Published online Apr 6, 2019. doi: 10.12998/wjcc.v7.i7.819
Peer-review started: December 29, 2018
First decision: January 30, 2019
Revised: February 26, 2019
Accepted: March 11, 2019
Article in press: March 11, 2019
Published online: April 6, 2019
Porphyromonas gingivalis (P. gingivalis) is an anaerobic gram-negative bacterium that colonizes in the epithelium and has been strongly associated with periodontal disease. Recently, various degrees of associations between P. gingivalis and digestive system cancers, including oral squamous cell carcinoma in the oral cavity, oesophageal squamous carcinoma in the digestive tract, and pancreatic cancer in pancreatic tissues, have been displayed in multiple clinical and experimental studies. Since P. gingivalis has a strong association with periodontal diseases, not only the relationships between P. gingivalis and digestive system tumours but also the effects induced by periodontal diseases on cancers are well-illustrated in this review. In addition, the prevention and possible treatments for these digestive system tumours induced by P. gingivalis infection are also included in this review. At the end, we also highlighted the possible mechanisms of cancers caused by P. gingivalis. One important carcinogenic effect of P. gingivalis is inhibiting the apoptosis of epithelial cells, which also plays an intrinsic role in protecting cancerous cells. Some signalling pathways activated by P. gingivalis are involved in cell apoptosis, tumourigenesis, immune evasion and cell invasion of tumour cells. In addition, metabolism of potentially carcinogenic substances caused by P. gingivalis is also one of the connections between this bacterium and cancers.
Core tip:Porphyromonas gingivalis (P. gingivalis) has been discovered in many digestive system cancers, such as oral squamous cell carcinoma, oesophageal squamous cell carcinoma, and pancreatic cancer. For the strong association between P. gingivalis and periodontal diseases, we also interpret how periodontal diseases push effects on digestive system tumours. This review also presents some preventions and possible treatments for these cancers associated with P. gingivalis infection. In addition, the mechanisms by which P. gingivalis affects the occurrence and development of carcinomas are covered, including immune evasion, tumourigenesis, inhibition of apoptosis and invasion of tumour cells.
- Citation: Zhou Y, Luo GH. Porphyromonas gingivalis and digestive system cancers. World J Clin Cases 2019; 7(7): 819-829
- URL: https://www.wjgnet.com/2307-8960/full/v7/i7/819.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i7.819
Porphyromonas gingivalis (P. gingivalis), one of several hundred bacterial species in the oral cavity, is an anaerobic gram-negative bacterium[1] and is highly associated with periodontal diseases[2]. Dental plaque is a biofilm on the surface of teeth[3] and functions as a highly organized and integrated microbial community in the mouth[4]. P. gingivalis is part of the composition of dental plaque and endows dental plaque with a panoply of critical virulence attributes[5]. This bacterium regulates the immune reaction of host to promote the proliferation of microbial biofilm, thereby destroying the homeostasis of the host and causing biological disorders and disease[6]. P. gingivalis can be found in the gingival epithelium[7]. To effectively colonize, P. gingivalis secretes protein adhesins, hemin‐binding proteins and proteinases. Such factors could cause toxicity as well[8-10]. Arg-specific gingipains (RgpA and RgpB) and Lys-specific gingipain (Kgp) are cysteine proteinases, which are extracellular proteases with multiple effects on both the innate and adaptive immune responses[11,12]. These cysteine proteinases are capable of degrading extensive connective tissues and large host defence molecules[13,14]. The R-gingipains (Rgp) and Kgp are demonstrated highly related to the relief of inflammation and host defence evasion by means of activating kinin cascade and liquid phase transformation of complement C3 and C5 proteins[15,16]. Apart from these defences, some other factors, such as fimbriae, haemagglutinins, lipopolysaccharide, capsule polysaccharides, and major outer membrane protein, have also been demonstrated for potential virulence[17,18]. Recently, an increasing number of investigations have focused on cancers related to P. gingivalis. With the deepening of the research concerning this bacterium, outer membrane protein has been reported to make contributions to the interaction and colonization of host, the evasion of immune defense, as well as the destruction of periodontal tissues[19], which may explain how P. gingivalis pushes impacts on several digestive cancers. However, more and complicated internal mechanisms still need to be uncovered. This article aims to review the current relationship between P. gingivalis and cancer.
Oral cavity consists of diverse boundary surfaces, such as the gingiva, the back of the tongue, tongue side, oral mucosa and so on which contribute to the colonization and growth of various microorganisms. In 2016, accompanying an approximate 48330 new diagnoses and 9570 deaths, oral cancer was considered the sixth most common carcinoma globally[20]. Among these, what most commonly happened in oral cavity is oral squamous cell carcinoma (OSCC)[21]. P. gingivalis has been discovered colonizing in periodontium and spreading in the original lesion locations of OSCC as well, such as oral and lingual mucosa[22]. It is well known that tobacco and alcohol are the major risk factors for oral cancer, and approximately three-fourths of all oral and pharyngeal cancers are owing to tobacco smoking and alcohol drinking in the United States[23]. Upon alcohol drinking, P. gingivalis would dehydrogenate ethanol to acetaldehyde, which is a carcinogenic derivative and capable of contributing to DNA damage, mutation and excessive proliferation of the epithelium[24,25].
The concentration rate of P. gingivalis was discovered to be higher among cancer cells than normal tissues of the mouth by Katz et al[26] in 2011. Meanwhile, these authors suggested that P. gingivalis may lead to mouth cancer and the induction of epithelial cell transformation into a tumour. In a meta-analysis, the prevalence of P. gingivalis was 40.7%, and P. gingivalis made the chances of cancer and periodontal disease increasing 1.36 times[27]. Kang et al[28]’s study also showed that the incidence of P. gingivalis in patients with head and neck cancer and in healthy subjects is significantly different. Besides, compared to non-infected with P. gingivalis controls, those with long-term infection showed the up-regulating expressions of CD44 and CD133 which are the well-known cancer stem cell markers, and promoted the tumorigenic properties[29]. By activating ERK1/2-Ets1, p38/HSP27, and PAR2/NF-kB pathways, P. gingivalis increases pro-matrix metalloproteinase-9 (proMMP-9) levels and consequently promotes cellular invasion of OSCC cell lines when the proenzyme is stimulated by gingipains[30]. Comparing to uninfected cells, those infected with P. gingivalis cells were monitored to increase MMP-1, -2, -9 and -10 levels in time dependence[31]. In addition, activated MMP-9 has been illustrated to enhance the invasion of tumour cells; this study was performed with the help of a specific anti-MMP-9 blocking monoclonal antibody[32]. As Ha et al[33]’s findings also suggested, P. gingivalis infection makes significant functions in promoting the invasion of OSCC cells, including SCC-25, OSC-20 and SAS cells, through upregulating levels of IL-8 and MMPs (particularly MMP-1 and MMP-2). By establishing a long-term model in which human immortalized oral epithelial cells were chronically challenged with P. gingivalis for up to 23 wk, Geng et al[34] found that long-term exposure to P. gingivalis accelerated the cell cycle and promoted cell migration as well as invasion abilities, both of which eventually induced metastatic proliferation in distant organs. Compared with non-infected P. gingivalis in mice, it was demonstrated that chronic infection would make the promotion of metastasis via the blood pathway in vivo[21].
On a global scale, oesophageal cancer, the eighth incidence of tumour also contributes to the sixth highest mortality. Asia, especially Central China, is a high-incidence area, whereas data recently revealed that newly-diagnosed cases are rising in Western Europe and America[35]. Oesophageal cancer is characteristic of difficulty in early diagnosis, rapid development and high mortality. Tobacco smoking and excess alcohol consumption are risk factors for oesophageal cancer[36]. This cancer has two major histological subtypes, squamous cell carcinoma and adenocarcinoma; the former is common in developing countries and the latter is more common in developed nations[37]. In this review, oesophageal squamous cell carcinoma (ESCC) is the focus. The similarity in histology between oesophageal squamous cells and oral squamous cells is well-known, and it has been revealed that there is a high frequency of P. gingivalis (61%) in ESCC[36]. In this study, the expression intensity of P. gingivalis antigen and the exclusive Lys-gingipain protease, as well as the detection of the P. gingivalis-specific 16S rDNA in ESCC patients, all of them were notably higher in tumour tissue than in ambient tissue or normal controls. In addition, the differentiation of tumour cells, the distant metastasis of ESCC, the survival ratio of ESCC patients and other clinicopathological factors all positively relate to the existence of P. gingivalis. Such conclusions not only firstly proved that infection with P. gingivalis would act as a new risk factor of ESCC, but also indicated that P. gingivalis could serve as a prognostic biomarker for ESCC[36]. The inhibition of epithelial cell apoptosis, the promotion of tumour cell immune invasion and the metabolism of potentially carcinogenic substances induced by P. gingivalis all contribute to the occurrence and development of ESCC[12,24,25,32,38,39]. Gao et al[40] firstly reported the implication between host immunologic reaction to P. gingivalis and malignant proliferation of ESCC cells. They also suggested that IgG and IgA for P. gingivalis played a role as possible serum markers of ESCC, and combining the both would advance the diagnosis and improve prognosis[40]. Finally, interestingly, the rate of infection of P. gingivalis is much higher in oesophageal cancers than in cardiac cancers and is approximately as low as zero in gastric cancer, which is due to lack of acid adaptation[41].
Pancreatic cancer, which has a low frequency, is unexpectedly the fourth leading cause of death among various carcinomas, with an overall five-year survival rate of merely 8.2% (in 2007–2013) for all stages[42,43]. There are several factors leading to a poor prognostic performance of this devastating cancer, such as the deficiency of serum biomarkers for diagnosis and prognosis, the absence of biomarkers which could function as guidelines for individual therapy, and the broad and primary resistance to chemotherapy[43]. The bacterial colony is critical to developing gastrointestinal mucosal immune system, maintaining a normal physiological circumstance, and providing necessary nourishment, which has been of significance in various disease states[44,45]. Multiple observations have shown that there is an overlap between oral microbiota and digestive tract microbiota partly due to mastication and daily oral hygiene, such as tooth brushing and flossing[42], which promotes multiple avenues for the dissemination of dysbiosis[44]. Furthermore, poor oral health is linked with an increased risk of pancreatic cancer development[46]. Those who suffer from 2 types of periodontitis caused by oral microorganism tend to be subsequent at higher risks of increasing the occurrence of pancreatic cancer[47]. A large European prospective cohort study found that elevated serum antibodies to the ATTC 53978 strain of P. gingivalis could triple the risks of pancreatic carcinoma[48]. As Dr Miller recently demonstrated, P. gingivalis initiated the Toll-like receptor (TLR) signalling pathways[49], while TLR activation critically contributed to pancreatic carcinogenesis in animal models[50]. This result was considered direct evidence that P. gingivalis increases pancreatic cancer development. Additionally, high rates of tumour suppressor gene p53 mutations were detected in pancreatic cancer patients, and it was concluded that abnormality of the p53 gene is a significant event in human pancreatic tumourigenesis[51]. In addition, in the progression of P. gingivalis inhibiting epithelial cell apoptosis, p53 can be activated by P. gingivalis invasion[52,53]. As a result, mutations in p53 can play a role as a bridge that links P. gingivalis with the development of pancreatic cancer, but further investigations are needed to enrich this link.
In summary, P. gingivalis may be considered a biomarker in the occurrence and development of pancreatic cancer. It is urgent to conduct more research to explain the mechanism by which P. gingivalis acts on pancreatic cancer and to improve the prevention and treatment of pancreatic cancer. Through these studies, it is hopeful to contain the high mortality of pancreatic cancer and release the tense status quo in pancreatic cancer.
Periodontal disease is an inflammation that occurs in gums, which could induce the recession of gum, the damage of soft tissue, and the loss of bone and tooth (severe periodontitis)[54]. The identification of the “red complex”, which consists of Tannerella forsythia, P. gingivalis and Treponema denticola, is a major milestone in the research of periodontal microbiology. In terms of esophageal adenocarcinoma risk, Tannerella forsythia has to be found increasing this risk, while the exhaustion of Neisseria and Streptococcus pneumoniae was related to lower risk, and the abundance of P. gingivalis tended to increase the risk of ESCC[55]. Among these bacterial species, P. gingivalis shows the strongest association with periodontitis[56]. Additionally, P. gingivalis acts as an independent risk factor for the above-mentioned cancers. Therefore, we further discuss the relationship between periodontitis and these cancers. Substantial investigations have accumulated evidence supporting that periodontitis causes the host maintain a chronic inflammatory state[57] and confirming that cancer is associated with chronic infections or inflammation[58]. Taking the receptor to advanced glycation end products (RAGE) as an example, which is a multi-ligand receptor that can express on numerous cytomembrane and link with chronic infections, has been suggested to play a role in carcinogenesis[59]. Consequently, we can conclude that periodontitis has direct effects on cancers. As a prospective cohort study revealed, periodontal disease might play the role of being a marker for a susceptible immune system or having a direct impact on cancer risk[60]. Some others also suggested that there are positive connections between periodontitis and several tumours, including pancreatic carcinoma, lung tumours and digestive cancers[61,62]. Tezal et al[63] concluded that chronic periodontal disease could function as a risk factor for oral premalignant lesions and cancers. OSCC patients are highly sensitive to chronic periodontitis, which suggests the existence of OSCC cells in a chronic inflammatory state. Since periodontitis is attributed to multiple bacterial pathogens, OSCC patients infected with such inflammation are in consistent exposure to excessive periodontal bacteria[64,65]. These bacterial pathogens may subsequently affect the biological behaviour of oral cancer by modulating inflammatory mediators and invading related molecules[31]. Chronic periodontitis not only affects the occurrence and development of oral cancer but also contributes to the metastasis of this cancer[29,31]. The relationship between periodontitis and pancreatic cancer has also been revealed from a great deal of research. An evidence-based review showed that inflammation plays a key role in pancreatic cancer[66], and in a prospective cohort study of male health, professionals confirmed the strong positive association between periodontal disease and pancreatic cancer. In this cohort study, the existence of periodontal disease increases the risk of pancreatic cancer by 64%[46]. The systemic dissemination of oral microorganism and their toxic substance may also cause systemic inflammation and immune reaction, which could trigger the incidence of pancreatic cancer[66-68]. However, the role of oral microbiota in the development of pancreatic cancer is still under study. As mentioned in a former section of this review, periodontal disease could induce the loss of a tooth[54]. A prospective study also suggested that tooth loss has increased the incidence of esophageal and gastric cancers, which may owe to the alterations of oral microflora and subsequent increased carcinogens in vivo[69,70]. As a result, periodontal disease is connected with such tumours. In addition, the risk of lung tumour has been increased for those who suffering from periodontal disease in cohort studies, which may be because of the aspiration of oral pathogens into the lungs, the modification of the mucosal surface, the destruction of salivary pellicles, and the alteration of the respiratory epithelium[71]. Periodontal disease may establish more connections with other cancers, such as bladder cancer, colorectal cancer and so on, but a large amount of research is needed to verify this connection.
The prevention and treatment to those digestive cancers are specific and complicated for the existence of P. gingivalis. As a result, the intervention strategies are separate to antimicrobial therapy[72] and tumour site[73], but which both devote to thoroughly extracting carcinomas and managing good prognosis.
For that P. gingivalis owns the characteristics of high genetic capability, this bacterium could resist any adverse environment that hindering its development; P. gingivalis could also be capable of resisting various antibiotics that in use currently. In addition, the results of Deshpande et al[74]’s study indicated that P. gingivalis can actively invade endothelial cells with the help of fimbriae. Because of the specificity of this bacterium, the cancer treatment related to P. gingivalis is more directional and targeted. First, screening for P. gingivalis in dental plaques may identify susceptible subjects, which could help decrease the occurrence of cancers. Second, we can improve oral hygiene to reduce the risk of cancer. Third, the use of antibiotics or other antibacterial strategies may prevent the progression of cancers[36]. Blue light-emitting diode (BL) could inhibit the growth of P. gingivalis and play antimicrobial roles, and antimicrobial photodynamic therapy combining BL and rose bengal was expected to be a new technology for the elimination of bacteria[72]. Besides, AM404, an active metabolite of paracetamol, is an inhibitor of the growth and biofilm formation of P. gingivalis and could potentially make guidance in developing new drugs that is specific to infections and cancers with regard to P. gingivalis[75]. There are data suggesting that Kgp, a factor that playing the role of nutrition and toxicity for P. gingivalis, may act as the therapeutic point in controlling P. gingivalis infections. On account of it relating to the inhibition of other bacterial pathogens, protease inhibitors may have a potential to be an emerging antibacterial drug[76]. However, when cancers occur, treatment decisions are often complicated. The location, staging and resectability of the primary tumour, individual factors, such as swallowing function and airway condition, wishing to keep organ preserved, and coexisting diseases, all play roles in guiding accurate therapy. The main therapeutic approaches towards cancers are surgical therapy and radiotherapy[73]. During the last decade, the introduction of intensity modulated radiation therapy and concomitant che-moradiation have substantially changed the treatment techniques in head and neck cancer[77] and solved problems caused by radiotherapy, such as rapidly progressing dental caries as well as fungal and bacterial infections[78,79]. Recently, the chronological order between dental extractions and radiotherapy has also been fully demonstrated[80]. As for oesophageal cancer, the avoidance of radiotherapy is a valid choice. And the combination of platinum-based chemotherapy and radiotherapy before operation is the first choice in treating resectable local oesophageal carcinoma. In addition, the use of cisplatin and 5-FU combined with chemo-radiotherapy is an effective strategy for the treatment of ESCC. Surgical retention is a salvage procedure for patients with persistent or recurrent diseases. Several multicentre trials regarding neoadjuvant treatment for pancreatic cancer to improve survival are in progress[81]. With regard to pancreatic cancer, considering its characteristic of diagnosis in advanced stages, surgery tends not to be a selection; radiotherapy or chemotherapy is mainly used for palliative care[54]. Neoadjuvant therapy has become increasingly important in recent years, and further changes in standards are still needed[82]. The improvements in surgery, the advance of radiotherapy and the coordinated use of systemic drugs during treatment have clinically contributed to better outcomes of cancer patients[73]. In summary, the combination of antimicrobial therapy against P. gingivalis and tumour clearance with surgery, radiotherapy or chemotherapy, and neoadjuvant therapy is a good treatment for these digestive system cancers caused by P. gingivalis. However, the prognosises of digestive cancers are not eventually same. As Woo et al[21] put, those OSCC patients who were infected with P. gingivalis are discovered more metastatic foci in the lung than those who were non-infected; ESCC patients those with high levels of IgA or IgG against P. gingivalis tend to worse prognosis and those who were detected with high both IgA and IgG were associated with worst prognosis[40]; Pancreatic cancer patients have suffered poor prognosis, partly for lacking diagnostic and prognostic biomarkers. It is better now for discovering the role of P. gingivalis in this devastating carcinoma, which would guide personalized treatments, improve prognosis and enhance the quality of life[43].
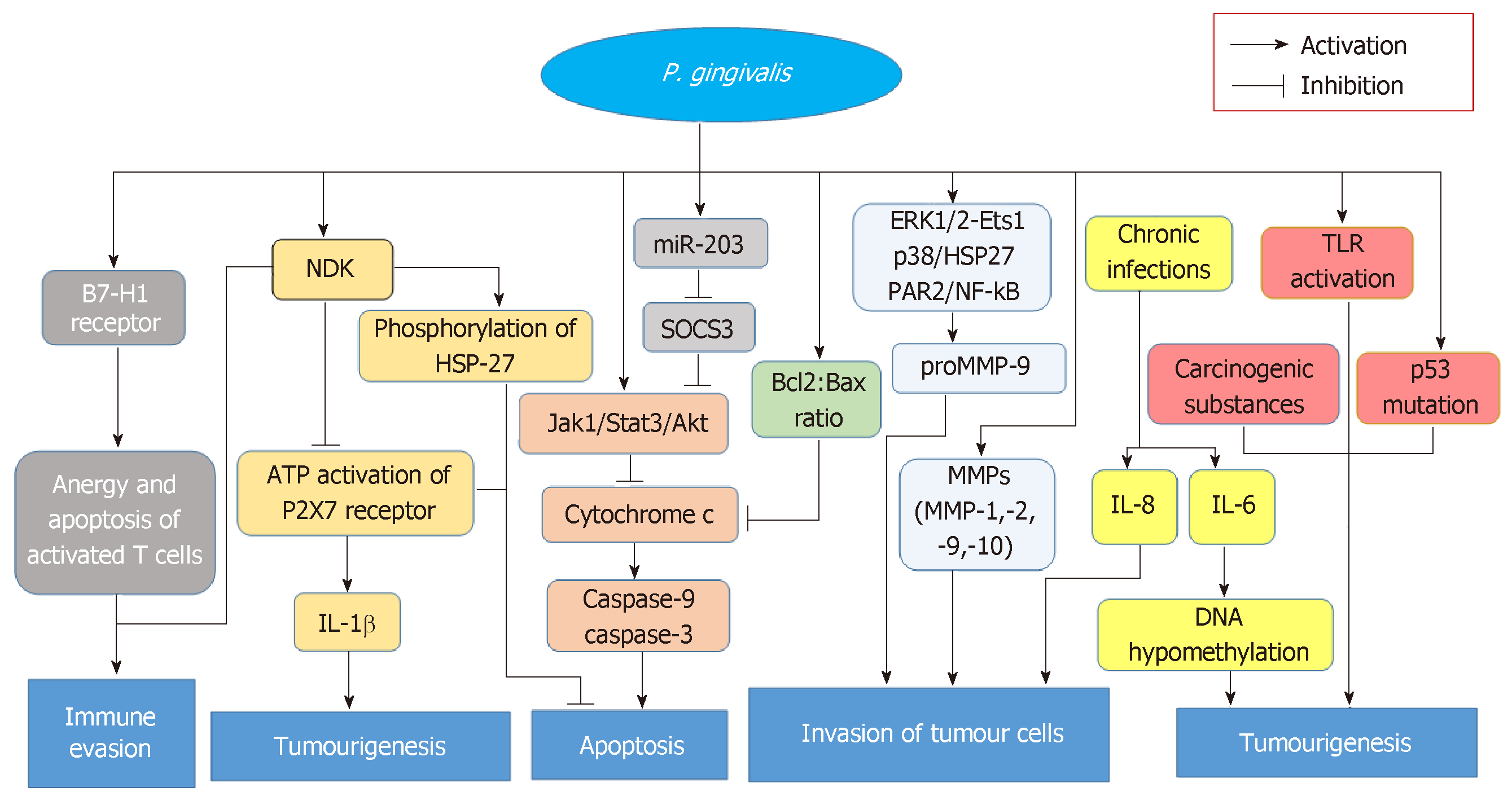
Regarding cancer, diverse degrees of connections between P. gingivalis and tumours of the buccal cavity, digestive tract or pancreas have been displayed in multiple clinical and experimental studies[26,48,62]. The correlation of orodigestive cancer mortality to P. gingivalis was first and directly illustrated in a cohort study from Ahn et al[62], who pinpointed that P. gingivalis would also act as a microbial marker full of value in such carcinomas. In this study, non-parametric trend analysis for all subjects also showed an increase in orodigestive cancer mortality with the increase of anti-P. gingivalis IgG levels[62]. P. gingivalis interacts with host epithelium in varieties of aspects, which may provide basis at molecular level for potential mechanisms carcinogenesis mediated by this bacterium. In addition to P. gingivalis inducing the invasion of tumour cells and activating TLR mentioned above[30,31,33,50], other possible mechanisms have also been identified. First, nucleoside diphosphate kinase (NDK) excreted by P. gingivalis is capable of promoting tumourigenesis. The NDK inhibits ATP activation of purinergic receptor (P2X7) receptors and consequently depress the production of IL-1β in epithelium[83]. In that IL-1β plays a crucial role in priming IFNγ which could produce CD8+ T cells that specific to tumour antigen, and NDK secreted by P. gingivalis can also make tumour escape from immune surveillance[39]. The degradation of ATP mediated by NDK also suppresses apoptosis, which is dependent on ATP activation of P2X7 receptors[84]. Moreover, the phosphorylation of heat shock protein 27 (HSP27) by NDK from P. gingivalis confers an antiapoptotic phenotype to primary gingival epithelial cells, suggesting that HSP27 is a critical molecule for the suppression of host cell apoptosis caused by P. gingivalis[85]. Second, Yilmaz et al[84] have noted that the inhibition of epithelial cell apoptosis is an important carcinogenic effect of P. gingivalis and is also an intrinsic protective mechanism of cancerous cells. Activating the Jak1/Akt/Stat3 signalling, increasing the Bcl2 (anti-apoptosis): Bax (pro-apoptosis) ratio, curtailing releasing pro-apoptotic factor cytochrome c, and blocking activating both caspase-9 and the executioner caspase-3 may all contribute to this progression[38,86,87]. In addition, P. gingivalis modulates the expressing levels of microRNAs (miRNAs), and the upregulation of miR-203 caused by P. gingivalis results in the decreasing levels of negative regulator SOCS3 and subsequently suppressing apoptosis of epithelium[88]. Since SOCS3 can bind to phosphorylated JAK receptors, SOCS3 consequently inhibits JAK/STAT3 signalling[89]. Third, P. gingivalis infection induces expression of the B7-H1 receptor which belongs to the B7 family and plays a significant regulatory role in immunologic reaction mediated by cells[90,91], suggesting that this pathogen is involved in transferring to the distant and advancing nuclear grading of carcinoma cells[92]. On the other hand, B7-H1 receptors-mediated costimulatory signal could cause the anergy and apoptotic effect of those activated T cells, which subsequently make tumours evade immune reaction[93]. Fourth, those carcinogenesis caused by P. gingivalis also contributes to metabolising potential carcinogen. For instance, P. gingivalis could dehydrogenate ethanol to acetaldehyde which is a carcinogenic derivative and capable of making the damage of DNA, the mutation and excessive proliferation of the epithelial cells[24,25]. Certainly, the above contributions could suitably explain why excessive drinking is a risk factor for orodigestive cancer[39]. Lastly, P. gingivalis establishes chronic infections that involve intracellular persistence within epithelial cells[94]. Chronic inflammation has a close connection with the development of cancer for the release of inflammatory factors, such as IL-6, which can promote tumourigenesis by causing DNA hypomethylation as well as aberrant changes in promoter hypermethylation[95]. All the above-mentioned possible mechanisms of cancers caused by P. gingivalis are shown in Figure 1.
P. gingivalis is involved in periodontal disease and several cancers. This bacterium not only independently affects the development of cancers by RgpA, RgpB and Kgp[11,12] but also indirectly impacts cancers via periodontal disease that causes the host be in an inflammatory state[57,58]. One critical carcinogenesis caused by P. gingivalis is inhibiting the apoptotic effects of epithelium, which also function as the intrinsic protective mechanism of cancer cells[87]. P. gingivalis has a strong association with oral cancer, and the treatment of oral cancer is abundant and has been improved in the clinic. The significant function of P. gingivalis in oesophageal cancer was conducted by Gao et al[36] in 2017, which was considered a breakthrough in the research of oeso-phageal cancer and indicated that P. gingivalis can be an important biomarker for monitoring the occurrence and progression of this cancer. For pancreatic cancer, the evidence concerning the role of P. gingivalis is still limited, while the relationship between periodontal diseases and pancreatic cancer is very large[66-68]. Therefore, more investigations are still needed to reduce the incidence, increase the five-year survival rate and improve the treatments for this devastating cancer. Collectively, maintaining oral hygiene, seeking more biomarkers, improving therapeutic measures, and improving the prognosis of these diseases are the focus for clinical research and work in the future.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YK, Sridharan G, Vynios D S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1082] [Cited by in F6Publishing: 1060] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 2. | Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988;15:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 529] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Struzycka I. The oral microbiome in dental caries. Pol J Microbiol. 2014;63:127-135. [PubMed] [Cited in This Article: ] |
| 5. | Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 946] [Cited by in F6Publishing: 1067] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 7. | Colombo AV, Silva CM, Haffajee A, Colombo AP. Identification of oral bacteria associated with crevicular epithelial cells from chronic periodontitis lesions. J Med Microbiol. 2006;55:609-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Travis J, Pike R, Imamura T, Potempa J. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodontal Res. 1997;32:120-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kuramitsu HK. Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol Immunol. 1998;13:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 12. | Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242-7250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007;42:589-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Inomata M, Ishihara Y, Matsuyama T, Imamura T, Maruyama I, Noguchi T, Matsushita K. Degradation of vascular endothelial thrombomodulin by arginine- and lysine-specific cysteine proteases from Porphyromonas gingivalis. J Periodontol. 2009;80:1511-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902-18907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Discipio RG, Daffern PJ, Kawahara M, Pike R, Travis J, Hugli TE, Potempa J. Cleavage of human complement component C5 by cysteine proteinases from Porphyromonas (Bacteroides) gingivalis. Prior oxidation of C5 augments proteinase digestion of C5. Immunology. 1996;87:660-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol. 2000;15:341-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontol 2000. 1999;20:168-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 19. | Gui MJ, Dashper SG, Slakeski N, Chen YY, Reynolds EC. Spheres of influence: Porphyromonas gingivalis outer membrane vesicles. Mol Oral Microbiol. 2016;31:365-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö. Human Primary Epithelial Cells Acquire an Epithelial-Mesenchymal-Transition Phenotype during Long-Term Infection by the Oral Opportunistic Pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Woo BH, Kim DJ, Choi JI, Kim SJ, Park BS, Song JM, Lee JH, Park HR. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget. 2017;8:46981-46992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Atanasova KR, Yilmaz Ö. Prelude to oral microbes and chronic diseases: past, present and future. Microbes Infect. 2015;17:473-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282-3287. [PubMed] [Cited in This Article: ] |
| 24. | Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci. 2003;40:183-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Homann N, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18:1739-1743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 202] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Sayehmiri F, Sayehmiri K, Asadollahi K, Soroush S, Bogdanovic L, Jalilian FA, Emaneini M, Taherikalani M. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. Int J Immunopathol Pharmacol. 2015;28:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Kang MS, Oh JS, Kim HJ, Kim HN, Lee IK, Choi HR, Kim OJ, Ko YJ, Lim WB, Park HJ, Yu MG, Yi CK. Prevalence of Oral Microbes in the Saliva of Oncological Patients. J Bacteriol Virol. 2009;39:277. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Ha NH, Woo BH, Kim DJ, Ha ES, Choi JI, Kim SJ, Park BS, Lee JH, Park HR. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015;36:9947-9960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, Morisaki I, Lamont RJ, Amano A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16:131-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Cho BH, Jung YH, Kim DJ, Woo BH, Jung JE, Lee JH, Choi YW, Park HR. Acetylshikonin suppresses invasion of Porphyromonas gingivalis‑infected YD10B oral cancer cells by modulating the interleukin-8/matrix metalloproteinase axis. Mol Med Rep. 2018;17:2327-2334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066-13076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 450] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 33. | Ha NH, Park DG, Woo BH, Kim DJ, Choi JI, Park BS, Kim YD, Lee JH, Park HR. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine. 2016;86:64-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Geng F, Liu J, Guo Y, Li C, Wang H, Wang H, Zhao H, Pan Y. Persistent Exposure to Porphyromonas gingivalis Promotes Proliferative and Invasion Capabilities, and Tumorigenic Properties of Human Immortalized Oral Epithelial Cells. Front Cell Infect Microbiol. 2017;7:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 36. | Gao S, Li S, Ma Z, Liang S, Shan T, Zhang M, Zhu X, Zhang P, Liu G, Zhou F, Yuan X, Jia R, Potempa J, Scott DA, Lamont RJ, Wang H, Feng X. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer. 2016;11:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 37. | Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, Stavropoulos MF, Yilmaz O, Lamont RJ. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997-2007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 40. | Gao SG, Yang JQ, Ma ZK, Yuan X, Zhao C, Wang GC, Wei H, Feng XS, Qi YJ. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer. 2018;18:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Yuan X, Liu Y, Kong J, Gu B, Qi Y, Wang X, Sun M, Chen P, Sun W, Wang H, Zhou F, Gao S. Different frequencies of Porphyromonas gingivalis infection in cancers of the upper digestive tract. Cancer Lett. 2017;404:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, Ravel J, Hayes RB, Ahn J. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67:120-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 43. | Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel). 2017;9:pii: E157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 44. | Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101:4250-4255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 45. | Öğrendik M. Oral bacteria in pancreatic cancer: mutagenesis of the p53 tumour suppressor gene. Int J Clin Exp Pathol. 2015;8:11835-11836. [PubMed] [Cited in This Article: ] |
| 46. | Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 47. | Jacob JA. Study Links Periodontal Disease Bacteria to Pancreatic Cancer Risk. JAMA. 2016;315:2653-2654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjønneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Kaaks R, Boeing H, Foerster J, Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Sieri S, Palli D, Tumino R, Panico S, Siersema PD, Peeters PH, Lund E, Barricarte A, Huerta JM, Molina-Montes E, Dorronsoro M, Quirós JR, Duell EJ, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Travis RC, Vineis P, Bueno-de-Mesquita HB, Riboli E. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 49. | Shaik-Dasthagirisaheb YB, Huang N, Weinberg EO, Shen SS, Genco CA, Gibson FC. Aging and contribution of MyD88 and TRIF to expression of TLR pathway-associated genes following stimulation with Porphyromonas gingivalis. J Periodontal Res. 2015;50:89-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, Jonnadula S, Torres-Hernandez A, Tippens D, Pushalkar S, Eisenthal A, Saxena D, Ahn J, Hajdu C, Engle DD, Tuveson D, Miller G. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med. 2015;212:2077-2094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 51. | Barton CM, Staddon SL, Hughes CM, Hall PA, O'Sullivan C, Klöppel G, Theis B, Russell RC, Neoptolemos J, Williamson RC. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991;64:1076-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 287] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 52. | Yu Y, Richardson DR. Cellular iron depletion stimulates the JNK and p38 MAPK signaling transduction pathways, dissociation of ASK1-thioredoxin, and activation of ASK1. J Biol Chem. 2011;286:15413-15427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Inaba H, Amano A, Lamont RJ, Murakami Y, Matsumoto-Nakano M. Cell Cycle Arrest and Apoptosis Induced by Porphyromonas gingivalis Require Jun N-Terminal Protein Kinase- and p53-Mediated p38 Activation in Human Trophoblasts. Infect Immun. 2018;86:pii: e00923-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34:2193-2197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Peters BA, Wu J, Pei Z, Yang L, Purdue MP, Freedman ND, Jacobs EJ, Gapstur SM, Hayes RB, Ahn J. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res. 2017;77:6777-6787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 56. | Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3166] [Cited by in F6Publishing: 3116] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 57. | Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727-752, table of contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 274] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 58. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6912] [Cited by in F6Publishing: 7709] [Article Influence: 481.8] [Reference Citation Analysis (0)] |
| 59. | Katz J, Wallet S, Cha S. Periodontal disease and the oral-systemic connection: "is it all the RAGE?". Quintessence Int. 2010;41:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 60. | Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 61. | Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13:312-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 62. | Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 63. | Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, Lillis C, Wactawski-Wende J, Scannapieco FA. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406-2412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 64. | Wolff L, Dahlén G, Aeppli D. Bacteria as Risk Markers for Periodontitis. J Periodontol. 1994;65 Suppl 5S:498-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Abiko Y, Sato T, Mayanagi G, Takahashi N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res. 2010;45:389-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9:411-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Joshipura KJ, Wand HC, Merchant AT, Rimm EB. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. 2004;83:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Meurman JH. Oral microbiota and cancer. J Oral Microbiol. 2010;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 69. | Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR, Dawsey SM. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Yin XH, Wang YD, Luo H, Zhao K, Huang GL, Luo SY, Peng JX, Song JK. Association between Tooth Loss and Gastric Cancer: A Meta-Analysis of Observational Studies. PLoS One. 2016;11:e0149653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60:15-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 424] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 72. | Chui C, Aoki A, Takeuchi Y, Sasaki Y, Hiratsuka K, Abiko Y, Izumi Y. Antimicrobial effect of photodynamic therapy using high-power blue light-emitting diode and red-dye agent on Porphyromonas gingivalis. J Periodontal Res. 2013;48:696-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1341] [Cited by in F6Publishing: 1417] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 74. | Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337-5343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Gerits E, Spincemaille P, De Cremer K, De Brucker K, Beullens S, Thevissen K, Cammue BPA, Vandamme K, Fauvart M, Verstraeten N, Michiels J. Repurposing AM404 for the treatment of oral infections by Porphyromonas gingivalis. Clin Exp Dent Res. 2017;3:69-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Curtis MA, Aduse Opoku J, Rangarajan M, Gallagher A, Sterne JA, Reid CR, Evans HE, Samuelsson B. Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect Immun. 2002;70:6968-6975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Bortfeld T. IMRT: a review and preview. Phys Med Biol. 2006;51:R363-R379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 78. | Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001;61:271-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Epstein JB, Lunn R, Le N, Stevenson-Moore P. Periodontal attachment loss in patients after head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:673-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Koga DH, Salvajoli JV, Alves FA. Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis. 2008;14:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Chiappa A, Andreoni B, Dionigi R, Spaggiari L, Foschi D, Polvani G, Orecchia R, Fazio N, Pravettoni G, Cossu ML, Galetta D, Venturino M, Ferrari C, Macone L, Crosta C, Bonanni B, Biffi R. A rationale multidisciplinary approach for treatment of esophageal and gastroesophageal junction cancer: Accurate review of management and perspectives. Crit Rev Oncol Hematol. 2018;132:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Kawai M, Yamaue H. [Evidence of Neoadjuvant Treatment for Pancreatic Cancer]. Gan To Kagaku Ryoho. 2018;45:1410-1414. [PubMed] [Cited in This Article: ] |
| 83. | Morandini AC, Ramos-Junior ES, Potempa J, Nguyen KA, Oliveira AC, Bellio M, Ojcius DM, Scharfstein J, Coutinho-Silva R. Porphyromonas gingivalis fimbriae dampen P2X7-dependent interleukin-1β secretion. J Innate Immun. 2014;6:831-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Lee J, Roberts JS, Atanasova KR, Chowdhury N, Yilmaz Ö. A novel kinase function of a nucleoside-diphosphate-kinase homologue in Porphyromonas gingivalis is critical in subversion of host cell apoptosis by targeting heat-shock protein 27. Cell Microbiol. 2018;20:e12825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Yao L, Jermanus C, Barbetta B, Choi C, Verbeke P, Ojcius DM, Yilmaz O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25:89-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743-3751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 88. | Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632-2637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4:339-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 304] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 90. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1806] [Cited by in F6Publishing: 1867] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 91. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3572] [Cited by in F6Publishing: 3731] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 92. | Groeger S, Domann E, Gonzales JR, Chakraborty T, Meyle J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology. 2011;216:1302-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 93. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2838] [Cited by in F6Publishing: 3288] [Article Influence: 149.5] [Reference Citation Analysis (0)] |
| 94. | Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 95. | Alevizos I, Mahadevappa M, Zhang X, Ohyama H, Kohno Y, Posner M, Gallagher GT, Varvares M, Cohen D, Kim D, Kent R, Donoff RB, Todd R, Yung CM, Warrington JA, Wong DT. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene. 2001;20:6196-6204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |