | 1.00 |
| Patchy shadowing | 44/56 (78.6) | 33/45 (73.3) | 11/11 (100.0) | 0.097 | 5/5 (100.0) | 39/51 (76.5) | 0.574 |
| Bilateral ground-glass opacity/bilateral patchy shadowing | 38/56 (67.8) | 27/45 (60.0) | 11/11 (100.0) | 0.011 | 5/5 (100.0) | 33/51 (64.7) | 0.014 |
| Laboratory findings– n/total, n (%) | |||||||
| Blood leukocyte count | 5.76 (1.8-16.7) | 5.14 (1.8-13.1) | 7.62 (2.96-16.7) | 0.057 | 9.42 (3.9-16.7) | 5.01 (1.8-13.1) | < 0.001 |
| > 9.5 × 109/L | 10/56 (17.8) | 6/45 (13.3) | 4/11 (36.4) | 0.093 | 2/5 (40.0) | 8/51 (15.7) | 0.214 |
| < 3.5 × 109/L | 14/56 (25.0) | 10/45 (22.2%) | 4/11 (36.4) | 0.439 | 1/5 (20.0) | 13/51 (25.5) | 1.000 |
| Neutrophil count | 4.2 (1.34-16.08) | 3.49 (0.5-11.94) | 6.34 (1.77-16.08) | 0.036 | 8.02 (2.4-16.08) | 3.37 (0.5-11.94) | < 0.001 |
| < 1.8 × 109/L | 12/56 (21.4) | 9/45 (20.0) | 3/11 (27.3) | 0.686 | 1/5 (20.0) | 11/51 (21.5) | 1.000 |
| Lymphocyte count | 1.09 (0.2-2.1) | 1.2 (0.2-2.1) | 0.78 (0.2-1.0) | 0.008 | 0.66 (0.2-1.0) | 1.2 (0.2-2.1) | < 0.001 |
| < 1.1 × 109/L | 44/56 (78.6) | 34/45 (75.5) | 10/11 (90.9) | 0.424 | 5/5 (100.0) | 29/51 (56.8) | 0.054 |
| Platelet count | 221.4 (40-384) | 214.7 (40-382) | 241.4 (124-384) | 1.000 | 247.8 (181-384) | 215.6 (40-382) | 1.000 |
| < 125 × 109/L | 8/56 (14.3) | 6/45 (13.3) | 2/11 (18.2) | 0.649 | 0/5 (0.0) | 8/51 (15.7) | 1.000 |
| Hemoglobin level, g/dL | 144.1 (108-174) | 144.6 (108-174) | 142.7 (130-162) | 0.889 | 141.2 (130-162) | 144.8 (108-174) | 0.875 |
| C-reactive protein level | 31.9 (0.17-128.32) | 25.6 (0.17-128.32) | 50.9 (5.43-108.28) | 0.047 | 51.7 (5.43-108.28) | 27.7 (0.17-128.32) | 0.046 |
| ≥ 8 mg/L, n/total, n (%) | 32/56 (57.1) | 23/45 (51.1) | 9/11 (81.8) | 0.093 | 4/5 (80.0) | 28/51 (54.9) | 0.379 |
| Lactose dehydrogenase | 563.1 (385-882) | 517.2 (385-882) | 700.7 (581-820) | 0.048 | 720 (628-820) | 529 (385-882) | 0.047 |
| ≥ 618 U/L, n/total, n (%) | 18/56 (32.1) | 8/45 (17.8) | 10/11 (90.9) | 0.000 | 5/5 (100.0) | 13/51 (25.5) | 0.002 |
| Procalcitonin level | 0.05 (0.04-0.13) | 0.05 (0.04-0.11) | 0.06 (0.04-0.13) | 1.000 | 0.06 (0.04-0.1) | 0.05 (0.04-0.13) | 1.000 |
| ALT > 40 U/L, n/total n (%) | 13/56 (23.2) | 5/45 (11.1) | 8/11 (72.7) | < 0.001 | 5/5 (100.0) | 8/51 (15.7) | < 0.001 |
| AST > 40 U/L, n /total, n (%) | 16/56 (28.6) | 8/45 (17.8) | 8/11 (72.7) | 0.001 | 3/5 (60.0) | 8/51 (15.7) | 0.047 |
| Albumin < 35 g/L, n /total, n (%) | 28/56 (50.0) | 18/45 (40.0) | 10/11 (90.9) | 0.005 | 5/5 (100.0) | 23/51 (45.1) | 0.051 |
| Creatinine kinase > 22 mmol/L, n/total, n (%) | 18/56 (32.1) | 13/45 (28.9) | 5/11 (54.4) | 0.305 | 3/5 (60.0) | 15/51 (29.4) | 0.314 |
| Creatinine kinase ≥ 170 U/L, n/total, n (%) | 8/56 (14.3) | 4/45 (8.9) | 4/11 (36.3) | 0.057 | 2/5 (40.0) | 6/51 (11.8) | 0.144 |
| Blood urea nitrogen ≥ 6.1 mmol/L, n/total, n (%) | 3/56 (5.3) | 1/45 (2.2) | 2/11 (18.2) | 0.095 | 1/5 (20.0) | 2/51 (3.9) | 0.249 |
| Creatinine ≥ 110 mmol/L, n/total, n (%) | 2/56 (3.6) | 1/45 (2.2) | 1/11 (9.1) | 0.357 | 1/5 (20.0) | 1/51 (2.0) | 0.172 |
| D-dimer ≥ 0.55 mg/L, n/total, n (%) | 12/56 (21.4) | 20/45 (44.4) | 7/11 (63.6) | 0.322 | 3/5 (60.0) | 25/51 (49.0) | 1.000 |
| Co-infection | |||||||
| Other viruses | 0/56 (0.0) | 0/45 (0.0) | 0/11 (0.0) | - | 0/5 (0.0) | 0/51 (0.0) | - |
| Bacterial | 1/56 (1.8) | 0/45 (0.0) | 1/11 (9.1) | 0.196 | 0/5 (0.0) | 1/51 (2.0) | 1.000 |
| Fungus | 0/56 (0.0) | 0/45 (0.0) | 0/11 (0.0) | - | 0/5 (0.0) | 0/51 (0.0) | - |
| Mycoplasma pneumoniae | 2/56 (3.6) | 2/45 (4.4) | 0/11 (0.0) | 1.000 | 0/5 (0.0) | 2/51 (3.9) | 1.000 |
All 56 COVID-19 patients underwent blood tests upon admission. Among them, 44 cases (78.6%) showed reduction in lymphocyte counts, 32 (57.1%) showed increases in their C-reactive protein levels, 28 (50.0%) showed decreases in their albumin levels, and 18 (32.1%) showed increases in their lactate dehydrogenase levels. Reduction in platelet and leukocyte counts and rise in alanine aminotransferase, aspartate aminotransferase, creatine kinase, and D-dimer levels were not common. Compared to the non-serious cases, the serious cases displayed higher levels of indicator anomalies at higher degrees, such as decreases in lymphocyte counts (0.78 × 109/L vs 1.2 × 109/L, P < 0.001), increases in C-reactive protein levels (50.9 mg/L vs 25.6, P < 0.05), increase in lactate dehydrogenase level (700.7 U/L vs 517.2 U/L, P < 0.05), and an increase in alanine aminotransferase level and aspartate aminotransferase level (P < 0.05).
Treatment, comorbidities, and outcomes are listed in Table 3. The percentages of patients receiving oxygen therapy, mechanical ventilation, intravenous antibiotics, glucocorticoid, and lopinavir/ritonavir treatments were 44.6%, 8.9%, 39.9%, 17.8% and 100.0%, respectively. Among them, 4 cases (7.1%) received non-invasive mechanical ventilation, and 1 case (1.8%) received invasive mechanical ventilation and ECMO. The proportion of patients receiving these treatments was significantly higher among the serious cases than the non-serious cases (P < 0.05). The contrast was even starker for mechanical ventilation (non-invasive: 36.4% vs 0%, P < 0.001; invasive: 9.1% vs 0%, P < 0.001, for the serious cases vs non-serious cases). This was also true for oxygen therapy (100.0% vs 31.1%, P < 0.001).
| Disease severity | Composite endpoint | ||||||
| All patients, n = 56 | Non-severe, n = 45 | Severe, n = 11 | P value | Yes, n = 5 | No, n = 51 | P value | |
| Complications, n (%) | |||||||
| Pneumonia | 51/56 (91.1) | 40/45 (88.9) | 11/11 (100.0) | 0.571 | 5/5 (100.0) | 46/51 (90.2) | 1.00 |
| ARDS | 2/56 (3.9) | 0/45 (0.0) | 2/11 (18.2) | 0.036 | 2/5 (40.0) | 0/51 (0.0) | 0.006 |
| Acute lung injury | 4/56 (7.1) | 1/45 (2.2) | 3/11 (27.3) | 0.026 | 1/5 (20.0) | 3/51 (5.9) | 1.00 |
| Acute myocardial injury | 2/56 (3.9) | 0/45 (0.0) | 2/11 (18.2) | 0.056 | 1/5 (20.0) | 1/51 (1.9) | 0.172 |
| Acute liver injury | 13/56 (23.2) | 7/45 (15.5) | 6/11 (54.5) | 0.013 | 3/5 (60.0) | 10/51 (19.6) | 0.076 |
| Treatment, n (%) | |||||||
| Oxygen therapy | 25/56 (44.6) | 14/45 (31.1) | 11/11 (100.0) | 0.000 | 5/5 (100.0) | 20/51 (39.2) | 0.014 |
| Mechanical ventilation | |||||||
| Invasive | 4/56 (7.1) | 0/45 (0.0) | 4/11 (36.4) | 0.001 | 4/5 (80.0) | 0/51 (0.0) | 0.000 |
| Non-invasive | 1/56 (1.8) | 0/45 (0.0) | 1/11 (9.1) | 0.196 | 1/5 (20.0) | 0/51 (0.0) | 0.089 |
| Use of extracorporeal membrane oxygenation | 1/56 (1.8) | 0/45 (0.0) | 1/11 (9.1) | 0.196 | 1/5 (20.0) | 0/51 (0.0) | 0.089 |
| Antiviral therapy | 56/56 (100.0) | 45/45 (100.0) | 11/11 (100.0) | - | 5/5 (100.0) | 51/51 (100.0) | - |
| Administration of antibiotics | 22/56 (39.3) | 13/45 (28.9) | 9/11 (81.8) | 0.002 | 5/5 (100.0) | 17/51 (33.3) | 0.000 |
| Administration of glucocorticoid | 10/56 (17.8) | 3/45 (6.7) | 7/11 (63.6) | 0.000 | 4/5 (80.0) | 6/51 (11.7) | 0.003 |
| Use of intravenous immunoglobin | 7/56 (12.5) | 2/45 (4.4) | 5/11 (45.4) | 0.002 | 5/5 (100.0) | 2/51 (3.9) | 0.000 |
| Clinical outcomes, n (%) | |||||||
| Discharge from hospital | 21/56 (37.5) | 20/45 (44.4) | 1/11 (9.1) | 0.039 | 0/5 (0.0) | 21/51 (41.2) | 0.045 |
| Death | 0/56 (0.0) | 0/45 (0.0) | 0/11 (0.0) | - | 0/5 (0.0) | 0/5 (0.0) | - |
| Staying in hospital | 35/56 (62.5) | 25/45(55.5) | 10/11 (90.9) | 0.039 | 5/5 (100.0) | 30/51 (58.8) | 0.145 |
Pneumonia was the most common complication during treatment (91.1%), followed by liver injury (23.2%) and acute lung injury (7.2%). There were 2 cases (3.6%) of ARDS. Comorbidities were observed in all 11 serious cases, significantly higher than the non-serious cases (88.9%, P < 0.001). The percentages of patients admitted to intensive care unit and progressing to ARDS were 19.6% and 3.5% respectively, and 5 cases (8.9%) developed primary endpoint events.
After proactive and effective treatment, all 56 patients showed a gradual recovery. As of February 21, 2020, 21 patients had been discharged, including 1 serious case. No deaths had been recorded at the time of writing.
This is a descriptive study performed using the epidemiological and clinical data of 56 COVID-19 patients. These patients all received centralized treatment in the Sixth People’s Hospital of Shenyang (provincial-level COVID-19 treatment center of Liaoning province). Most of the enrolled patients were residents of the province and were infected by coming into close contact with imported cases with a history of travel to or residence in Wuhan. This study is a preliminary demonstration of the characteristics of COVID-19 in a northern province of China (about 1800 km from Wuhan).
Human coronavirus is a key type of pathogen that causes respiratory system infections. SARS-CoV and MERS-CoV are two highly contagious viruses that have caused severe respiratory syndromes in humans, while four other human coronaviruses (HCoV-OC43, HCoV-229E, HCoV-NL63, and HCoV-HKU1) can result in mild upper respiratory diseases. The outbreak of SARS-CoV epidemic from 2002 to 2003 resulted in 8422 cases of infection globally, across 29 countries[22,23]. MERS-CoV appeared in Middle East countries in 2012 and was also imported to China[24,25]. SARS-CoV-2 carries very different genetic traits from those of SARS-CoV and MERS-CoV and is currently found to have a homology with bat SARS-like coronavirus (bat-SL-CoVZC45) of over 85%. More importantly, the transmission routes of SARS-CoV-2 facilitate its rapid spread. Respiratory droplets and direct contact are typical manners of transmission, which are the same as those of SARS-nCoV, MERS-nCoV, and highly pathogenic influenza viruses[26-28]. Transmission via the digestive tract and other routes remains to be confirmed. In recent studies, fecal samples, rectal swabs, gastrointestinal tract, saliva, and urine showed positive test results for SARS-CoV-2[19]. The alarm should be raised for possible transmission through gastrointestinal secretion, as it would help in improving our defense systems and the inhibition of the rapid spread of this virus globally.
Our research offers more definite evidence of human-to-human transmission. None of the 56 patients had been in direct contact with wild animals. Most had either lived or traveled in Wuhan or had contact with COVID-19 patients. Only one patient had an unclear exposure history. In most cases, the onset of disease happened after January 24, 2020, with the earliest on January 10, 2020. Our results show clear evidence of familial cluster outbreak, involving 25 patients from 10 families. One family even had 5 successive cases of infection in family members, which is in high agreement with the latest reports[10,12,14]. It should be noted that infection through contact with diagnosed second-generation patients has appeared, namely so-called “third-generation cases.” In this study, research was conducted on patients living in a region distant from Wuhan (1800 km away), who were infected mainly through different levels of exposure to patients with COVID-19, with the hope of laying the foundations for improving the prevention and control of this infection. No infection has been identified in children during the course of the study, and two families with cluster outbreak have been found. For the same exposure time and in the same environment, the children in the families were not affected. The reason for this warrants in-depth investigation and may help uncover the pathogenesis of this disease.
In terms of its name, the disease was temporarily assigned the name of novel coronavirus pneumonia (NCP) on February 8, 2020 by the prevention and control task force for novel coronavirus-infected pneumonia of China’s State Council; it was later re-named as COVID-19 by WHO. Six versions of interim guidelines have been released at different times of the epidemic, including by WHO on January 12, 2020 for the clinical management of patients with severe respiratory infection possibly caused by the novel coronavirus and the five issued by the Chinese government for the diagnosis and treatment of novel coronavirus-infected pneumonia. The definition and diagnostic criteria for mild cases were put forward in the fifth edition of the guidelines by China. In our study, 5 patients (8.9%) belonged to the mild cases. They exhibited no clinical symptoms, and their lung CT images were normal. These patients were identified during the screening of personnel in contact with COVID-19 cases and showed a significantly lower disease severity, a shorter isolation and treatment time, and lower treatment costs. Our results suggest the importance of the early identification as well as timely and accurate treatment and management of patients in the early stage of the disease, when pulmonary inflammation is not yet severe.
There were significantly more male patients than females in the 56 COVID-19 cases enrolled in this study. The reduced vulnerability of females to SARS-CoV-2 infection is perhaps related to the protection provided by the X chromosome and by female hormones, which play an important role in the innate and acquired immunity of humans[29]. Nearly half of the patients suffered from a chronic disease, including cardiovascular and cerebrovascular diseases, diabetes, and chronic liver diseases. These chronic health conditions are known to lower the immunity of the affected patients[30-32]. The condition of these 56 COVID-19 patients was also found to be significantly milder than cases from Wuhan reported in two recent papers[1,18]. This could be closely related to the appropriate prevention and control measures and the diagnosis and treatment plans adopted as well as the availability of healthcare resources. The clinical symptoms and pneumonia of the imported cases (i.e. those infected due to living or traveling in Wuhan) were significantly more serious than those of patients local to Liaoning province. The only critical case encountered in the study had a long history of residence in Wuhan, and lived only 3 km from Huanan Seafood Market at the time of the epidemic. With age (41 years) and immune function (no chronic disease) excluded in this case, the analysis of factors related to disease severity was more indicative of a large number of viruses at the center of the epidemic, their strong pathogenicity, and a higher possibility of immune disorder induced by cytokine storm in prime-age people in good health. Only 3 (9.1%) out of the 33 local patients of Liaoning province (i.e. patients infected by COVID-19 cases from Wuhan, or “third-generation cases”) were diagnosed as severe, and 2 had comorbidities of chronic respiratory diseases. The latter is an indication for the COVID-10 diagnosis and treatment centers in Liaoning province to remain alert to COVID-19 patients with chronic respiratory conditions, as they are at much higher risk of disease progression. In this study, a 17-wk pregnant woman was diagnosed with mild COVID-19. The reduced immunity yet only mild illness observed in this patient will require an in-depth investigation from multiple aspects, such as virus, host, and the interaction between the two.
In this study, the clinical characteristics of 56 COVID-19 patients were analyzed. Fever and cough were common, while symptoms affecting the digestive system were rare. This suggests that unlike SARS-nCoV, MERS-nCoV, and influenza viruses, respiratory epithelial cells are the primary target of SARS-CoV-2[33-36]. As 58.9% of local NCP patients only showed mild symptoms, a comprehensive evaluation, including lung CT and nucleic acid tests, should be used when monitoring cases outside of Wuhan to avoid omission. As shown in our laboratory test results, over 3/4 of the cases showed a decrease in lymphocyte count. It is thus speculated that SARS-CoV-2 mainly attacks lymphocytes, especially T-lymphocytes, which can be used as a reference indicator in clinical diagnosis. The reduction in lymphocyte count is even more prominent in serious cases. The lowest lymphocyte count was 0.2 × 109/L in the critical case. None of the 10 severe cases progressed to a critical state, and all recovered gradually after proactive and comprehensive treatment[37]. Hence, the early identification and timely treatment of infection are very important for serious cases. No deaths were recorded in this study for of 56 COVID-19 patients. The death rate was remarkably lower than the recently reported mortality rates of 15% and 11%[1,18]. This could be related to the early isolation, early identification, early treatment, and centralized management of the patients in this cohort.
The limitations of this study are two-fold: (1) This study only includes 56 confirmed COVID-19 cases in Liaoning province, while more cases from cities outside of Wuhan and even from other countries should be included; Such an inclusive investigation would provide a deeper understanding of COVID-19; and (2) As some patients are still in the hospital, information on their outcomes is not available at the time of data analysis. However, the data used and analyzed in this study provide a preliminary assessment of the epidemiological and clinical characteristics of COVID-19 in areas outside of Wuhan.
The transmission of SARS-CoV-2 virus has been explosive, with a recent increase in the rate of human-to-human transmission. This virus strain is highly infectious and more likely to affect males with underlying diseases. Moreover, familial cluster outbreak is possible. Pneumonia is the most common comorbidity. Bilateral ground-glass opacity and patchy opacity are typically observed in lung CT images. Cases infected via contact with COVID-19 patients without any history of travel or residence in Wuhan usually show mild symptoms, a good prognosis, and a relatively low mortality. The strict, timely, and effective prevention and control of the disease in a comprehensive manner is essential to curb the rapid spread of this virus. More efforts will be needed to study the infection mechanism of SARS-CoV-2 and explore effective treatments.
SARS-CoV-2 can be transmitted among humans. Most COVID-19 patients show symptoms of fever, cough, lymphocyte reduction, and typical lung CT manifestations. Most are moderate cases. Infection of SARS-CoV-2 and seriousness of COVID-19 is related to old age and underlying diseases.
In December 2019, coronavirus disease 2019 (COVID-19) associated with the severe acute respiratory coronavirus 2 (SARS-CoV-2) emerged in Wuhan and spread quickly across China and world. The World Health Organization declared the SARS-CoV-2 epidemic a global health emergency. Other than its rapid transmission, the epidemiological, pathogenesis, and clinical characteristics of COVID-19 remain unclear.
By analyzing clinical cases, we summarized the risk factors, clinical manifestations, and curative treatment of COVID-19. More efforts will be needed to study the basic and clinical research related to the infection of SARS-CoV-2.
We analyzed the epidemiological and clinical characteristics of confirmed cases of COVID-19 in Liaoning province, a Chinese region far north of Wuhan. The research would help provide measures for controlling the spread of SARS-CoV-2 and clinical diagnosis and treatment of COVID-19.
The clinical data of 56 laboratory-confirmed COVID-19 cases due to 2019 novel coronavirus infection were collected from eight cities in Liaoning Province. Laboratory diagnosis was performed in the SARS-CoV-2 laboratory of Shenyang Center for Disease Control and Prevention. Epidemiological characteristics were analyzed. SPSS (version 26.0) was used for the statistical analysis of clinical, laboratory, and imaging data.
Most patients had resided in or traveled to epidemic area and had contact with confirmed COVID-19 patients. Fever and cough were the most common symptoms. The typical manifestations in lung computed tomography (CT) included bilateral ground-glass opacity and patchy shadows. Severe cases and critical cases were respectively 10 (17.9%) and 1 (1.8%). No deaths were reported.
SARS-CoV-2 can be transmitted among humans. This virus strain is highly infectious and more likely to affect males with underlying diseases. Most COVID-19 patients are mild cases or moderate cases. The risk factors for seriousness of the disease included old age, underlying diseases, significant lymphocytopenia, and serious lesions shown in lung computed tomography.
More efforts will be needed to study the infection mechanism of SARS-CoV-2, early identification of severe cases, and effective treatments.
We gratefully acknowledge Professor Ma YN (China Medical University, China) for technical assistance in statistical analysis. We are grateful to head nurse Song JJ (The Sixth People’s Hospital of Shenyang, China) for communicating with patients to confirm relevant information.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Campanale M, Orbell J S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28482] [Article Influence: 7120.5] [Reference Citation Analysis (3)] |
| 2. | Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1670] [Cited by in F6Publishing: 1693] [Article Influence: 423.3] [Reference Citation Analysis (0)] |
| 3. | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2274] [Cited by in F6Publishing: 1861] [Article Influence: 465.3] [Reference Citation Analysis (0)] |
| 4. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7915] [Cited by in F6Publishing: 7185] [Article Influence: 1796.3] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. [cited 2020 Jan 11]. Available from: http://www.who. int. [Cited in This Article: ] |
| 6. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18987] [Cited by in F6Publishing: 16536] [Article Influence: 4134.0] [Reference Citation Analysis (0)] |
| 7. | Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 632] [Cited by in F6Publishing: 628] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 8. | Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3277] [Cited by in F6Publishing: 3208] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 9. | Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4030] [Cited by in F6Publishing: 3819] [Article Influence: 318.3] [Reference Citation Analysis (0)] |
| 10. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6159] [Cited by in F6Publishing: 5253] [Article Influence: 1313.3] [Reference Citation Analysis (0)] |
| 11. | Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N Engl J Med. 2020;382:872-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 673] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 12. | Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2799] [Cited by in F6Publishing: 2433] [Article Influence: 608.3] [Reference Citation Analysis (0)] |
| 13. | Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3012] [Cited by in F6Publishing: 2400] [Article Influence: 600.0] [Reference Citation Analysis (0)] |
| 14. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10402] [Cited by in F6Publishing: 8966] [Article Influence: 2241.5] [Reference Citation Analysis (0)] |
| 15. | World Health Organization main website. [cited 2020 Feb 17]. Available from: http://www.who.int. [Cited in This Article: ] |
| 16. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4155] [Cited by in F6Publishing: 3698] [Article Influence: 924.5] [Reference Citation Analysis (1)] |
| 17. | National Health Commission of the People′s Republic of China. [cited 2020 Feb 17]. Available from: http://www.nhc.gov.cn. [Cited in This Article: ] |
| 18. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12369] [Article Influence: 3092.3] [Reference Citation Analysis (1)] |
| 19. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19202] [Cited by in F6Publishing: 17996] [Article Influence: 4499.0] [Reference Citation Analysis (5)] |
| 20. | National Health Commission. New coronavirus pneumonia prevention and control program (5th ed.) (in Chinese). 2020. Available from: http://www.nhc.gov.cn/. [Cited in This Article: ] |
| 21. | World Health Organization. Laboratory diagnosis for novel coronavirus. 2020. Available from: http://www.who.int/. [Cited in This Article: ] |
| 22. | Hu B, Zeng LP, Yang XL, Ge XY, Zhang W, Li B, Xie JZ, Shen XR, Zhang YZ, Wang N, Luo DS, Zheng XS, Wang MN, Daszak P, Wang LF, Cui J, Shi ZL. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13:e1006698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 617] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 23. | Song HD, Tu CC, Zhang GW, Wang SY, Zheng K, Lei LC, Chen QX, Gao YW, Zhou HQ, Xiang H, Zheng HJ, Chern SW, Cheng F, Pan CM, Xuan H, Chen SJ, Luo HM, Zhou DH, Liu YF, He JF, Qin PZ, Li LH, Ren YQ, Liang WJ, Yu YD, Anderson L, Wang M, Xu RH, Wu XW, Zheng HY, Chen JD, Liang G, Gao Y, Liao M, Fang L, Jiang LY, Li H, Chen F, Di B, He LJ, Lin JY, Tong S, Kong X, Du L, Hao P, Tang H, Bernini A, Yu XJ, Spiga O, Guo ZM, Pan HY, He WZ, Manuguerra JC, Fontanet A, Danchin A, Niccolai N, Li YX, Wu CI, Zhao GP. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc Natl Acad Sci USA. 2005;102:2430-2435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 522] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 24. | Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 447] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 25. | Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499-2505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 604] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 26. | Lei H, Li Y, Xiao S, Lin CH, Norris SL, Wei D, Hu Z, Ji S. Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: Comparative analyses. Indoor Air. 2018;28:394-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 27. | Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 645] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 28. | Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 781] [Cited by in F6Publishing: 749] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 29. | Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol. 2019;56:308-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 327] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 30. | Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, Li J, Charbonneau C, Baillon-Plot N, Haider S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21 Suppl 2:S27-S32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 439] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 32. | Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol. 2017;198:4046-4053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 531] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 33. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 338] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 34. | Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R, Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, Memish ZA; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 888] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 35. | Minodier L, Charrel RN, Ceccaldi PE, van der Werf S, Blanchon T, Hanslik T, Falchi A. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol J. 2015;12:215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Shi T, Feng X, Jie Z. Progress and Current Status of Influenza Researches in China. J Transl Int Med. 2019;7:53-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Rawal G, Yadav S, Kumar R. Acute Respiratory Distress Syndrome: An Update and Review. J Transl Int Med. 2018;6:74-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |