Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4200
Peer-review started: July 7, 2020
First decision: July 24, 2020
Revised: August 2, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: September 26, 2020
Mucinous adenocarcinomas of the buttock are rare and have an uncertain etiology and natural course. They are usually related to chronic anal fistulas, hidradenitis suppurativa, or Crohn's disease. Here, we report a case of mucinous adenocarcinoma associated with hidradenitis and contradictory immunochemistry results.
A 62-year-old man complained of recurrent abscesses of the buttock for 3 years. He had several scars and nodules in bilateral buttocks, with purulent discharge. The skin lesions did not appear to originate from the anus. The patient was diagnosed with recurrent abscesses due to hidradenitis suppurativa at the first visit. He showed purulent and subsequent mucin discharge in the first operation and was diagnosed with mucinous adenocarcinoma. Several examinations were performed to determine disease origin and staging. There were no significant findings or evidence of anal fistulas. Hence, he underwent wide local excision and V-Y advancement flap in the second operation. The final diagnosis was mucinous adenocarcinoma without any evidence of anal fistulas. Additional immunochemistry test results were negative for cytokeratin (CK) 7 and positive for CK20 and CDX2, with a colorectal origin. A pathologist suggested that the disease originated from a chronic anal fistula. The patient has remained free of recurrence for 24 mo.
Although the patient with mucinous adenocarcinoma showed an atypical course, immunochemistry helped detect the disease origin.
Core Tip: In a case of mucinous adenocarcinoma, medical history and examinations suggested hidradenitis suppurativa as the origin. Pathologic results suggested that the disease was related to chronic anal fistulas. Results of the immunochemistry test showed that the disease had a colorectal origin. However, there was no evidence of anal fistulas, and the case did not match the diagnostic criteria previously known. Hence, the authors suggest that this case showed an atypical course related to anal fistulas.
- Citation: Kim SJ, Kim TG, Gu MJ, Kim S. Mucinous adenocarcinoma of the buttock associated with hidradenitis: A case report. World J Clin Cases 2020; 8(18): 4200-4206
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4200.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4200
Adenocarcinomas of the buttock and perianal area are rare, with most the articles published in the literature being case reports. Perianal adenocarcinomas can develop from perianal fistula[1]. Adenocarcinomas related to the fistula tract type almost present mucinous type[1,2]. Mucinous adenocarcinomas of the buttock and perianal area are also reported in hidradenitis suppurativa (HS) or Crohn's disease, which rarely occur[3-5].
The etiology or natural course of the mucinous adenocarcinoma of the buttock is not clearly known because of the rarity of the disease. Its diagnostic criteria were as follows: (1) The fistula should predate the carcinoma; (2) There should be no synchronous colorectal carcinoma; and (3) The internal opening of the fistula should be into the anal canal and not the malignancy[6]. However, it is often difficult to identify the disease origin since these criteria do not appear in all cases[3,7]. Here, we report a case of mucinous adenocarcinoma of the buttock with an atypical diagnostic course.
A 62-year-old man, who complained of recurrent abscesses of the buttock, was referred to the Division of Colon, Rectum and Anus for treatment.
The patient had recurrent abscesses of the buttock for 3 years and had undergone incision and drainage at a local clinic. Although the abscess was resolved with treatment, it recurred. The patient had several abscess sites on the buttock, but no anal discharge, bloody stools, fever, chills, or weight loss were noted. He was otherwise healthy.
He had been taking anti-hypertensive medication and antiplatelet drugs for hypertension for 4 years. However, he had no significant past medical or surgical history. He had no history of anal diseases, such as anal fistulas, hemorrhoids, or perianal abscesses. He had no history of other skin diseases.
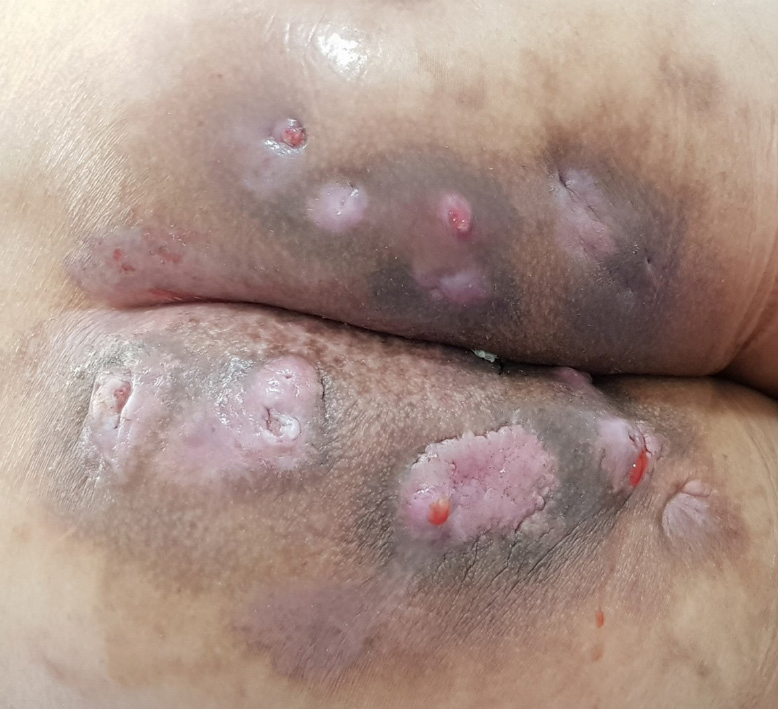
His vital signs were normal. He had several scars and nodules on the bilateral buttocks (Figure 1). Purulent discharge drained from one site on the left buttock. Other sites on the left buttock, which showed no drainage, were composed of granulation tissue. Several nodules with skin lesions on the right buttock were observed; however, the discharge was not purulent but serous. There were no lesions in the anus on digital rectal examination. The site of the skin nodule did not originate from the anus through physical examination. He had no symptoms indicative of other diseases. Finally, he was diagnosed with recurrent abscess due to HS at the first visit.
The laboratory test included complete blood count, liver function, kidney function, and electrolyte and coagulation profiles, which were within normal limits. Carcinoembryonic antigen and CA19-9 levels were 4.93 ng (normal range, 0-10 ng) and 39.69 U/mL (normal range, 0-37 U/mL), respectively.
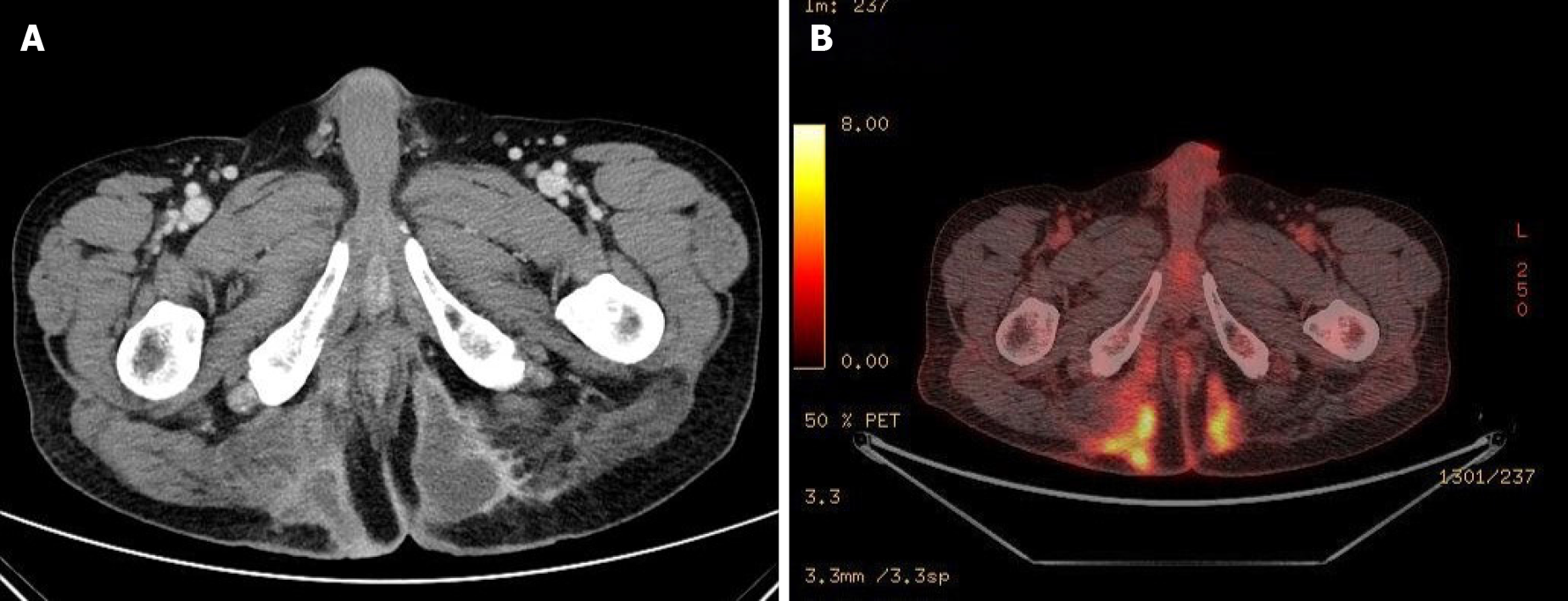
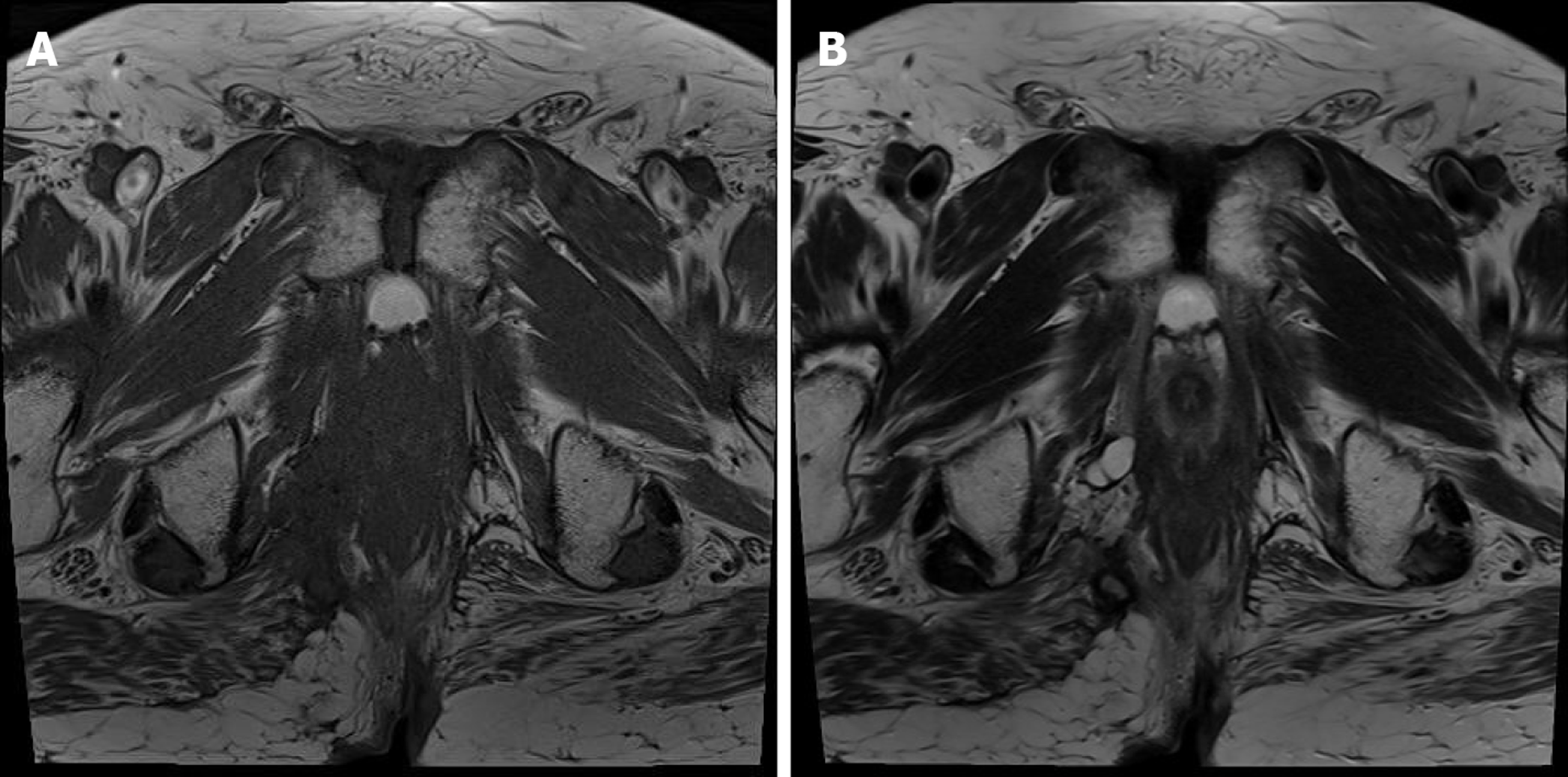
He underwent pelvic computed tomography (CT) before the operation because of the prolonged duration of symptoms suggesting the possibility of undiagnosed disease. CT showed an approximately 10 cm in length of abscess in the subcutaneous tissue of the bilateral buttocks (Figure 2A). There was no evidence of its relationship with the anus. After the first operation, he underwent gastroscopy, colonoscopy, transanal ultrasonography (TRUS), and positron emission tomography (PET) to determine the disease origin and condition, as he was diagnosed with mucinous adenocarcinoma. The results of all examinations other than PET were unremarkable. There was no evidence of anal fistula on TRUS. PET showed increased fluorodeoxyglucose uptake in the abscess cavity, buttock, and perianal wall (Figure 2B). It also showed increased fluorodeoxyglucose uptake in the prostate, which suggested inflammation or malignancy. The patient was diagnosed with inflammation of the prostate based on prostate biopsy findings.
He had no significant personal or familial history. Moreover, there was no history of cancer in his family.
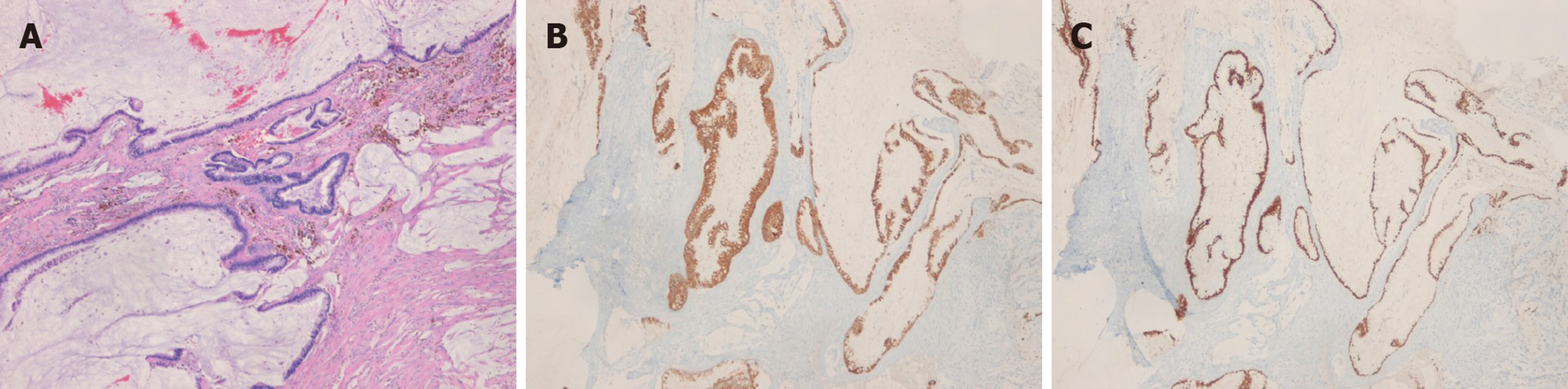
The histopathologic results after 2nd surgery showed free resection margins on the left-side specimen and positive margins on the right-side specimen. The positive resection margin was close to the right ischium. The final diagnosis of the patient was mucinous adenocarcinoma without any evidence of anal fistulas. We performed additional immunochemistry tests, which yielded negative results for cytokeratin 7 (CK7) and positive for CK20 and CDX2 (Figure 3). A pathologist suggested that the disease originated from a chronic anal fistula. However, there was no evidence of anal fistulas.
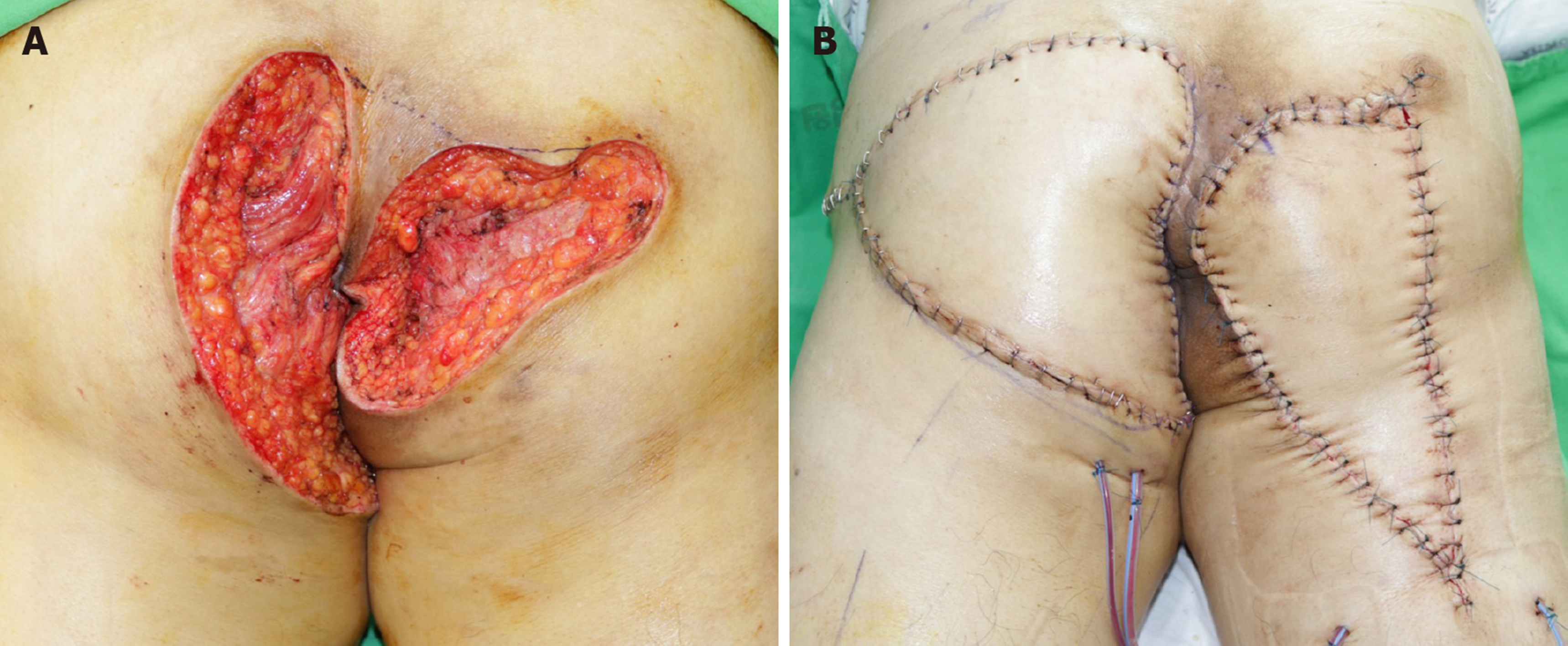
The patient underwent incision and drainage in the first operation. All nodules on each buttock were connected. The cavity of the abscess was deep, and it was not limited to the subcutaneous area. The discharge was purulent and subsequently mucinous. The patient was diagnosed with mucin-containing adenocarcinoma and treated with wide excision of the abscess cavity in the bilateral buttocks and V-Y advancement flap in the second operation (Figure 4). The surgeons resected the cavities carefully to prevent rupture. The abscess cavities in the bilateral buttocks were connected through the deep posterior anal space. The surgeons checked the anus during the second operation but did not find any indication of hidden disease.
The patient has undergone clinical evaluation every 6 mo with a physical examination, laboratory test, and radiologic study. He has remained free of recurrence for 24 mo (Figure 5).
It is difficult to diagnose mucinous adenocarcinoma of the perineum. They are usually associated with chronic inflammation and manifest as persistent symptoms, regardless of the conventional treatment[8,9]. Most patients present with perianal purulent discharge, a palpable peripheral mass, an ulcer or a recurrent abscess, which are non-specific symptoms regardless of original disease[3,4,9,10]. Patients with a tumor originating from a perianal fistula have a history of a fistula or persistent disease[3,11,12]. Although diagnostic criteria have been developed, the diagnosis of adenocarcinoma from a perianal fistula is difficult as the symptoms are nonspecific[6]. Therefore, mucinous discharge from the anal fistula provides evidence suggestive of carcinoma[13]. T2-weighted magnetic resonance imaging shows a high signal intensity in some cases[3,14,15]. However, many patients are diagnosed on the basis of official or operative biopsy[16,17].
Patients with a tumor originating from HS also have a history of HS and symptoms similar to those of patients with a perianal fistula[4,18]. Patients with a tumor originating from HS present with HS for at least 10 years[19,20]. The tumor originating from HS may be located in the perianal region, perineum, and buttocks[4,20]. The most common tumor type originating from HS is squamous adenocarcinoma, but it could be mucinous adenocarcinoma in some cases, in contrast to the tumor types originating from a perianal fistula[19,20].
Immunochemistry is commonly performed to determine the site of origin of the carcinoma[21,22]. CKs are an epithelial class of intermediate-sized filament proteins in the cytoskeleton[23]. CK7 is present in lung, breast, and female genital tract carcinomas[23]. CK20 is found in the gastrointestinal, urothelium, and Merkel cell carcinomas[23,24]. Adenocarcinomas of the small bowel, appendix, and colorectum express negative CK7 and positive CK20[23-25]. Although a report of a case of mucinous adenocarcinoma from perianal abscesses stated that CK7 and CK20 were immunopositive, the immunochemistry results in this study indicated that the carcinoma had a colorectal origin[3]. Another useful marker is CDX2, which indicates intestinal differentiation. CDX2 is a caudal homeobox gene related to the proliferation and differentiation of intestinal epithelial cells[26]. CDX2 expression presents in at least 85% of overall colorectal adenocarcinomas[26,27]. In this study, the first abscess of the patient developed in the left buttock, and the lesion persisted. The patient had no perianal symptoms. The initial diagnosis made in the outpatient clinic was recurrent HS. However, the immunohistochemistry results showed that the disease originated from the lower gastrointestinal tract. The authors suggested that this mucinous adenocarcinoma might be related to the anal fistulas that healed but remained as an abscess.
The treatment of mucinous adenocarcinomas of the buttock and perianal region depends on the location of the lesion and origin of the disease. Mostly, the surgical treatment is abdominoperineal resection (APR) with or without a wide local excision. Patients with mucinous adenocarcinoma involved with an anal fistula undergo APR, similar to patients with Crohn's anal fistula[5,11]. Even if the disease does not involve the anus, most patients with associated HS undergo APR[4,19]. Patients with wide excision underwent reconstructive operations[12,17,28]. In a case report similar to this one, the patient underwent a local wide resection, and there was no gross evidence of anal canal involvement[10]. The authors also considered APR as a curative treatment; however, the patient refused permanent colostomy, as there was no evidence of fistula. Moreover, many patients with perianal mucinous adenocarcinoma underwent further aggressive therapy, such as chemotherapy with or without radiotherapy[16,29,30]. Neoadjuvant or adjuvant chemoradiotherapy showed better results, although the number of patients was small[16,29,30]. The authors considered performing adjuvant chemoradiotherapy; however, the patient refused further treatment.
Mucinous adenocarcinomas of the buttock are rare and have uncertain etiologies and diagnostic criteria. They must be suspected in cases of continuous inflammation with an atypical course. Although the patient in the present case showed no evidence of fistula, immunochemistry was performed to detect the disease origin. Therefore, we need larger studies to confirm the etiology, diagnosis, and treatment of mucinous adenocarcinomas of the buttock.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leal RF, Long X S-Editor: Gong ZM L-Editor: A P-Editor: Liu JH
| 1. | Hamilton SR, Aadltonen LA. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the digestive system. IARC press Lyon. 2000;147-155. [Cited in This Article: ] |
| 2. | Hobbs CM, Lowry MA, Owen D, Sobin LH. Anal gland carcinoma. Cancer. 2001;92:2045-2049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Prasad SN, Razik A, Siddiqui F, Lal H. Mucinous adenocarcinoma arising from chronic perianal fistula mimicking horseshoe abscess. BMJ Case Rep. 2018;2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Alkeraye S, Nguyen T, Le Guern A, Alhaddad M, Mortier L. Mucinous adenocarcinoma in association with hidradenitis suppurativa. Clin Exp Dermatol. 2017;42:550-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Papaconstantinou I, Mantzos DS, Kondi-Pafiti A, Koutroubakis IE. Anal adenocarcinoma complicating chronic Crohn's disease. Int J Surg Case Rep. 2015;10:201-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Gordon PH. Principles and Practice of Surgery for the Colon, Rectum, and Anus. 3rd ed. Informa Healthcare, 2007: 226. [Cited in This Article: ] |
| 7. | Pai VD, Jatal S, Engineer R, Ostwal V, Saklani AP. Multidisciplinary management of colorectal adenocarcinoma associated with anal fistula: an Indian series. Colorectal Dis. 2015;17:O240-O246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Anderson BB, Cadogan CA, Gangadharam D. Hidradenitis suppurativa of the perineum, scrotum, and gluteal area: presentation, complications, and treatment. J Natl Med Assoc. 1982;74:999-1003. [PubMed] [Cited in This Article: ] |
| 9. | Yang BL, Shao WJ, Sun GD, Chen YQ, Huang JC. Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: a review from single institution. Int J Colorectal Dis. 2009;24:1001-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ilbawi AM, Simianu VV, Millie M, Soriano P. Wide local excision of perianal mucinous adenocarcinoma. J Clin Oncol. 2015;33:e16-e18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Alvarez-Laso CJ, Moral S, Rodríguez D, Carrocera A, Azcano E, Cabrera A, Rodríguez R. Mucinous adenocarcinoma on perianal fistula. A rising entity? Clin Transl Oncol. 2018;20:666-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Sato H, Maeda K, Maruta M, Kuroda M, Nogaki M, Nogaki M. Mucinous adenocarcinoma associated with chronic anal fistula reconstructed by gracilis myocutaneous flaps. Tech Coloproctol. 2006;10:249-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Sierra EM, Villanueva Saenz E, Martínez PH, Rocha JR. Mucinous adenocarcinoma associated with fistula in ano: report of a case. Tech Coloproctol. 2006;10:51-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kim MJ, Park JS, Park SI, Kim NK, Kim JH, Moon HJ, Park YN, Kim WH. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr. 2003;27:48-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Leal RF, Ayrizono ML, Coy CS, Fagundes JJ, Góes JR. Mucinous adenocarcinoma derived from chronic perianal fistulas: report of a case and review of the literature. Tech Coloproctol. 2007;11:155-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Gaertner WB, Hagerman GF, Finne CO, Alavi K, Jessurun J, Rothenberger DA, Madoff RD. Fistula-associated anal adenocarcinoma: good results with aggressive therapy. Dis Colon Rectum. 2008;51:1061-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Suleiman IE, Pindiga UH, Waziri AM, Abubakar BM. Adenocarcinoma arising in a chronic fistula-in-ano and presenting as a gluteal mass. Arch Int Surg. 2016;6:47-50. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Mukai N, Pinheiro LV, Ayrizono Mde L, Barreiro GC, Kharmandayan P, Akinaga MH, Bento AM, Martinez CA, de Carvalho RB, Ward M, Coy CS, Leal RF. Mucinous adenocarcinoma associated with chronic suppurative hidradenitis: Report of a case and review of the literature. Int J Surg Case Rep. 2016;26:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Makris GM, Poulakaki N, Papanota AM, Kotsifa E, Sergentanis TN, Psaltopoulou T. Vulvar, Perianal and Perineal Cancer After Hidradenitis Suppurativa: A Systematic Review and Pooled Analysis. Dermatol Surg. 2017;43:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | do Val IC, Almeida Filho GL, Corrêa A, Neto N. Chronic hidradenitis suppurativa and perianal mucinous adenocarcinoma. A case report. J Reprod Med. 2007;52:100-102. [PubMed] [Cited in This Article: ] |
| 21. | Stelow EB, Yaziji H. Immunohistochemistry, carcinomas of unknown primary, and incidence rates. Semin Diagn Pathol. 2018;35:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Pecciarini L, Cangi MG, Doglioni C. Identifying the primary sites of metastatic carcinoma: the increasing role of immunohistochemistry. Curr Diagn Pathol. 2001;7:168-175. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Campbell F, Herrington CS. Application of cytokeratin 7 and 20 immunohistochemistry to diagnostic pathology. Curr Diagn Pathol. 2001;7:113-122. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 736] [Cited by in F6Publishing: 613] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 25. | Bayrak R, Haltas H, Yenidunya S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: cytokeratin 7-/20+ phenotype is more specific than CDX2 antibody. Diagn Pathol. 2012;7:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Kaimaktchiev V, Terracciano L, Tornillo L, Spichtin H, Stoios D, Bundi M, Korcheva V, Mirlacher M, Loda M, Sauter G, Corless CL. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17:1392-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 475] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 28. | Erhan Y, Sakarya A, Aydede H, Demir A, Seyhan A, Atici E. A case of large mucinous adenocarcinoma arising in a long-standing fistula-in-ano. Dig Surg. 2003;20:69-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Hongo K, Kazama S, Sunami E, Kitayama J, Watanabe T. Perianal adenocarcinoma associated with anal fistula: a report of 11 cases in a single institution focusing on treatment and literature review. Hepatogastroenterology. 2013;60:720-726. [PubMed] [Cited in This Article: ] |
| 30. | Díaz-Vico T, Fernández-Martínez D, García-Gutiérrez C, Suárez-Sánchez A, Cifrián-Canales I, Mendoza-Pacas GE, Sánchez-Farpón H, Truán-Alonso N. Mucinous adenocarcinoma arising from chronic perianal fistula-a multidisciplinary approach. J Gastrointest Oncol. 2019;10:589-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |