Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3697
Peer-review started: May 1, 2020
First decision: June 13, 2020
Revised: June 24, 2020
Accepted: August 13, 2020
Article in press: August 13, 2020
Published online: September 6, 2020
Neuroendocrine tumors of appendix (ANETs) known as carcinoids, are rare endocrine neoplasms originated from enterochromaffin cells of gastrointestinal tract. ANETs are the third most frequent (16.7%) gastrointestinal neuroendocrine tumors, with the incidence of 0.08-0.2 cases/100000 during one year. Incidental ANETs occur in 0.2%-0.7% of emergency surgical resections because of suspected appendicitis which is usually the first manifestation of ANET. Although there are a lot of papers about application of somatostatin receptor scintigraphy in gastrointestinal neuroendocrine tumors, there are very rare sporadic cases described about ANETs particularly.
To establish the role of somatostatin receptor scintigraphy (SRS) in the management of patients with neuroendocrine tumors of appendix (ANET).
The total of 35 patients was investigated, 23 females and 12 males, average age (43.7 ± 17.3 years). All patients had histological diagnosis of ANET (34 carcinoids of appendix and one tubular carcinoid). Majority of tumors have been found incidentally during surgery of: Acute appendicitis (n = 15), perforated appendicitis (n = 2), ileus (n = 3), hysterectomy (n = 3), ruptured ovarian cyst (n = 2), caecal volvulus (n = 1), while 9 patients had diagnosis of appendiceal tumor before the surgery. Seventeen patients had tumor grade (G) G1, 12 G2 and 6 G3. The right hemicolectomy was performed in 13, while the rest of the patients had appendectomy only. SRS was done early (2 h) and late (24 h) after i.v. application of 740 MBq technetium-99m ethylenediamine-N, N'-diacetic acid Hydrazinonicotinyl-Tyr3-Octreotide (technetium-99m-Tektrotyd, Polatom, Poland). SRS was performed for restaging in all the patients after surgery.
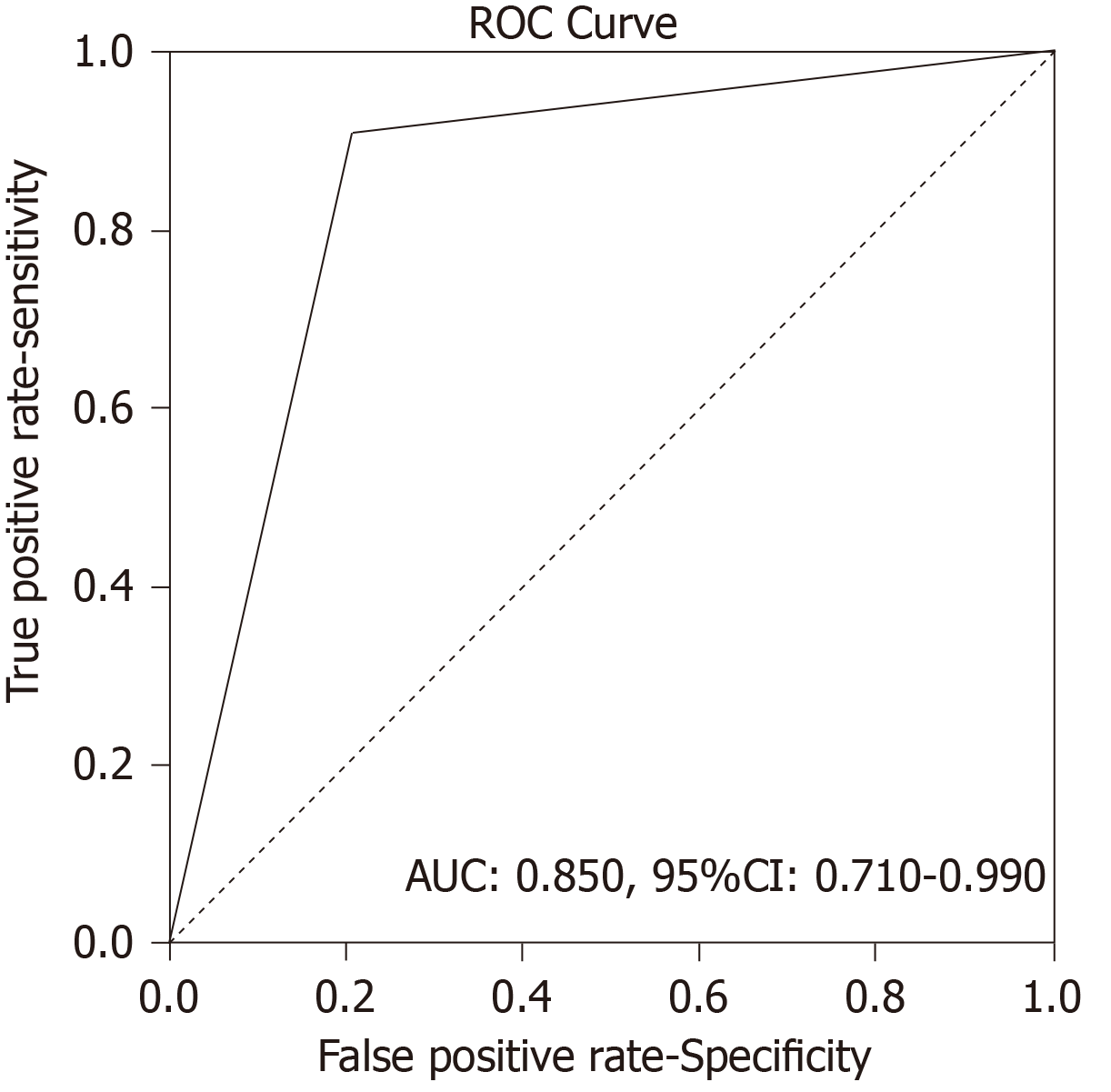
There were 12 true positive (TP), 19 true negative, 3 false positive and 1 false negative SRS result. Sensitivity of the method was 92.31%, specificity was 86.36%, positive predictive value was 80.00%, negative predictive value was 95.00% and accuracy 88.57%. Receiver operating characteristics analysis showed that SRS scintigraphy is a good test for detection TP cases [area under the curve of 0.850, 95% confidence interval (CI): 0.710-0.990, P < 001]. Single photon emission computed tomography contributed diagnosis in 7 TP findings. In 10 patients Krenning score was 4 and in 2 was 3. In 8 patients SRS significantly changed the management of the patients (in two surgery was repeated, in 4 somatostatin analogues and in two peptide receptor radionuclide therapy). Median progression-free survival in SRS positive patients was 52 months (95%CI: 39.7-117.3 mo) while in SRS negative patients it was 60 months (95%CI: 42.8-77.1 mo), without statistically significant difference between the two groups (P = 0.434).
In conclusion, our results confirmed the value of SRS in the follow-up of the patients with ANET after surgery, if recurrences or metastases are suspected.
Core tip: The aim is to establish the role of somatostatin receptor scintigraphy (SRS) in the management of 35 patients with neuroendocrine tumors of appendix. Sensitivity of the method was 92.31%, negative predictive value was 95.00% and accuracy 88.57%. In 8 patients SRS significantly changed the management. Median progression-free survival in SRS positive patients was 52 months while in SRS negative patients it was 60 months, without statistically significant difference between the two groups (P = 0.434). Our results confirmed the value of SRS in the follow-up of the patients with neuroendocrine tumors of appendix after surgery, if recurrences or metastases are suspected.
- Citation: Saponjski J, Macut D, Sobic-Saranovic D, Ognjanovic S, Bozic Antic I, Pavlovic D, Artiko V. Somatostatin receptor scintigraphy in the follow up of neuroendocrine neoplasms of appendix. World J Clin Cases 2020; 8(17): 3697-3707
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3697.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3697
Neuroendocrine tumors of appendix (ANETs) known as carcinoids, are rare endocrine neoplasms originated from enterochromaffin cells of gastrointestinal tract. ANETs are the third most frequent (16.7%) gastrointestinal neuroendocrine tumors, with the incidence of 0.08-0.2 cases/100000 during one year[1,2]. Incidental ANETs occur in 0.2%-0.7% of emergency surgical resections because of suspected appendicitis which is usually the first manifestation of ANET[3]. Over half of the ANET discovered accidentally following appendectomy are the most often at the early stage, implicating high survival rate. Majority (89%) of the tumors of appendix detected during surgery are smaller than 1 cm, which metastasize in only 2%. Metastatic rate for the tumors between 1 cm and 2 cm, and over 2 cm is 50% and 80%–90%, respectively[3,4]. According to the literature, 100% of these patients without lymph node metastases survive 10 years, and over 90% if they have metastases, regardless of the size of the initial tumor[5].
Symptoms typical for carcinoid syndrome are detected in approximately 20%–30% of patients with tumors usually with distant metastases[1]. For the diagnosis of the neuroendocrine neoplasms of appendix, besides biochemical analyses, different imaging methods and histopathology analyses with immunohistochemical staining, we could use somatostatin receptor scintigraphy (SRS) or positron emission tomography with computed tomography (PET/CT). Surgery represent the first-line therapeutic option while in patients with advanced disease it could be considered long-acting somatostatin analogues, targeted therapies (everolimus) or peptide receptor radionuclide therapy (PRRT)[6,7]. According to Spallitta et al[8] SRS has an 86% sensitivity in detection of the carcinoid of appendix and can be used for staging as well as for planning an appropriate surgery. Also, in the patients with liver metastases octreotide can relieve symptoms and delay the progression of the disease, which emphasize the role of SRS and PRRT. Safioleas et al[9] emphasize the role of SRS in extended surgical treatment. Candela et al[10] emphasized the role of SRS and PET in the diagnosis of the presence of locoregional metastases in ANET patients. Although there are a lot of papers about application of somatostatin receptor scintigraphy in gastrointestinal neuroendocrine tumors, there are very rare sporadic cases described about ANETs particularly. Considering that theses tumors have specific symptoms and are discovered mainly accidentally, the aim of this paper was to draw more attention about appropriate management and particularly follow up of these tumors using radionuclide methods. The aim of this investigation is to estimate the role of SRS in the follow up of the patients operated for carcinoid of appendix.
The study is retrospective analysis of SRS scintigraphies in the patients with ANETs. Demographic and clinical characteristics of 35 studied patients are shown in Table 1. Majority of tumors have been found incidentally during surgery of: Acute appendicitis (n = 15), perforated appendicitis (n = 2), ileus (n = 3), hysterectomy (n = 3), ruptured ovarian cyst (n = 2), cercal volvulus (n = 1), while 9 patients had diagnosis of appendiceal tumor before the surgery. All the patients gave informed consent for the SRS investigation. The study was approved by Ethical committee of the Faculty of Medicine University of Belgrade.
| Characteristics | Value |
| Age (yr) | |
| Mean ± SD (range) | 43.7 ± 17.3 |
| Sex, n (%) | |
| Male | 12 (34.3) |
| Female | 23 (65.7) |
| Histopathology, n (%) | |
| Carcinoid of appendix | 34 (97.1) |
| Tubular carcinoid | 1 (2.9) |
| Initial clinical grade, n (%) | |
| G1 | 17 (48.6) |
| G2 | 12 (34.3) |
| G3 | 6 (17.1) |
| Initial clinical stage, n (%) | |
| IIa | 10 (28.57) |
| IIb | 6 (17.14) |
| IIIa | 6 (17.14) |
| IIIb | 7 (20.00) |
| IV | 6 (17.14) |
| Surgery, n (%) | |
| Appendectomy | 22 (62.9) |
| Right hemicolectomy | 13 (37.1) |
SRS was performed for follow-up of the patients after surgery because of ANET in the cases when the results of other imaging methods were not conclusive enough. Clinical assessment of majority of patients with ANETs during follow up after the surgery was performed in the intervals 6–12 months. Initially, laboratory diagnostics was performed following by ultrasound, computed tomography, magnetic resonance imaging (US, CT, MRI) as well as endoscopy. SRS findings were confirmed by surgery, biopsy and clinical follow up of 5 years. The histopathological diagnosis included immunohistochemistry of the tumor in regard to chromogranin A and the Ki-67 index.
Whole body scintigraphy was performed 2 h and 24 h after i.v. administration of 740 MBq of technetium-99m, ethylenediamine-N, N'-diacetic acid, hydrazinonicotinyl-Tyr3-Octreotide 99mTc-EDDA//HYNIC TOC (99mTc-Tektrotyd, Polatom), with ECAM gamma camera and computer, using high resolution collimator and one photopeak activity (140keV ± 20%). After whole body scintigraphy, single photon emission computed tomography of particular region was performed (360º orbit, step and shoot mode, 30 s/view). Computer matrix was 128 x 128. Reconstruction was done using filtered back-projection and iterative reconstruction. Patients were prepared with good hydration and mild laxatives. Therapy with somatostatin analogs was temporarily withdrawn. The images were evaluated and analysed by two nuclear medicine physicians. Increased focal uptake of tracer apart of physiological accumulation was considered a positive finding. Semiquantitative analysis was performed in some cases in order to compare the tumor uptake of radiopharmaceutical to non-tumor tissue.
The results were presented as mean ± standard deviation. Diagnostic performance of SRS was estimated by determination of sensitivity, specificity, positive and negative predictive values (PPV, NPV) and accuracy. Receiver operating characteristics (ROC) of scintigraphy was performed, and area under the curve (AUC) was calculated. Student t test was used to determine statistically significant difference between Ki 67 and chromogranin A (CgA) values in true positive and true negative patients. Progression-free survival was assessed by Kaplan Meier survival analyses. Statistical hypotheses were tested using statistical level of significance P < 0.05. IBM SPSS Statistics 20, Chicago Illinois program was used for statistical analysis.
Somatostatin receptor scintigraphy was performed for follow up the patients after surgery for ANETs. The SRS results were as follows: 12 true positives (TP), 19 true negative (TN), 3 false positive (FP) and 1 false negative (FN). Sensitivity was 92.31%, specificity was 86.36%, positive predictive value (PPV) was 80.00%, negative predictive value (NPV) was 95.00% and accuracy 88.57% (Table 2).
| Parameter | (%) | 95%CI |
| Sensitivity | 92.31 | 63.97-99.81 |
| Specificity | 86.36 | 65.09-97.09 |
| Positive predictive value | 80.00 | 58.01-92.05 |
| Negative predictive value | 95.00 | 74.15-99.21 |
| Accuracy | 88.57 | 73.26-96.80 |
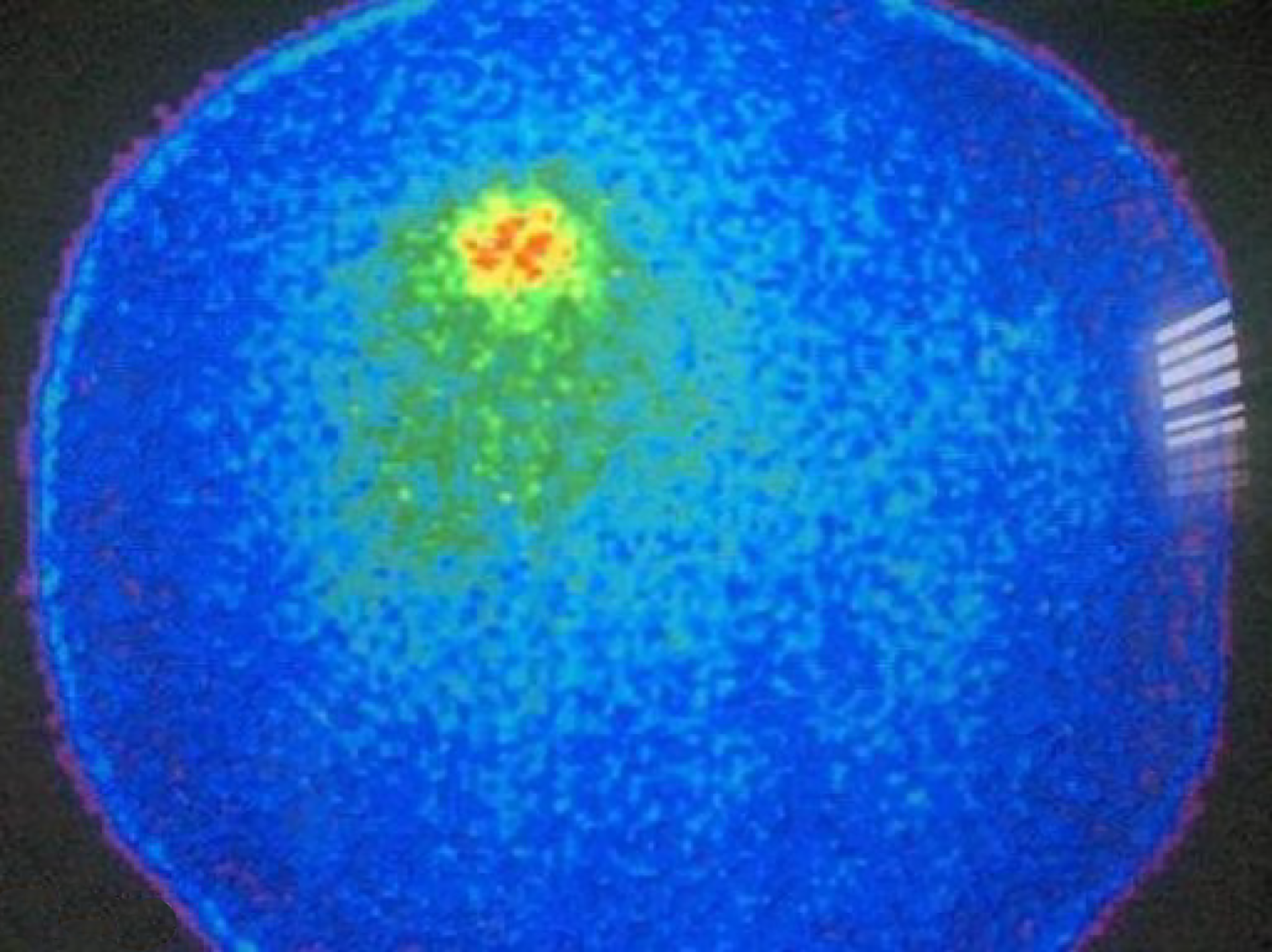
Analysis of Receiver Operating Characteristics (ROC) shows AUC of 0.850 [95% confidence interval (CI): 0.710-0.990, P < 001] (Figure 1). Single-photon emission computed tomography (SPECT) contributed diagnosis in 7 TP findings. In 10 patients Krenning score was 4 and in 2 was 3. The majority of our patients, n = 16 were stage II (a, b), smaller group was stage III while only 6 patients were stage IV. The best results, obviously very high negative predictive value (13 patients TN, without TP) were obtained in stage II (a, b), in spite of 3 FP findings mainly due to local inflammation. In 7 patients with stage III, there was an equal number of TN and TP findings (n = 3) with one FN due to very small size of the lesion. All the patients in stage IV were TP (very high positive predictive value). In 8/35 (22.9%) patients SRS significantly changed the management of the patients (in two surgery was repeated, in 4 somatostatin analogues and in two peptide receptor radionuclide therapy were performed, Figures 2 and 3). In 6 of them (6/35, 17.1%) tumor node metastasis (TNM) classification was corrected after SRS results. Distribution of SRS findings (TP, TN, FP, FN) according the stage of the disease are shown in Table 3.
| Stage | TP | TN | FP | FN | Total |
| IIa | 0 | 8 | 2 | 0 | 10 |
| IIb | 0 | 5 | 1 | 0 | 6 |
| IIIa | 0 | 6 | 0 | 0 | 6 |
| IIIb | 6 | 0 | 0 | 1 | 7 |
| IV | 6 | 0 | 0 | 0 | 6 |
| TOTAL | 12 | 19 | 3 | 1 | 35 |
Average Ki 67 index values for TP were 5.62% ± 3.17%, which was not significantly different (P < 0.05, the t-value is 0.83491, the P value is 0.206583) from Ki-67 index values in TN patients (3.54% ± 2.12%). Chromogranin A values for TP patients 5081 ± 2146 µg/L were significantly (P < 0.05, the t-value is 2.40933, the P value is 0.014193) higher in comparison to the values in TN patients 43.35 ± 16.92 µg/L.
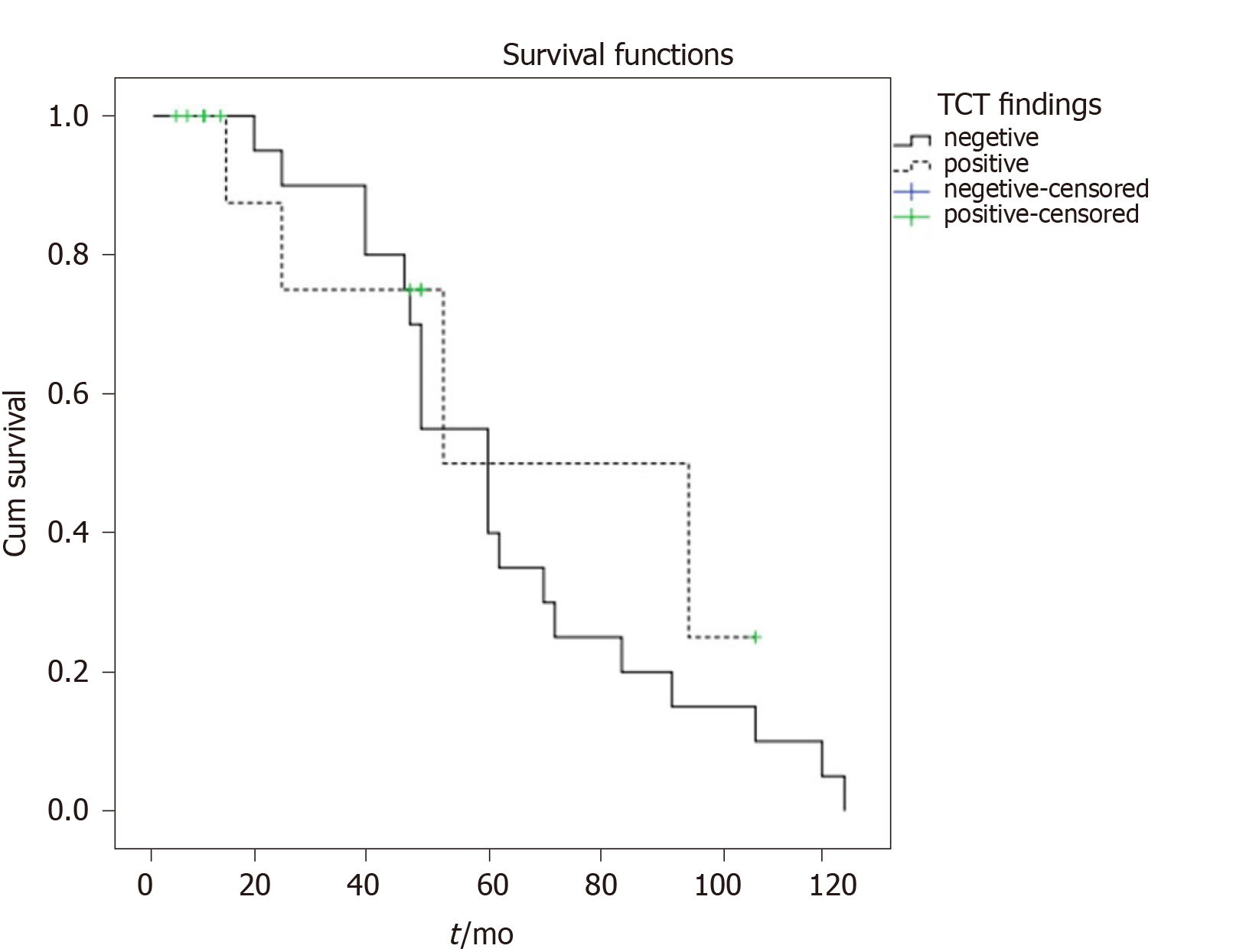
Median progression- free survival in SRS positive patients was 52 months (95%CI: 39.7-117.3) months while in SRS negative patients it was 60 months (95%CI: 42.8-77.1 months), without statistically significant difference between the two groups (P = 0.434) (Figure 4).
Our results proved high sensitivity, specificity, accuracy, as well as PPV and negative predictive value of SRS in the follow-up of ANETs. The additional value of SPECT, because of the increased resolution in comparison to planar images is confirmed in 20% of the patients. FP results were caused either by inflammation or by increased uptake of radiopharmaceutical on particular site caused by previous surgery. In the patient with FN finding, very small tumor below resolution of gamma camera was confirmed. Our results prove the value of SRS in follow-up of ANETs, if recurrences or metastases are suspected. The majority of our patients, n = 16 were stage II (a, b), smaller group (n = 13) was stage III while only 6 patients were stage IV. According to our results, number of TP are higher in advanced stages of the disease while number of TN was higher in lower stages. The obviously very high negative predictive value (13 patients TN, without TP) were obtained in stage II (a, b), in spite of 3 FP findings mainly due to local inflammation. In 7 patients with stage III, there was an equal number of TN and TP findings (n = 3) with one FN due to very small size of the lesion. All the patients in stage IV were TP (very high positive predictive value). Similar to our results Maxwell et al[11] in small bowel NETs, obtained that the SRS localizing group (TP) had a greater number of patients with multifocal disease, a greater number of lymph nodes excised at surgery, a higher lymph node ratio (number of positive lymph nodes divided by the total number of lymph nodes excised), and higher somatostatin receptor 2 expression compared to the nonlocalizing group (FN), although these differences were not significant. Likewise, according to van Adrichem et al[12] primary tumor site, disease stage and ENETS TNM classification were not significantly different between patients with negative and positive expression of somatostatin receptors. In 8/35 (22.9%) patients SRS significantly changed the management of the patients (in two surgery was repeated, in 4 somatostatin analogues and in two peptide receptor radionuclide therapy were performed) and is also valuable tool for the choice of therapy. In 6 of them (6/35, 17.1%) TNM classification was corrected after SRS results. Similar to our results, Lebtahi et al[13] proved that SRS results modified patient classification in 24% of the cases, while surgical therapeutic strategy was changed in 25%. In all SRS positive patients, Krenning score was very high.
According to Bednarczuk et al[14], SRS is more sensitive than radiological methods with the sensitivity around 80% for the detection of the primary tumor site. Investigations with positron emitting radiopharmaceuticals [Gallium-68(68Ga)-peptides] are the preferred imaging method, particularly if the lesions are smaller than 1 cm[4]. For localization of primary tumor and assess the disease stage, SRS in addition to CT and MRI is recommended, but in the case of incomplete surgery of the ANET or if distant metastases are suspected, SRS should be performed[14]. According to other investigations sensitivity, specificity, accuracy, positive and negative predictive values of 99mTc- SRS were; 96%, 100%, 97%, 100% and 94% respectively, in NENs in general, with high negative predictive value in ANET[15], which is in concordance with our results. Likewise, a lot of SRS investigations showed high sensitivity for gastroenteropancreatic tumors, from 80% to 90%. However, sensitivities for metastatic disease is even higher, and in many studies, Spallitta et al[8] recommend SRS in all the patients with ANEN after the surgery, after US and CT examination as well as Spalitta et al[8] as well as other authors[16]. Hoegerle et al[17] concluded that there are some potential pitfalls for SRS soon after surgery as well as Fornaro et al[18]. Namely, they stated that there is a possibility of FP results, which is in concordance with our results. SRS could detect lesions not seen with radiology imaging modalities[19], thus influencing on the further patient management like in our study. In the case of high expression of somatostatin receptors, even during somatostatin analogue treatment, radionuclide therapy with somatostatin analogues should be considered as a first line treatment[20]. However, in patients with negative SRS and evidence of metaiodobenzylguanidine labelled with iodine-131 (131I-MIBG) accumulation in the tumor or metastases, therapy with 131I-MIBG should be considered.
Our results prove that Ki-67 index was not very high in majority of the patients and that it was in concordance with the number of positive findings, although there were no significant differences between TP and TN patients (P > 0.05), which was confirmed by the results of other authors[12]. However, this indicator can be valuable tool in diagnosis and prediction of prognosis in goblet cell carcinomas, which are not considered as ANET in the newest ENETs classification[21,22].
In our study, CgA was significantly increased in TP in comparison to TN patients (P < 0.05). The value of CgA was particularly increased in 4 patients which can be considered as an indicator for poor prognosis[5-7]. Perakakis et al[23] emphasized the role of PET/CT with 18F-fluoro-L-dihydroxyphenylalanine for diagnosis of adreno cortico tropic hormone (ACTH) secreting ANET. It was proved that SRS was more sensitive than CgA in diagnosis of ectopic ACTH-syndrome due to a ANET with equivalent specificity[24]. Moreover, SRS and CgA are recommended as useful methods in the diagnostic approach of NET patients[24] and carcinoid patients[25]. Similar to our results, other authors suggested that serum CgA is useful indicator for the diagnosis and follow-up of gastrointestinal NETs, while radionuclide imaging contributes to the more precise localization of the primary tumors and metastases, as well as, to the appropriate medical treatment[16]. According to Stokkel et al[26] who also emphasized the higher sensitivity of SRS in comparison to CgA in staging and follow up of well-differentiated NETs, both methods should be used at the initial stage while disease spread, symptoms, and metastasis have an influence on both SRS results and CgA values. However, the results of van Adrichem et al[12] point out that highest serum CgA level was not significantly different between patients with negative and positive SRS findings.
Median progression- free survival in SRS positive patients was 52 months, in SRS negative patients it was 60 months without statistically significant difference between the two groups (P = 0.434). ANETs have a good prognosis, meaning that survival after 5-years is 85.9%-100%[7] which is in accordance with our results. Modlin et al[27] concluded that patients with local disease survive 5 years in 92% of cases, those with regional metastases 81% and the few with distant metastases of 31%[27] while similar results (94%, 85% and 34%). Some authors obtained the 7-year survival rate of 100%[8,28]. This is in accordance with our results considering grade and stage of our investigated patients. Similar to our findings, SRS nor the Krenning score in SRS in general did not relate significantly to progression-free survival[29] nor can be used as prognostic markers.
Bearing in mind that still the ideal radiopharmaceutical for scintigraphic diagnosis of NETs has not been discovered, there are a lot of them under investigation[30-32] such as: 99mTc-EDDA--tricine-HYNIC-NATE, 99mTc-EDDA/HYNIC-Tyrosine3-octreotate,
Our results point out that SRS with 99mTc-Tektrotyd is useful for follow up of the patients after surgery of ANETs, and that the results influence significantly to the change in TNM classification as well as the further management of the patients. SPECT and estimation of Krenning score had important role in diagnosis. SRS is also valuable tool for the choice of therapy (surgery, somatostatin analogues or peptide receptor radionuclide therapy). If PET/CT with 68Ga-labeled peptides cannot be performed, the special emphasize should be given to hybrid SPECT/CT imaging and to the radioguided surgery. In spite of being a reliable, noninvasive technique for detection of locoregional or distant metastases, it cannot be used as an ANET predictive technique. Although there are not many data in the literature dealing particularly with ANETs, considering that these tumors have specific symptoms and are discovered mainly accidentally, in the emergency conditions, the aim of this paper was to draw more attention about due time and appropriate management and particularly follow up of this tumors using radionuclide methods.
Neuroendocrine tumors of appendix (ANETs) known as carcinoids, are rare endocrine neoplasms originated from enterochromaffin cells of gastrointestinal tract. Over half of the ANET discovered accidentally following appendectomy are the most often at the early stage, implicating high survival rate. Symptoms typical for carcinoid syndrome are detected in approximately 20%–30% of patients with tumors usually with distant metastases. For the diagnosis of the neuroendocrine neoplasms of appendix, besides biochemical analyses, different imaging methods and histopathology analyses with immunohistochemical staining, we could use somatostatin receptor scintigraphy (SRS) or positron emission tomography with computed tomography (PET/CT). Surgery represent the first-line therapeutic option while in patients with advanced disease can be considered long-acting somatostatin analogues, targeted therapies (everolimus) or peptide receptor radionuclide therapy.
Although there are a lot of papers about application of somatostatin receptor scintigraphy in gastrointestinal neuroendocrine tumors, there are very rare sporadic cases described about ANETs particularly. Considering that these tumors have specific symptoms and are discovered mainly accidentally, the aim of this paper was to draw more attention about appropriate management and particularly follow up of this tumors using radionuclide methods.
The aim of this investigation is to estimate the role of SRS in the follow up of the patients operated for carcinoid of appendix.
The total of 35 patients was investigated, 23 females and 12 males, average age (43.7 ± 17.3 years). All patients had histological diagnosis of ANET (34 carcinoids of appendix and one tubular carcinoid). Majority of tumors have been found incidentally during surgery of: Acute appendicitis (n = 15), perforated appendicitis (n = 2), ileus (n = 3), hysterectomy (n = 3), ruptured ovarian cyst (n = 2), caecal volvulus (n = 1), while 9 patients had diagnosis of appendiceal tumor before the surgery. Seventeen patients had tumor grade (G) G1, 12 G2 and 6 G3. The right hemicolectomy was performed in 13, while the rest of the patients had appendectomy only. SRS was done early (2h) and late (24h) after i.v. application of 740 MBq technetium-99m ethylenediamine-N, N'-diacetic acid Hydrazinonicotinyl-Tyr3-Octreotide (technetium-99m-Tektrotyd, Polatom, Poland). SRS was performed for restaging in all the patients after surgery.
There were 12 true positive (TP), 19 true negative, 3 false positive and 1 false negative SRS result. Sensitivity of the method was 92.31%, specificity was 86.36%, positive predictive value was 80.00%, negative predictive value was 95.00% and accuracy 88.57%. Receiver Operating Characteristics analysis showed that SRS scintigraphy is a good test for detection TP cases (area Under the Curve of 0.850, 95% confidence interval/CI: 0.710-0.990, P < 001). Single-photon emission computed tomography (SPECT) contributed diagnosis in 7 TP findings. In 10 patients Krenning score was 4 and in 2 was 3. In 8 patients SRS significantly changed the management of the patients (in two surgery was repeated, in 4 somatostatin analogues and in two peptide receptor radionuclide therapy). Median progression-free survival in SRS positive patients was 52 months (95%CI: 39.7-117.3) while in SRS negative patients it was 60 months (95%CI: 42.8-77.1), without statistically significant difference between the two groups (P = 0.434).
Our results point out that SRS with 99mTc-Tektrotyd is useful for follow up of the patients after surgery of ANETs, and that the results influence significantly to the change in tumor node metastasis classification as well as the further management of the patients. SPECT and estimation of Krenning score had important role in diagnosis. SRS is also valuable tool for the choice of therapy (surgery, somatostatin analogues or peptide receptor radionuclide therapy). If PET/CT with 68Ga-labeled peptides cannot be performed, the special emphasize should be given to hybrid SPECT/CT imaging and to the radioguided surgery. In spite of being a reliable, noninvasive technique for detection of locoregional or distant metastases, it cannot be used as an ANET predictive technique. Although there are not many data in the literature dealing particularly with ANETs, considering that these tumors have specific symptoms and are discovered mainly accidentally, in the emergency conditions, the aim of this paper was to draw more attention about due time and appropriate management and particularly follow up of tumors using radionuclide methods.
The ideal radiopharmaceutical for scintigraphic diagnosis of NETs has not been discovered, there are a lot of them under investigation. Wider application of hybrid systems (SPECT/CT, SPECT/magnetic resonance imaging) as well as new cadmium-zinc-telluride SPECT and SPECT/CT cameras increased and widened application and increased the accuracy of somatostatin receptor scintigraphy. These radiopharmaceuticals can also be used for radio-guided surgery thus increasing sensitivity and specificity of the method. (18 F)-Fluoro-2-deoxy-D-glucose PET/CT is recommended for detecting of low differentiated or heterogeneous neuroendocrine tumors. Recently, positron emitting radiopharmaceuticals are preffered, such as 68Ga labeled peptides or 18F-fluorodopamine. These radiopharmaceuticals as well as PET/CT provide superior resolution, faster investigation, shorter imaging time and visualization in three dimensions. However, because of their price and availability their application is still not wide enough.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Association of Nuclear Medicine, No. 12531.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang WY, Larentzakis A S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Hofland J, Kaltsas G, de Herder WW. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr Rev. 2020;41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 2. | Morais C, Silva E, Nuno Brandão P, Correia R, Foreid S, and Vítor Valente. Neuroendocrine tumor of the appendix—a case report and review of the literature. J Surg Case Rep. 2019;2019:rjz086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Moris D, Tsilimigras DI, Vagios S, Ntanasis-Stathopoulos I, Karachaliou GS, Papalampros A, Alexandrou A, Blazer DG, Felekouras E. Neuroendocrine Neoplasms of the Appendix: A Review of the Literature. Anticancer Res. 2018;38:601-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, Krenning E, Reed N, O'Toole D; Vienna Consensus Conference participants. ENETS Consensus Guidelines for Neuroendocrine Neoplasms of the Appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology. 2016;103:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Bolanowski M, Bednarczuk T, Bobek-Billewicz B, Handkiewicz-Junak D, Jeziorski A, Nowakowska-Duława E, Steinhof-Radwańska K, Zajęcki W, Zemczak A, Kos-Kudła B, Consensus Conference; Polish Network of Neuroendocrine Tumours. Neuroendocrine neoplasms of the small intestine and the appendix - management guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2013;64:480-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Mullen JT, Savarese DM. Carcinoid tumors of the appendix: a population-based study. J Surg Oncol. 2011;104:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26:791-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Spallitta SI, Termine G, Stella M, Calistro V, Marozzi P. [Carcinoid of the appendix. A case report]. Minerva Chir. 2000;55:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Safioleas MC, Moulakakis KG, Kontzoglou K, Stamoulis J, Nikou GC, Toubanakis C, Lygidakis NJ. Carcinoid tumors of the appendix. Prognostic factors and evaluation of indications for right hemicolectomy. Hepatogastroenterology. 2005;52:123-127. [PubMed] [Cited in This Article: ] |
| 10. | Candela G, Varriale S, Di Libero L, Giordano M, Maschio A, Manetta F, Borrelli V, Nunziata A, Santini L. Carcinoid of the vermiform appendix. Description of three clinical cases and review of the literature. Minerva Chir. 2006;61:265-272. [PubMed] [Cited in This Article: ] |
| 11. | Maxwell JE, Sherman SK, Menda Y, Wang D, O'Dorisio TM, Howe JR. Limitations of somatostatin scintigraphy in primary small bowel neuroendocrine tumors. J Surg Res. 2014;190:548-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | van Adrichem RC, Kamp K, van Deurzen CH, Biermann K, Feelders RA, Franssen GJ, Kwekkeboom DJ, Hofland LJ, de Herder WW. Is There an Additional Value of Using Somatostatin Receptor Subtype 2a Immunohistochemistry Compared to Somatostatin Receptor Scintigraphy Uptake in Predicting Gastroenteropancreatic Neuroendocrine Tumor Response? Neuroendocrinology. 2016;103:560-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, Mignon M, le Guludec D. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853-858. [PubMed] [Cited in This Article: ] |
| 14. | Bednarczuk T, Bolanowski M, Zemczak A, Bałdys-Waligórska A, Blicharz-Dorniak J, Boratyn-Nowicka A, Borowska M, Cichocki A, Ćwikła JB, Falconi M, Foltyn W, Handkiewicz-Junak D, Hubalewska-Dydejczyk A, Jarząb B, Junik R, Kajdaniuk D, Kamiński G, Kolasińska-Ćwikła A, Kowalska A, Król R, Królicki L, Kunikowska J, Kuśnierz K, Lampe P, Lange D, Lewczuk-Myślicka A, Lewiński A, Lipiński M, Londzin-Olesik M, Marek B, Nasierowska-Guttmejer A, Nowakowska-Duława E, Pałucki J, Pilch-Kowalczyk J, Rosiek V, Ruchała M, Siemińska L, Sowa-Staszczak A, Starzyńska T, Steinhof-Radwańska K, Strzelczyk J, Sworczak K, Syrenicz A, Szawłowski A, Szczepkowski M, Wachuła E, Zajęcki W, Zgliczyński W, Kos-Kudła B. Neuroendocrine neoplasms of the small intestine and appendix - management guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2017;68:223-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Al Bulushi N, Al Suqri B, Al Aamri M, Al Hadidi A, Al Jahdami H, Al Zadjali M, Al Risi M. Diagnostic accuracy of technetium-99m-octreotide in imaging neuroendocrine tumors, Oman hospital experience with literature review. World J Nucl Med. 2019;18:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Nikou GC, Lygidakis NJ, Toubanakis C, Pavlatos S, Tseleni-Balafouta S, Giannatou E, Mallas E, Safioleas M. Current diagnosis and treatment of gastrointestinal carcinoids in a series of 101 patients: the significance of serum chromogranin-A, somatostatin receptor scintigraphy and somatostatin analogues. Hepatogastroenterology. 2005;52:731-741. [PubMed] [Cited in This Article: ] |
| 17. | Hoegerle S, Nitzsche EU, Stumpf A, Simon GH, Otte A, Schwarzkopf G, Moer E. Incidental appendix carcinoid. Value of somatostatin receptor imaging. Clin Nucl Med. 1997;22:467-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Fornaro R, Frascio M, Sticchi C, De Salvo L, Stabilini C, Mandolfino F, Ricci B, Gianetta E. Appendectomy or right hemicolectomy in the treatment of appendiceal carcinoid tumors? Tumori. 2007;93:587-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Carrasquillo JA, Chen CC. Molecular imaging of neuroendocrine tumors. Semin Oncol. 2010;37:662-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1702] [Cited by in F6Publishing: 1854] [Article Influence: 264.9] [Reference Citation Analysis (0)] |
| 21. | Toumpanakis C, Standish RA, Baishnab E, Winslet MC, Caplin ME. Goblet cell carcinoid tumors (adenocarcinoid) of the appendix. Dis Colon Rectum. 2007;50:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Holt N, Grønbæk H. Goblet cell carcinoids of the appendix. ScientificWorldJournal. 2013;2013:543696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Perakakis N, Laubner K, Keck T, Steffl D, Lausch M, Meyer PT, Burger D, Csanadi A, Seufert J. Ectopic ACTH-syndrome due to a neuroendocrine tumour of the appendix. Exp Clin Endocrinol Diabetes. 2011;119:525-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Cimitan M, Buonadonna A, Cannizzaro R, Canzonieri V, Borsatti E, Ruffo R, De Apollonia L. Somatostatin receptor scintigraphy versus chromogranin A assay in the management of patients with neuroendocrine tumors of different types: clinical role. Ann Oncol. 2003;14:1135-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Namwongprom S, Wong FC, Tateishi U, Kim EE, Boonyaprapa S. Correlation of chromogranin A levels and somatostatin receptor scintigraphy findings in the evaluation of metastases in carcinoid tumors. Ann Nucl Med. 2008;22:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Stokkel MP, Rietbergen DD, Korse CM, Taal BG. Somatostatin receptor scintigraphy and chromogranin A assay in staging and follow-up of patients with well-differentiated neuroendocrine tumors. Nucl Med Commun. 2011;32:731-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Modlin IM, Drozdov I, Alaimo D, Callahan S, Teixiera N, Bodei L, Kidd M. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr Relat Cancer. 2014;21:615-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Nandakumar G. Evidence Based Practices in Gastrointestinal and Hepatobiliary Surgery. New York, The Health Science Publisher, 2017: 665. [Cited in This Article: ] |
| 29. | Boutsikou E, Porpodis K, Chatzipavlidou V, Hardavella G, Gerasimou G, Domvri K, Papadopoulos N, Avramidou V, Spyratos D, Kontakiotis T, Zarogoulidis K. Predictive Value of 99MTC-hynic-toc Scintigraphy in Lung Neuroendocrine Tumor Diagnosis. Technol Cancer Res Treat. 2019;18:1533033819842586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Artiko V, Afgan A, Petrović J, Radović B, Petrović N, Vlajković M, Šobić-Šaranović D, Obradović V. Evaluation of neuroendocrine tumors with 99mTc-EDDA/HYNIC TOC. Nucl Med Rev Cent East Eur. 2016;19:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Artiko V, Sobic-Saranovic D, Pavlovic S, Petrovic M, Zuvela M, Antic A, Matic S, Odalovic S, Petrovic N, Milovanovic A, Obradovic V. The clinical value of scintigraphy of neuroendocrine tumors using (99m)Tc-HYNIC-TOC. J BUON. 2012;17:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Sobic-Saranovic DP, Pavlovic SV, Artiko VM, Saranovic DZ, Jaksic ED, Subotic D, Nagorni-Obradovic L, Kozarevic N, Petrovic N, Grozdic IT, Obradovic VB. The utility of two somatostatin analog radiopharmaceuticals in assessment of radiologically indeterminate pulmonary lesions. Clin Nucl Med. 2012;37:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Signore A, Artiko V. Hybrid fusion images in diagnostic and therapeutic procedures. Q J Nucl Med Mol Imaging. 2018;62:1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Peters A, Kumar J, Patil PV. Diagnostic implications of CZT SPECT and impact of CT attenuation correction. J Nucl Cardiol. 2019;26:246-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Cuccurullo V, Di Stasio GD, Mansi L. Radioguided surgery with radiolabeled somatostatin analogs: not only in GEP-NETs. Nucl Med Rev Cent East Eur. 2017;20:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Pollard J, McNeely P, Menda Y. Nuclear Imaging of Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020;29:209-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Danti G, Berti V, Abenavoli E, Briganti V, Linguanti F, Mungai F, Pradella S, Miele V. Diagnostic imaging of typical lung carcinoids: relationship between MDCT, 111In-Octreoscan and 18F-FDG-PET imaging features with Ki-67 index. Radiol Med. 2020;125:715-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Werner RA, Bengel FM, Derlin T. [Theranostics and hybrid imaging for somatostatin receptor-expressing tumors]. Radiologe. 2020;60:413-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |