Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3047
Peer-review started: May 23, 2019
First decision: August 1, 2019
Revised: August 20, 2019
Accepted: August 27, 2019
Article in press: August 27, 2019
Published online: October 6, 2019
Cervical disc arthroplasty (CDA) is an alternative treatment to traditional interbody fusion that maintains postoperative cervical spine mobility. However, the CDA postoperative period is impacted by osteolysis, subsidence, metallosis, or heterotopic ossification (HO). We report a case of severe HO in a seronegative spondyloarthritis patient after cervical Bryan disc arthroplasty.
A 34-year-old man received hybrid surgery for C4-C5 and C5-C6 arthroplasty with Bryan discs and C6-C7 arthrodesis with polyetheretherketone cage due to traumatic herniation of the intervertebral disc (HIVD). After four years, cervical spine radiographs revealed severe HO around the Bryan discs over the C4-C5 and C5-C6 levels. The magnetic resonance image revealed HIVD over the C3-C4 level with spinal cord compression. Seronegative spondyloarthritis was diagnosed after consultation with a rheumatologist. A second CDA for the adjacent segment disease HIVD with Baguera C disc over the C3-C4 level achieved an excellent outcome.
Minimizing intraoperative tissue trauma and achieving postoperative interbody stability avoid soft tissue traction to prevent HO formation after CDA.
Core tip: Severe traction spur type heterotopic ossification (HO) occurred after cervical disc arthroplasty (CDA) with Bryan discs and matured in the second year after operation. A second CDA for the adjacent segment disease herniation of the intervertebral disc with Baguera C disc over the C3-C4 level achieved excellent outcome. Based on the second operation experience, the avoidance of unnecessary soft tissue traction and trauma such as burring, milling, or keeling during the operation and the prophylactic use of non-steroidal anti-inflammatory drugs are mandatory to prevent HO formation.
- Citation: Huang CW, Tang CL, Pan HC, Tzeng CY, Tsou HK. Severe heterotopic ossification in a seronegative spondyloarthritis patient after cervical Bryan disc arthroplasty: A case report. World J Clin Cases 2019; 7(19): 3047-3054
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3047.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3047
Compared to intervertebral body fusion for herniated discs of a cervical spine lesion, cervical disc arthroplasty (CDA) is an alternative treatment to maintain postoperative cervical spine mobility. However, the CDA postoperative outcome may be affected by osteolysis, subsidence, metallosis, or heterotopic ossification (HO). HO is a pathologic process of extraskeletal bone formation in the muscle and soft tissues. Nongenetic HO commonly accompanies traumatic injuries such as spinal cord injury, burns, musculoskeletal trauma, or traumatic brain injury; inflammation plays an essential role in the pathogenesis of HO[1]. Patients who have paraplegia after spinal cord injury or who have undergone total hip arthroplasty (THA) are at risk for HO[2].
Radiological examinations, including computed tomography (CT), magnetic resonance imaging (MRI), and bone scan, are useful tools in the diagnosis of HO[1], by which irregular opacities without a clear zonal maturation pattern are observed at the early stage of HO, and complete ankylosis of the affected joint caused by a massive ossification lesion will be observed later. Sometimes, HO may result in a bone island that facilitates the differential diagnosis of pseudotumor[1].
Little is known about the potential effects of HO on the cervical spine after artificial disc replacement (ADR)[3,4]. We herein report a patient with seronegative spondyloarthritis who developed severe HO after cervical spine ADR with Bryan disc over the C4-C5 and C5-C6 levels. The development of HO after arthroplasty surgery may restrict the range of motion (ROM) around the replacement site and hinder joint mobility in patients. HO formation may increase the risk of adjacent segment disease and decrease the joint mobility after CDA.
A 36-year-old man suffered from progressive weakness and numbness in all four limbs for two weeks. Gait disturbance was also noted.
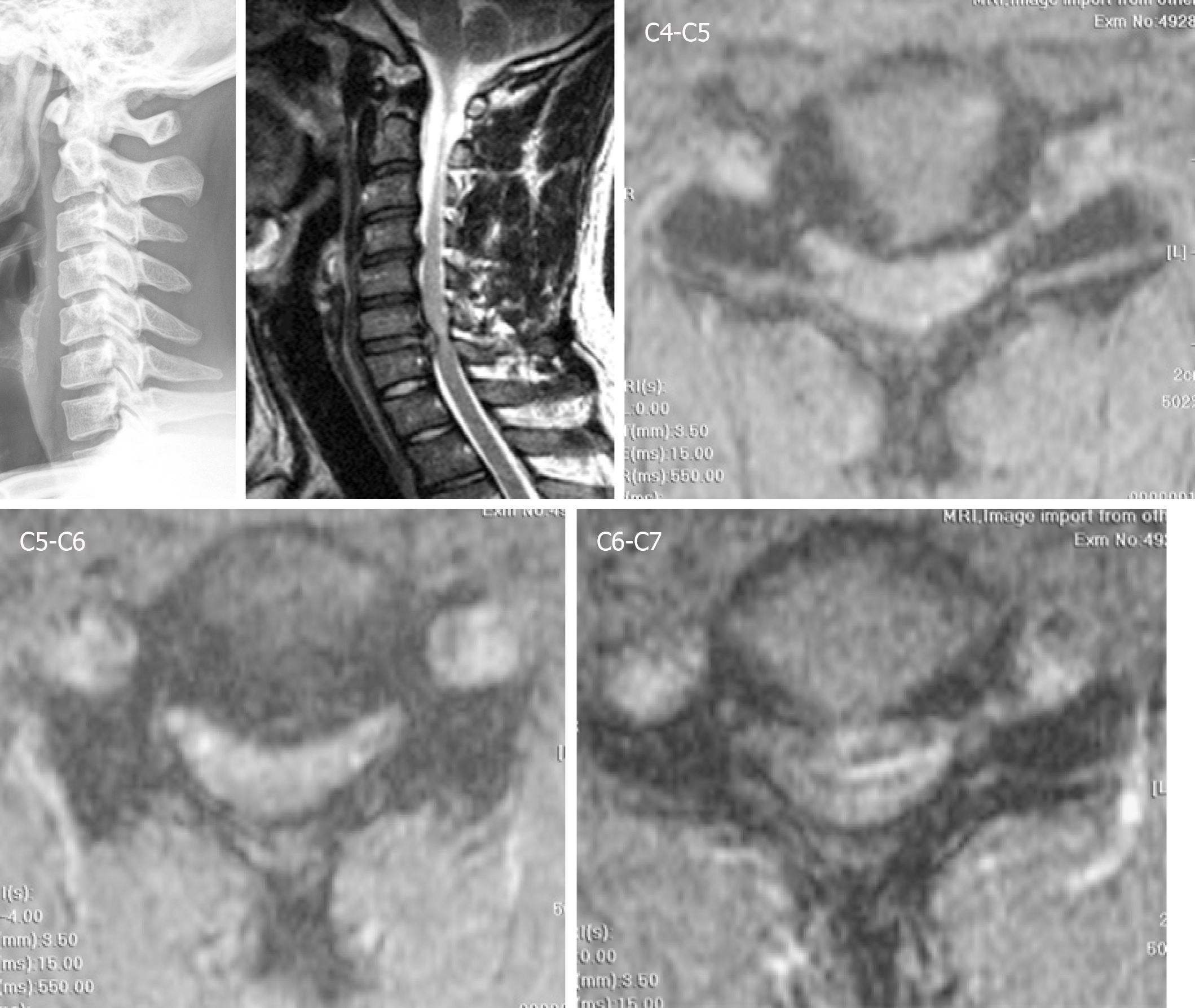
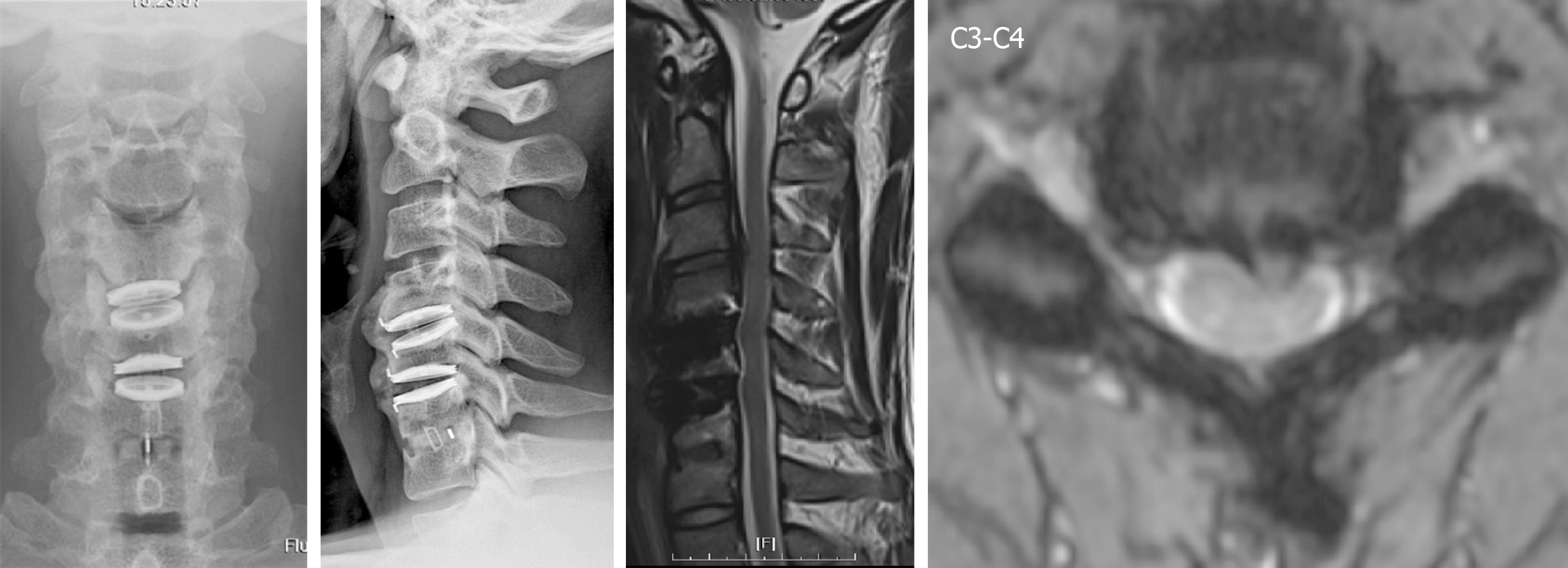
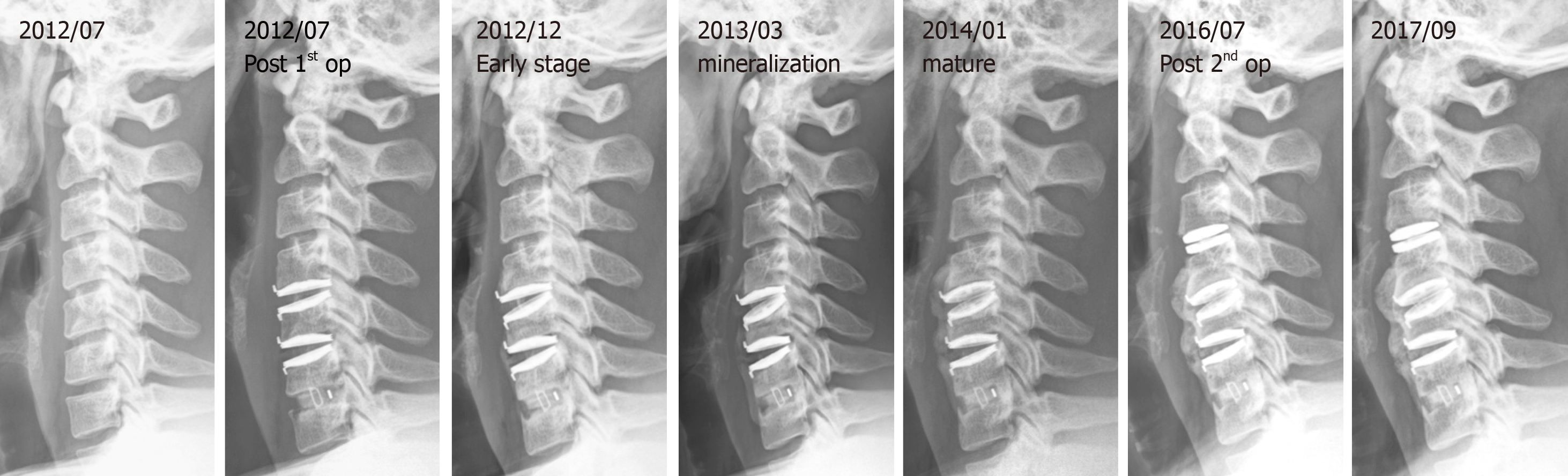
Tracing back the patient history, he suffered from a motorcycle crash about four years ago. Cervical spine radiographs at that time showed neuroforaminal narrowing of the bilateral C3-C4. MRI showed herniation of the intervertebral disc (HIVD) at the C4-C5, C5-C6, and C6-C7 levels (Figure 1). After the motorcycle accident, the patient received hybrid surgery for C4-C5 and C5-C6 arthroplasty with Bryan discs (Medtronic SofamorDanek, Memphis, TN, United States) and C6-C7 arthrodesis with polyetheretherketone cage (Figure 2). The symptoms soon resolved. The patient received regular follow-ups for 18 months and then was lost to follow-up. In the fourth year after the operation, the patient returned to our neurosurgical clinic due to left upper limb numbness and weakness for one week. These symptoms deteriorated into numbness in the upper back and all four limbs and eventually evolved into a gait disturbance.
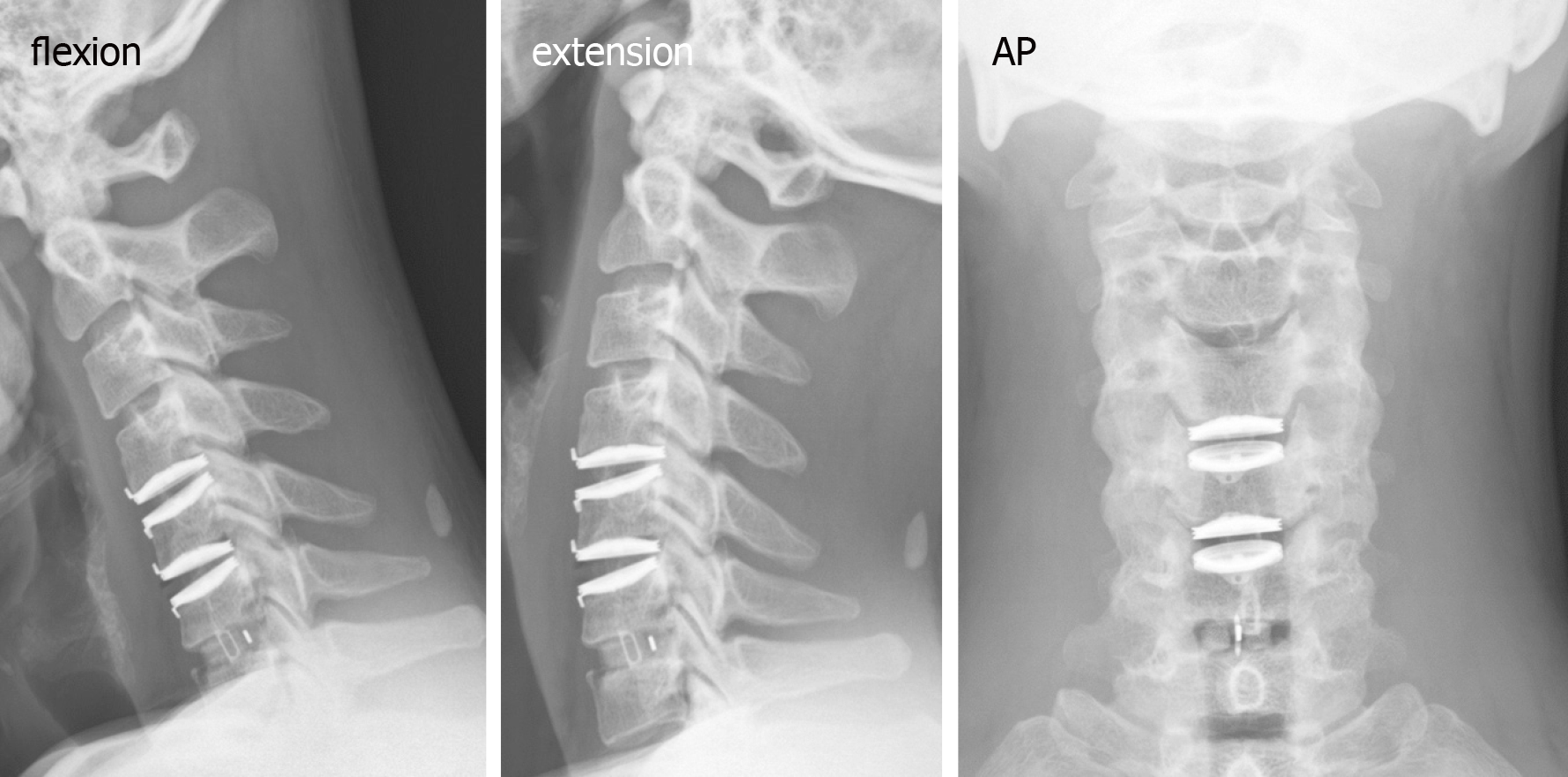
The cervical spine radiographs revealed severe HO around the Bryan discs, causing arthrodesis. However, there was no HO presentation in the anterior fusion level at level C6-C7 (Figure 3). The MRI image disclosed C3-C4 HIVD with spinal cord compression (Figure 3), a result compatible with the clinical presentation of myelopathy.
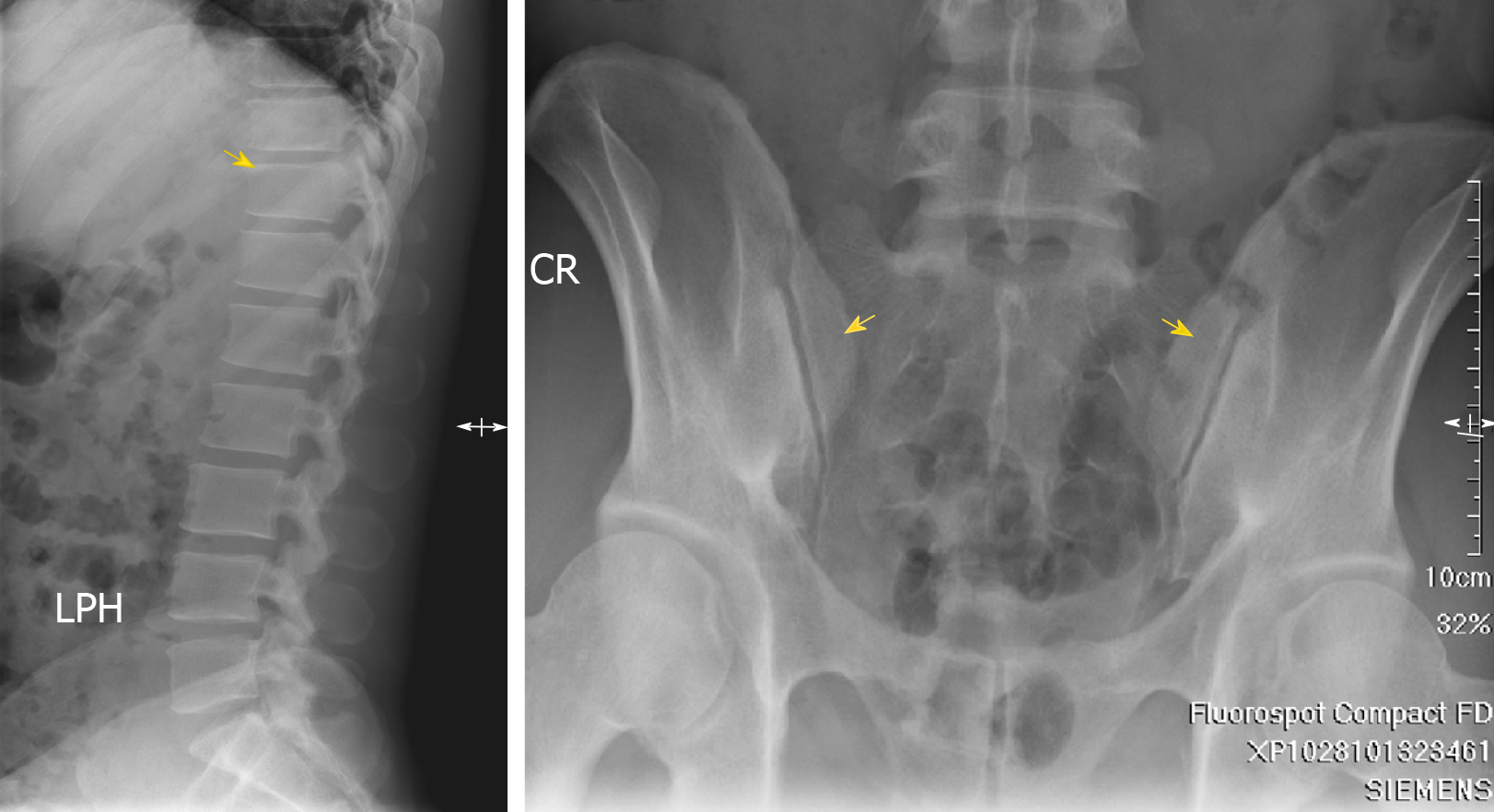
Image survey revealed sclerosis at the corners of the T-L spine (Shiny Corner Sign[5], Figure 4). Serum results were negative for rheumatoid arthritis factor and human leukocyte antigen B27. Seronegative spondyloarthritis was diagnosed in the following survey by our rheumatologist.
Due to the C3-C4 adjacent segment disease HIVD which developed four years later, the patient received arthroplasty with Baguera C disc (Spineart, Geneva, Switzerland) and felt much better soon after the operation. At this time, we used Baguera C disc to prevent HO because it involved less cortical bone damage, and prescribed the non-steroidal anti-inflammatory drug (NSAID) etoricoxib 60 mg daily.
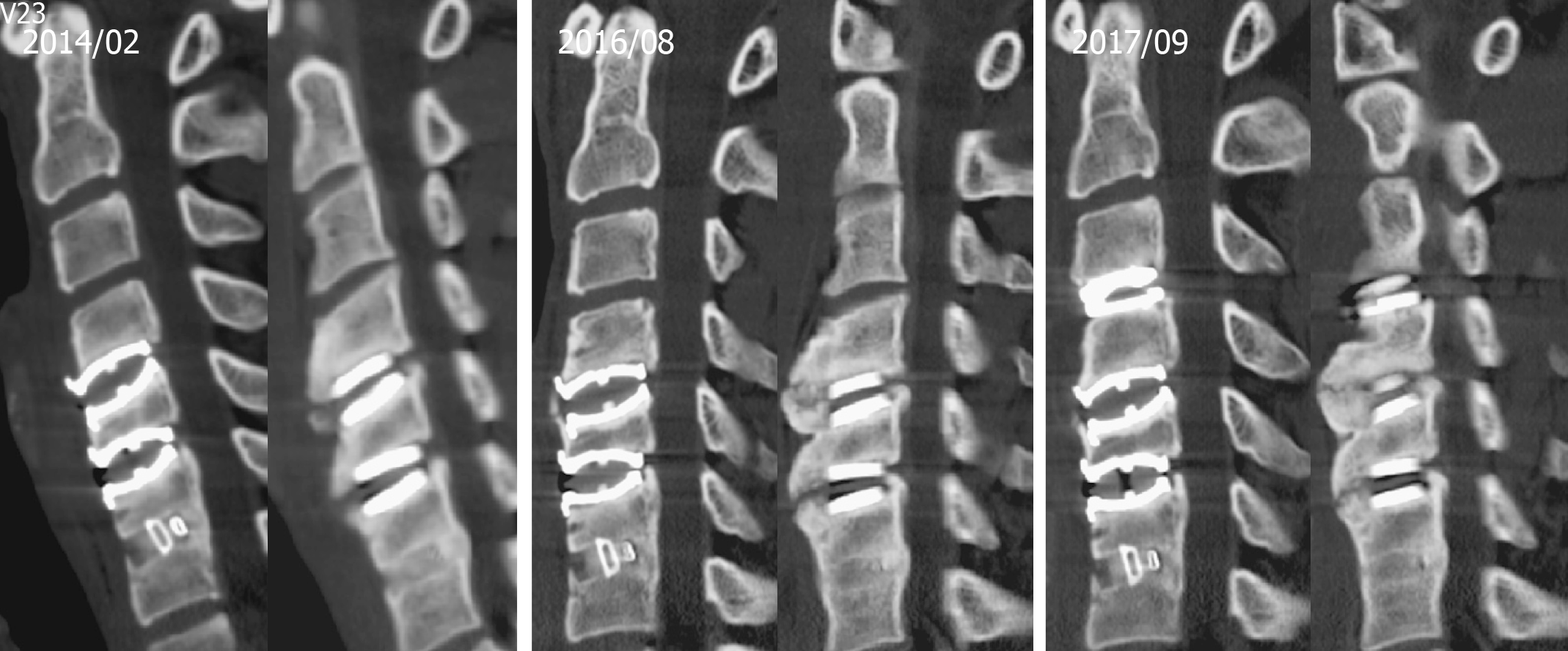
We collected a series of radiographs in this patient during follow-up (Figure 5). HO formation was noted from the C4-C5 and C5-C6 levels since the fifth month after the operation. The HO was at the mature stage 18 mo after the operation. There was no HO presentation at the arthrodesis level, C6-C7, nor at the arthroplasty level with the Baguera C disc, C3-C4. The cervical CT images showed anterior traction type spur formation without spinal stenosis (Figure 6).
The radiographic appearance of HO reveals the sequence of changes in bone maturation. The formation of HO is phasic and dynamic, making it easy to confuse with that of bone tumor; indeed, several nongenetic forms of HO, such as myositis ossificans, fibro-osseous pseudotumor of digits, and intraabdominal ossifying pseudotumors, have been designated as pseudotumors[1,6]. Hence, the diagnosis of HO should be established after ruling out primary bone tumors and paraspinal neurogenic tumors. Fortunately, the pre-operative imaging techniques of MRI, CT, and plain film X ray series offer sufficient information for a differential diagnosis of HO[6,7].
Many HO classifications have been developed based on the location, such as that of Brooker et al[8] in 1973 for the hip joint and that developed by Abrams in 1994 for the elbow joint. Over time, disc replacement was developed as an alternative therapy for spinal fusion. However, the preserved ROM in the operated segment is impaired over time, especially when HO occurs. HO is reported as a major cause of artificial disc dysfunction. HOs in total disc replacement (TDR) are quite different from HOs of the hip and knee, in terms of location, the absence of preceding inflammatory symptoms or signs, and the temporal pattern of growth[9]. The McAfee classification of HO in TDR[10] was developed by adjusting the HO classification of total hip replacement (THR) that was developed by Brooker in 1973[8].
As class III or IV HOs can limit the ROM of the implantation segment and may influence the clinical outcomes of CDA, they are defined as severe HOs[3]. The occurrence of severe HO in CDA ranges from 0% to 66.7%, and the overall prevalence is 17.0% (95% confidence interval). Because of clinical accessibility, image study is the most common tool for follow-up. Hence, in this case, an image study was performed and showed the ossification process evolving from early stage to mineralization and maturation (Figures 5 and 6).
Some studies of HO have focused on morphology, location, and their relationship to biomechanical stresses. HO is defined into three types resembling the spur formation mechanism in the aging spine[11]. Type 1 is the endplate type in the posterior that results from compressive force. Type 2 is the traction spur type found in the anterior or posterior view as a result of traction force. Type 3 is the teardrop type in the anterior position, and has no bony connection to the vertebral body.
Preventive measures include preventing unnecessary soft tissue traction and trauma such as burring, milling, or keeling[5]. In addition, the novel artificial disc with a mobile core has neither “lift off” phenomenon nor spontaneous movement under basic loading conditions[12], which may help to decrease micro-motion between the artificial disc and the endplate.
Early detection can occur with a series image study during postoperative follow-up. Plain radiographs are commonly the first imaging type used to detect HO, due to their low cost, accessibility, and dynamic view for functional tests. CT and MRI are needed in some cases, especially for final diagnosis. Technetium-99 bone scans can detect HO earlier than radiographs, but are limited by their cost and non-specificity to the inflammatory disease[13].
The most common and effective treatments for HO are NSAIDs and radiotherapy (RT). NSAIDs are efficacious in HO prophylaxis and can be used routinely for postoperative pain control. For indomethacin, a representative non-selective NSAID, the recommended dose is 75 mg twice per day or 25 mg three times per day for three to six weeks postoperatively[14]. Non-selective cyclooxygenase (COX)-1 and COX-2 inhibitors were found to have the same effect in HO prevention[15].
Cervical RT to prevent HO is not a common treatment. After THR, for example, HO is reported to range from 5% to 90%, with the prevalence after RT decreasing to 25%. Both preoperative and postoperative RT were found to have the same effectiveness as THR. Studies demonstrated no difference between NSAIDs and radiation in preventing HO (8%-22.2%) by the random-effects model[3].
The underlying pathophysiological mechanisms of HO are still to be investigated. One theory is that HO results from osteoprogenitor cells pathologically induced by an imbalance in local or systemic factors[16]. A transient increase in the biochemical markers of bone turnover and development of HO after THA, particularly the biochemical markers for osteoblast and osteoclast activity, has been suggested, as these changes are greater in patients who develop HO than in those who do not[17]. The conversion of progenitor cells into osteogenic precursor cells is affected by oxygen tension, pH, the availability of micronutrients, and mechanical stimuli[14]. Single nucleotide polymorphisms (SNPs) of the beta2-adrenergic receptor gene are associated with an increased risk of HO; however, SNPs in the genes of Toll-like receptor 4 and complement factor are associated with a decreased risk of HO[18]. Leptin receptors and beta2-adrenergic receptor mRNA expression in brain injury may also be related to HO[19]. In addition, neurogenic HO is a potential sequela of spinal cord injury or head injury[20].
The mechanism of HO formation after CDA is likewise unknown. In our patient, severe HO developed after the first CDA operation with Bryan discs over the C4-C5 and C5-C6 levels; however, no HO developed after the second operation with CDA over the C3-C4 level with Baguera C disc and after the first operation of interbody fusion over the C6-C7 level with PEEK cage. We postulate possible mechanisms to explain the phenomenon in this case. The CDA procedure is similar to creating two fracture fragments between two vertebral bodies. The endplates of the upper and lower vertebral bodies are cut and intervertebral disc tissue is excised, creating a phenomenon like a fracture gap in both ends. The structural support for upper and lower vertebral body stability is destroyed under conditions like a broken bone. Interbody fusion with a cage or a structural bone graft promotes bone fusion between two vertebral bodies to act biologically like a bone union. The stability of two vertebral bodies is greater in a cage or structural bone graft than with ADR. Secondary bone healing will be seen more in clinical follow-up of ADR cases than in cases with cage or structural bone grafts. For this reason, the ADR case is more likely to have HO, which acts biologically like a secondary bone healing process to provide stability for two unstable vertebral bodies. This process may explain why HO is more severe at the Bryan disc level compared with the PEEK cage level. Also, the mechanism of HO formation could be related to the transient joint instability after arthroplasty surgery due to damage to the soft tissue, such as the capsule, cartilage, or ligaments around or within the joint. To prevent movement around the joint after the operation, a signal pathway may be triggered to promote osteoblast or osteoclast activity, starting a chondrogenesis or osteogenesis process around the surgical site. In addition, the Baguera C disc is a lower profile prosthesis and produces less soft tissue damage than the Bryan Disc during the surgical procedure. Another reason may be related to the characteristics of seronegative spondyloarthritis itself, since peripheral extra-articular enthesitis is a clinical hallmark of seronegative spondyloar-thropathies[21]. In this unique case, we can exclude surgeon or patient factors as influencing the HO formation.
In this case, the severe traction spur type HO occurred after CDA with Bryan discs and matured in the second year after the operation. However, HO did not occur in the fusion level, nor in the CDA with Baguera C disc. Although this patient had severe HO with seronegative spondyloarthritis, ADR is not an absolute contraindication for seronegative spondyloarthritis. Based on the second operation experience, the avoidance of unnecessary soft tissue traction and trauma such as burring, milling, or keeling during the operation and the prophylactic use of NSAIDs are mandatory to prevent HO formation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd El-Razek A S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic Ossification: A Comprehensive Review. JBMR Plus. 2019;3:e10172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 2. | Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43:346-353. [PubMed] [Cited in This Article: ] |
| 3. | Kong L, Ma Q, Meng F, Cao J, Yu K, Shen Y. The prevalence of heterotopic ossification among patients after cervical artificial disc replacement: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e7163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Nunley PD, Cavanaugh DA, Kerr EJ, Utter PA, Campbell PG, Frank KA, Marshall KE, Stone MB. Heterotopic Ossification After Cervical Total Disc Replacement at 7 Years-Prevalence, Progression, Clinical Implications, and Risk Factors. Int J Spine Surg. 2018;12:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | ROMANUS R, YDEN S. Destructive and ossifying spondylitic changes in rheumatoid ankylosing spondylitis (pelvo-spondylitis ossificans). Acta Orthop Scand. 1952;22:88-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Abdel Razek AA, Castillo M. Imaging appearance of primary bony tumors and pseudo-tumors of the spine. J Neuroradiol. 2010;37:37-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Razek AAKA, Ashmalla GA. Assessment of paraspinal neurogenic tumors with diffusion-weighted MR imaging. Eur Spine J. 2018;27:841-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 8. | Brooker AF, Bowerman JW, Robinson RA, Riley LH. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Yi S, Shin DA, Kim KN, Choi G, Shin HC, Kim KS, Yoon DH. The predisposing factors for the heterotopic ossification after cervical artificial disc replacement. Spine J. 2013;13:1048-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003;16:384-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Jin YJ, Park SB, Kim MJ, Kim KJ, Kim HJ. An analysis of heterotopic ossification in cervical disc arthroplasty: A novel morphologic classification of an ossified mass. Spine J. 2013;13:408-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Lee JH, Park WM, Kim YH, Jahng TA. A Biomechanical Analysis of an Artificial Disc With a Shock-absorbing Core Property by Using Whole-cervical Spine Finite Element Analysis. Spine (Phila Pa 1976). 2016;41:E893-E901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification revisited. Orthopedics. 2011;34:177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Ranganathan K, Loder S, Agarwal S, Wong VW, Forsberg J, Davis TA, Wang S, James AW, Levi B. Heterotopic Ossification: Basic-Science Principles and Clinical Correlates. J Bone Joint Surg Am. 2015;97:1101-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 15. | Joice M, Vasileiadis GI, Amanatullah DF. Non-steroidal anti-inflammatory drugs for heterotopic ossification prophylaxis after total hip arthroplasty: A systematic review and meta-analysis. Bone Joint J. 2018;100-B:915-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006;65:1289-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Wilkinson JM, Stockley I, Hamer AJ, Barrington NA, Eastell R. Biochemical markers of bone turnover and development of heterotopic ossification after total hip arthroplasty. J Orthop Res. 2003;21:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Mitchell EJ, Canter J, Norris P, Jenkins J, Morris J. The genetics of heterotopic ossification: Insight into the bone remodeling pathway. J Orthop Trauma. 2010;24:530-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Chauveau C, Devedjian JC, Delecourt C, Jeanfils J, Hardouin P, Broux O. Leptin receptors and beta2-adrenergic receptor mRNA expression in brain injury-related heterotopic ossification. J Recept Signal Transduct Res. 2008;28:347-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Jensen LL, Halar E, Little JW, Brooke MM. Neurogenic heterotopic ossification. Am J Phys Med. 1987;66:351-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Barozzi L, Olivieri I, De Matteis M, Padula A, Pavlica P. Seronegative spondylarthropathies: Imaging of spondylitis, enthesitis and dactylitis. Eur J Radiol. 1998;27 Suppl 1:S12-S17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |