Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.668
Peer-review started: November 12, 2018
First decision: December 20, 2018
Revised: January 13, 2019
Accepted: January 30, 2019
Article in press: January 29, 2019
Published online: March 6, 2019
The tunnel endoscopic technique is the treatment of choice for submucosal tumors. However, the use of tunnel endoscopy to diagnose adenocarcinoma of the esophagus originating from the submucosa has not been well studied.
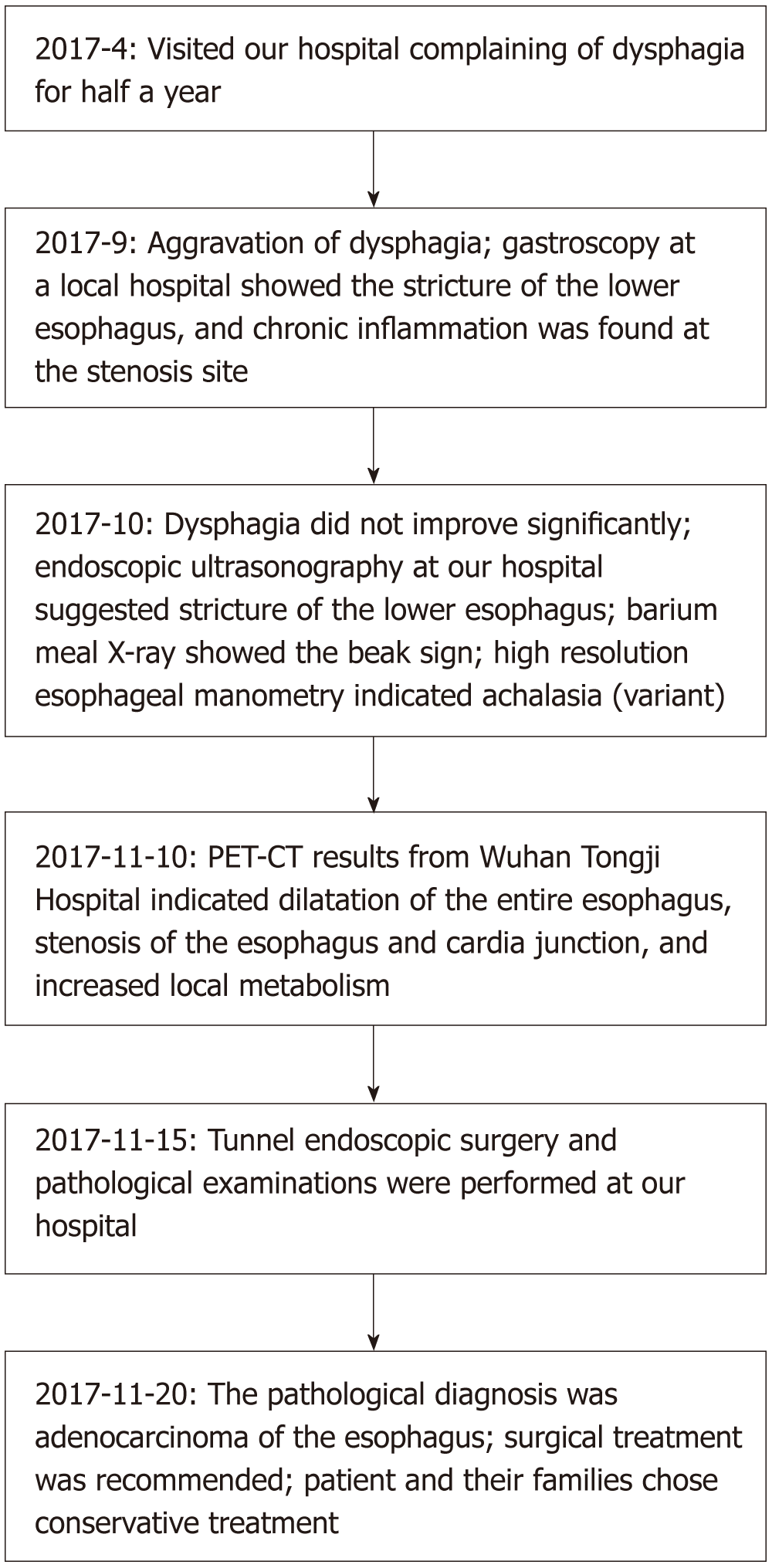
A 74-year-old man who presented with dysphagia for half a year underwent a series of checks, such as gastroendoscopy, X-ray contrast examination of the upper digestive tract, endoscopic ultrasonography, high-resolution esophageal manometry, and positron emission computed tomography. It should be noted that the stenosis of the esophagus was too narrow for endoscopic ultrasound-guided fine needle aspiration. The cause remained undiagnosed. Eventually, the tunnel endoscopic technique was perform for the pathological examination in the submucosa and the final diagnosis was adenocarcinoma of the esophagus. The patient and family members chose expectant treatment due to the patient’s age and the high costs of surgical treatment.
Tunnel endoscopy could be used to diagnose tumors. Moreover, we review the literature to provide guidance regarding the causes of esophagostenosis.
Core tip: Tunnel endoscopy is not only effective as a treatment for submucosal tumors, but also as a means of performing pathological examination for diagnosing tumors. Moreover, we review the literature to provide guidance regarding the causes of esophageal stenosis.
- Citation: Liu S, Wang N, Yang J, Yang JY, Shi ZH. Use of tunnel endoscopy for diagnosis of obscure submucosal esophageal adenocarcinoma: A case report and review of the literature with emphasis on causes of esophageal stenosis. World J Clin Cases 2019; 7(5): 668-675
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/668.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.668
Tumors of the esophagus are a severe health problem worldwide with crypticity and high mortality. According to the different pathologic types, tumors of the esophagus have been divided into esophageal squamous cell carcinoma and esophageal adenocarcinoma[1]. Esophageal squamous cell carcinoma is the predominant histologic type while esophageal adenocarcinoma remains rare[2]. It is generally accepted that pathological examination is the “gold standard” for the diagnosis of esophageal tumors. Moreover, imaging modalities such as endoscopy, endoscopic ultrasonography (EUS), and computed tomography (CT) have been applied to measure the depth of the invasion of the esophageal wall, tumor size, the presence of invasion into adjacent organs, lymph node metastasis, and distant metastasis[3]. The high mortality rate has been attributed to the fact that half of patients have a locally-advanced form of the disease at diagnosis[4]. Therefore, early pathological diagnosis can effectively improve patient prognosis.
The tunnel endoscopic technique is an innovative and minimally-invasive endoscopic surgical procedure. The first submucosal tunnel endoscopic resection in the world was conducted in China, when it was used to remove submucosal tumors (SMTs) originating from the muscularis propria of the esophagus[5]. Nevertheless, biopsy in a submucosal tunnel has not been well studied. We report herein an unusual case involving diagnosis of esophageal stenosis caused by adenocarcinoma through the tunnel endoscopic technique, and review the causes of esophageal stenosis. The patient has signed an informed consent form and data had been anonymized and unidentified.
A 74-year-old male patient visited our hospital complaining of dysphagia which he had experienced for half a year with no clear trigger.
He vomited after eating, bringing up the contents of the stomach. There was no obvious chest pain, hematemesis, or weight loss. He was in good physical condition.
There were no special circumstances in personal and family history.
On physical examination, he showed no evident positive characteristics.
With regard to laboratory values, only the serum tumor marker CA199 (47.85 U/mL; normal reference range: < 39 U/mL), albumin (35.6 g/L; normal reference range: 40-50 g/L), and hemoglobin (124 g/L; normal reference range: 130-175 g/L) were moderately changed. Renal function, electrolytes, blood sugar, cholesterol, erythrocyte sedimentation rate, blood coagulation, humoral immune function, antiphospholipid antibody, anti-autoantibody, antineutrophil cytoplasmic antibody, urine, and conventional stool concentration were all within normal limits.
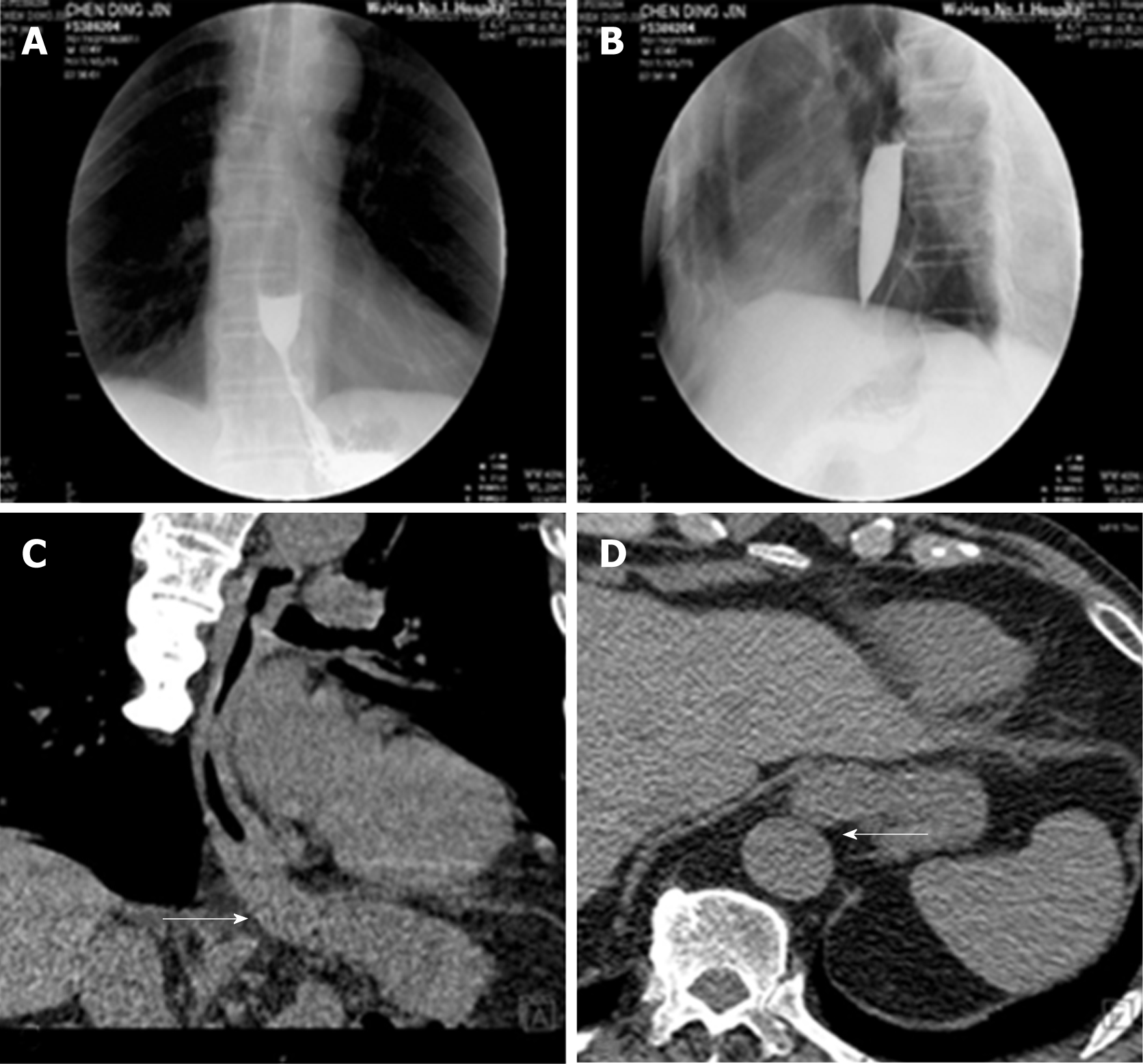
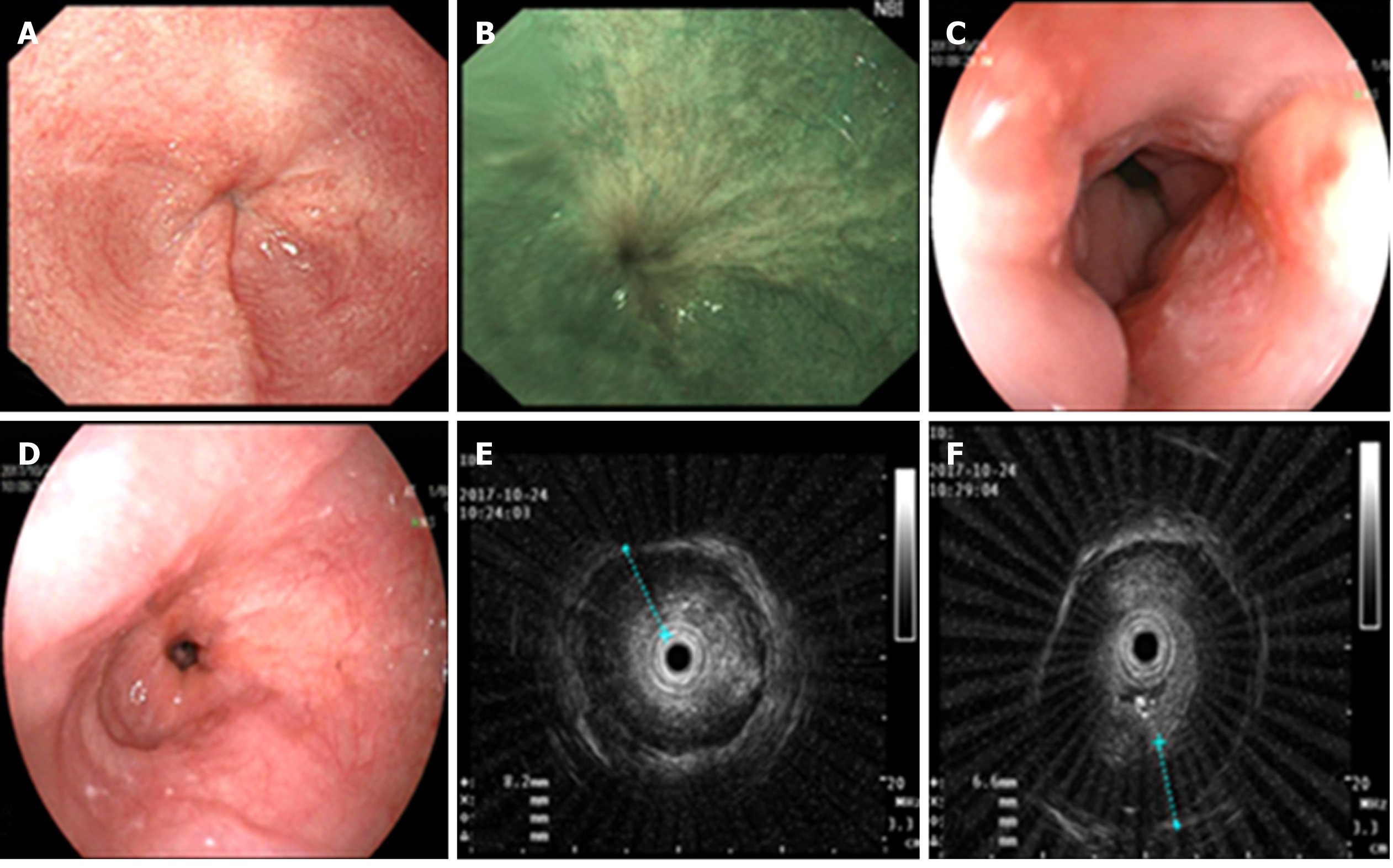
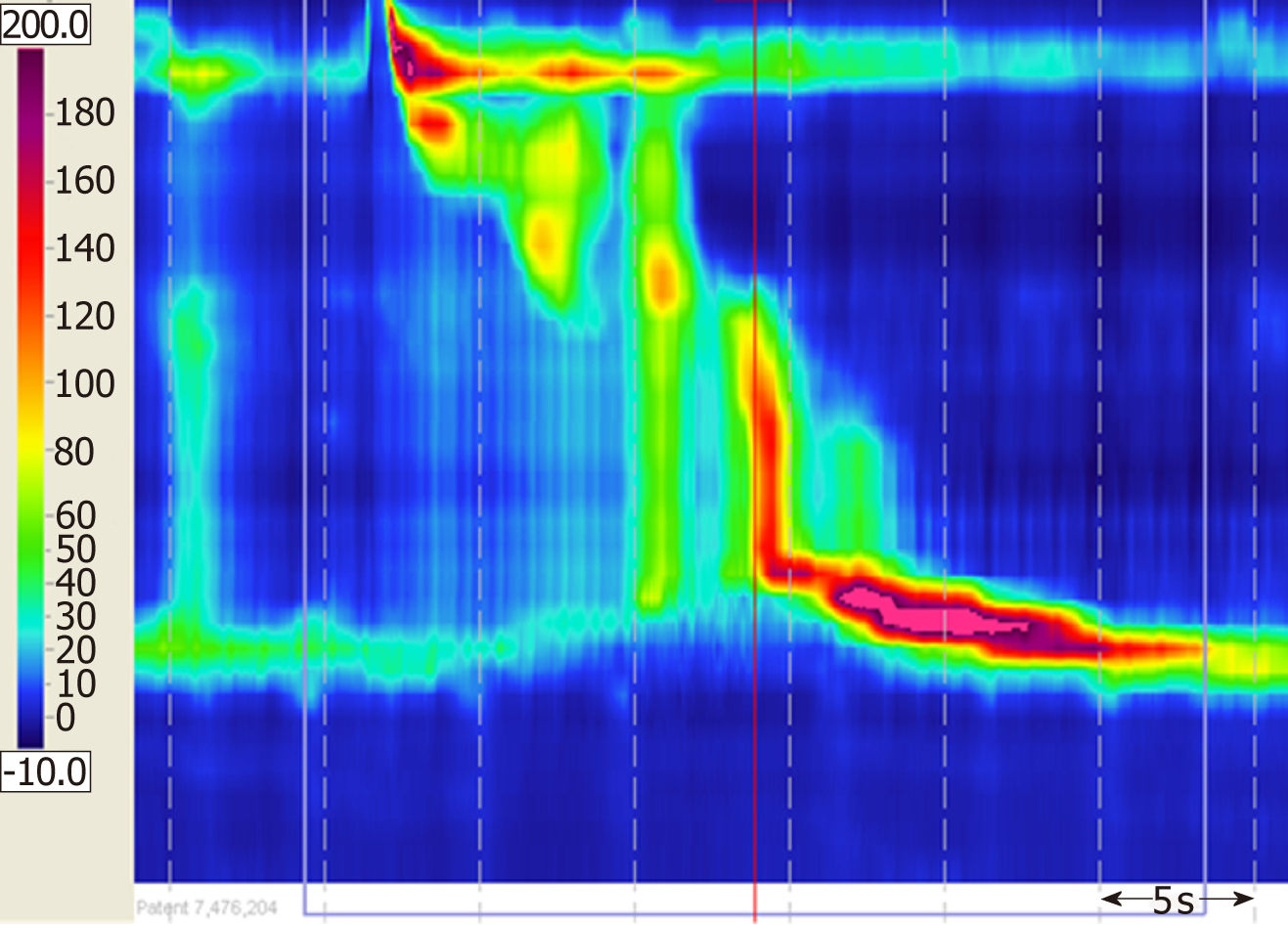
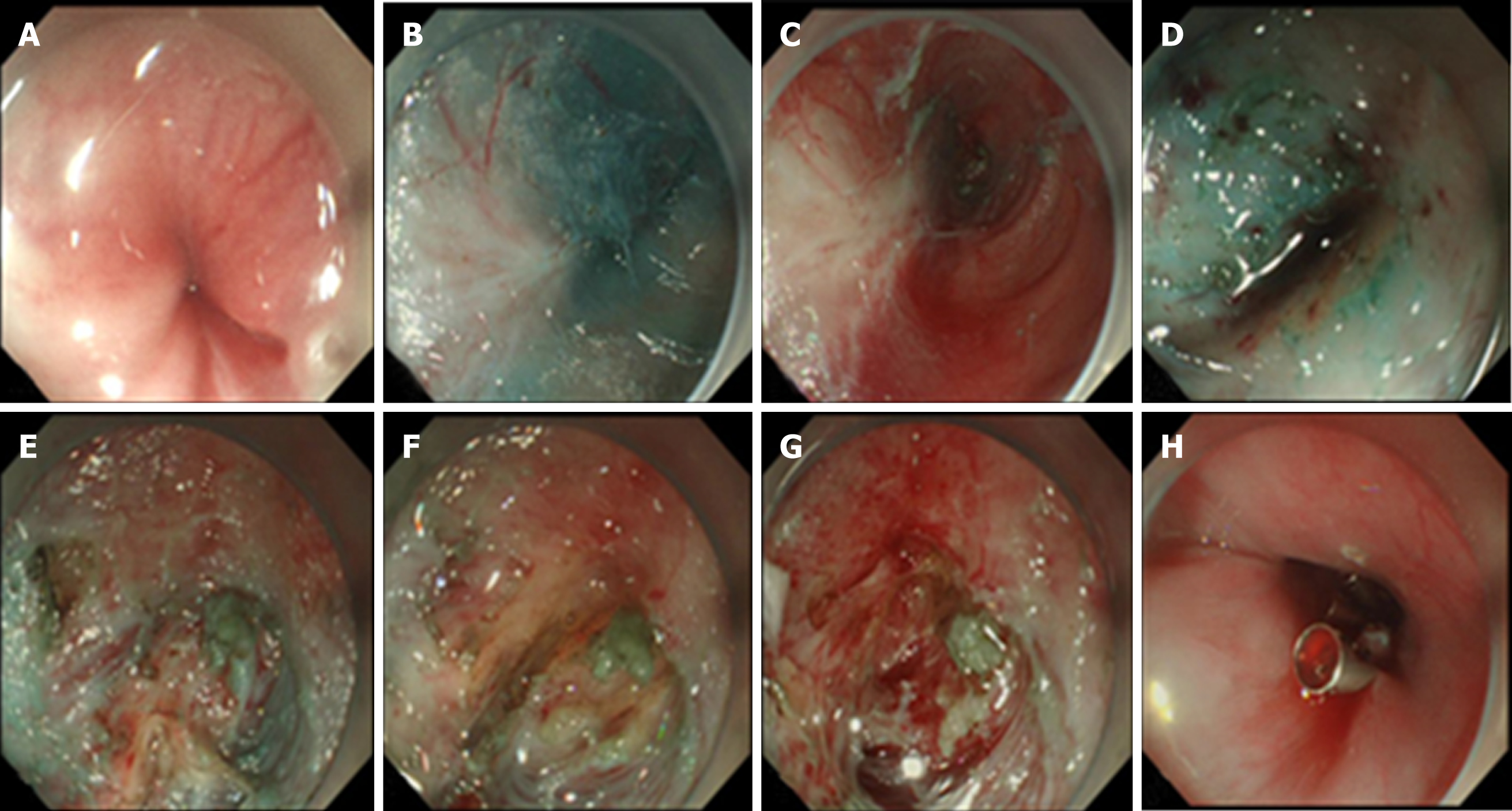
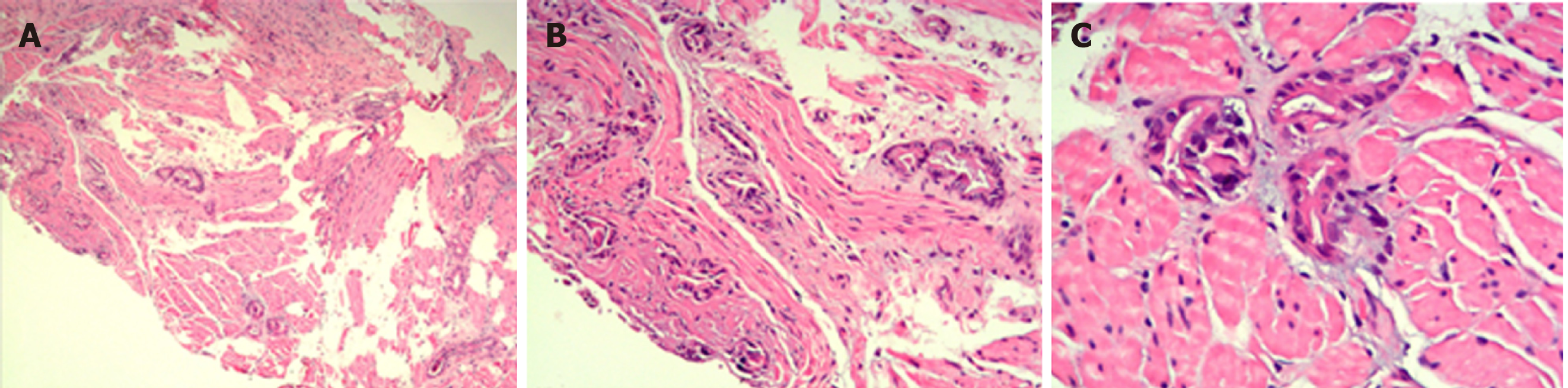
Color Doppler ultrasonography revealed chronic cholecystolithiasis. A chest CT scan revealed thickening of the esophageal wall (Figure 1A and B). Barium meal X-ray showed that the lower esophagus presented the beak sign, suggesting achalasia (Figure 1C and D). Regarding condition, no ulceration, prominence lesions, or Barrett's esophagus manifestation was found by endoscopy (Figure 2A-D). In addition, routine gastroscopy was performed at a local hospital, and no tumor cell was detected by pathological examination at esophageal stenosis. EUS was performed to clarify the cause of the narrow esophageal structure. On EUS, esophageal cavity stenosis was visible at a distance of 37 cm from the incisors, along with obvious thickening of the intrinsic muscularis which reached 0.8 cm, and the first to third layer structure was not clear (Figure 2E and F). High-resolution esophageal manometry suggested lower esophageal outflow obstruction (Figure 3). Based on these findings, a tumor that originated from the tunica muscularis esophagi was highly suspected. Therefore, we recommended the patient to undergo a positron emission CT (PET-CT) examination. The PET-CT results from Wuhan Tongji Hospital indicated dilatation of the entire esophagus, stenosis of the esophagus and cardia junction, and increased local metabolism. However, it was difficult to obtain pathological evidence as the esophageal mucosa was only roughened, with no ulceration, erosion, or bleeding. We invited a general thoracic vascular surgeon and a gastrointestinal surgeon to assist in the diagnosis and treatment. Eventually, a multidisciplinary consultation recommended that tunnel endoscopy was performed for biopsy. Therefore, the tunnel endoscopic technique was chose for pathological examination. We created a submucosal tunnel, advanced towards the stenosis of the esophagus, and obtained muscularis tissues (Figure 4).
The final diagnosis was adenocarcinoma of the esophagus (Figure 5).
Regretfully, the patient and family members chose expectant treatment due to the patient’s age and the high costs of surgical treatment. The flow chart of disease diagnosis can be referred to Figure 6.
Adenocarcinoma of the esophagus is a malignant tumor arising from the submucosal tissue of the esophagus or from the glands of the cardia. However, because early disease is asymptomatic in most patients, timely diagnosis of esophageal adenocarcinoma (especially arising from the submucosa) is relatively difficult. Even when treated by radical surgery combined with radiotherapy and chemotherapy, the 5-year survival rate remains low[6,7]. Thus, early diagnosis and treatment of esophageal tumors are of great significance.
Esophageal strictures can result from a wide variety of benign and malignant conditions. Meanwhile, dysphagia is the most common symptom which urges patients to seek medical treatment. Benign esophageal strictures can occur following peptic strictures[8], eosinophilic esophagitis[9], achalasia[10], pill-injury esophageal strictures[11], caustic strictures[12], anastomotic strictures[13], Crohn's disease-associated esophageal stricture[14], IgG4-related esophagitis[15], radiation-induced esophageal strictures[16], esophageal intramural pseudodiverticulosis[17], or epidermolysis bullosa[18] (Table 1). It is generally known that a malignant esophageal stricture refers to esophageal cancer. Some esophageal strictures can be treated by drug therapy such as with proton pump inhibitors or steroids[9,11,15,17], while others can be refractory to most optical endoscopic therapies such as dilation[15,16,18], stent placement[13], or peroral endoscopic myotomy.
| Ref. | Years | Cause analysis of stenosis | Clinical manifestation |
| Ramage Jr et al[8] | 2005 | Peptic strictures | Acid reflux, dysphagia |
| Moonen et al[10] | 2014 | Achalasia | Dysphagia, regurgitation aspiration, chest pain, and weight loss |
| Muir et al[9] | 2018 | Eosinophilic esophagitis | Dysphagia |
| Yeoh et al[11] | 2017 | Pill-injury esophageal strictures | Regurgitation, retrosternal pain |
| He et al[12] | 2018 | Caustic strictures | Dysphagia, hematemesis |
| Siddiqui et al[13] | 2012 | Anastomotic strictures | Dysphagia, when a standard endoscope could not pass through the post-ESD scar |
| Nandy et al[14] | 2017 | Crohn's disease induced esophageal strictures | Dysphagia and odynophagia |
| Obiorah et al[15] | 2017 | IgG4-related esophagitis | Dysphagia |
| Agarwalla et al[16] | 2015 | Radiation-induced esophageal strictures | Dysphagia |
| Abbes et al[17] | 2017 | Esophageal intramural pseudodiverticulosis | Dysphagia and weight loss |
| Michalak et al[18] | 2018 | Epidermolysis bullosa | Dysphagia, skin blistering, joint contractures and missing nails |
In the present case, the mucosa of the esophageal stenosis was only rough, with no obvious ulceration or erosion. It could well be that mucosal biopsies failed to achieve real results. Meanwhile, the stenosis of the esophagus was too narrow for a conventional endoscope to pass, let alone the large probe required for EUS. Therefore, it could not perform EUS guided fine needle aspiration for biopsy and we chose to use small probe endoscopic ultrasonography to clarify the cause of the esophageal stricture.
Subcutaneous emphysema, pneumothorax, and secondary infection are common complications of endoscopic resection[19-21]. As there was no serous layer of the esophagus, resection of the muscularis propria of the esophagus would be more prone to concurrent subcutaneous emphysema, pneumothorax, and secondary infection than the gastric muscularis propria. When the tunnel endoscopic technique is used to excise SMTs, the lesion mucosal surface remains intact and the mucosa of the tunnel opening is closed with a titanium clip, avoiding leakage of gas and digestive fluid into the chest and abdominal cavity, thus reducing the risk of secondary infection. Moreover, the tunnel endoscopic technique allows clear visualization of bleeding foci in the tunnel, reducing bleeding during the operation and postoperative delayed bleeding.
In conclusion, this was an unusual case of esophageal stenosis caused by adenocarcinoma and diagnosed by the tunnel endoscopic technique. As a method of diagnosis and treatment, the tunnel endoscopic technique would be a less complicated and less risky choice. We would like to emphasize the role of tunnel endoscopy in diagnostic treatment. Further, we analyzed the common causes of esophageal stenosis, hoping to provide some information which will help with clinical work.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ananthakrishnan N, Lee JI, Lim SC S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 900] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 2. | Shibata A, Matsuda T, Ajiki W, Sobue T. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993-2001. Jpn J Clin Oncol. 2008;38:464-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, Das P, Denlinger C, Fanta P, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Jasperson K, Keswani RN, Kleinberg LR, Korn WM, Leong S, Lockhart AC, Mulcahy MF, Orringer MB, Posey JA, Poultsides GA, Sasson AR, Scott WJ, Strong VE, Varghese TK, Washington MK, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H; National comprehensive cancer network. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13:194-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 278] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 4. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2115] [Cited by in F6Publishing: 2142] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 5. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75:195-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Fan YJ, Song X, Li JL, Li XM, Liu B, Wang R, Fan ZM, Wang LD. Esophageal and gastric cardia cancers on 4238 Chinese patients residing in municipal and rural regions: a histopathological comparison during 24-year period. World J Surg. 2008;32:1980-1988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Borghesi S, Hawkins MA, Tait D. Oesophagectomy after definitive chemoradiation in patients with locally advanced oesophageal cancer. Clin Oncol (R Coll Radiol). 2008;20:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ramage JI, Rumalla A, Baron TH, Pochron NL, Zinsmeister AR, Murray JA, Norton ID, Diehl N, Romero Y. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol. 2005;100:2419-2425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Muir AB, Wang JX, Nakagawa H. Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol. 2019;54:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Moonen A, Boeckxstaens G. Current diagnosis and management of achalasia. J Clin Gastroenterol. 2014;48:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Yeoh SW. Esophageal bezoar due to karaya gum granules used as a laxative. Clin J Gastroenterol. 2017;10:437-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | He K, Zhao L, Bu S, Liu L, Wang X, Wang M, Fan Z. Endoscopic mucosal autograft for treating esophageal caustic strictures: preliminary human experience. Endoscopy. 2018;50:1017-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Siddiqui AA, Sarkar A, Beltz S, Lewis J, Loren D, Kowalski T, Fang J, Hilden K, Adler DG. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc. 2012;76:44-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Nandy N, Gavin M, Martin D, McCarthy D. Crohn's Disease: Hard to Swallow! Dig Dis Sci. 2017;62:2690-2693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Obiorah I, Hussain A, Palese C, Azumi N, Benjamin S, Ozdemirli M. IgG4-related disease involving the esophagus: a clinicopathological study. Dis Esophagus. 2017;30:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Agarwalla A, Small AJ, Mendelson AH, Scott FI, Kochman ML. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Abbes L, Perrod G, Rahmi G, Cellier C. Esophageal intramural pseudodiverticulosis, a rare cause of stenosis. Clin Res Hepatol Gastroenterol. 2017;41:505-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Michalak A, Cichoż-Lach H, Prozorow-Król B, Buk L, Dzida M. A rare case of skin blistering and esophageal stenosis in the course of epidermolysis bullosa - case report and literature review. BMC Gastroenterol. 2018;18:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Soh JS, Kim JK, Lim H, Kang HS, Park JW, Kim SE, Moon SH, Kim JH, Park CK, Cho JW, Lim MS, Kim KO. Comparison of endoscopic submucosal dissection and surgical resection for treating gastric subepithelial tumours. Scand J Gastroenterol. 2016;51:633-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | He G, Wang J, Chen B, Xing X, Wang J, Chen J, He Y, Cui Y, Chen M. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc. 2016;30:4206-4213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Shen C, Chen H, Yin Y, Chen J, Han L, Zhang B, Chen Z, Chen J. Endoscopic versus open resection for small gastric gastrointestinal stromal tumors: safety and outcomes. Medicine (Baltimore). 2015;94:e376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |