Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.2963
Peer-review started: July 14, 2019
First decision: August 2, 2019
Revised: August 9, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: October 6, 2019
Osteoarthritis is a chronic degenerative disease with an incidence of 50% in people over 65 years old and 80% in people over 80 years old worldwide. It is the second leading reason of loss of working capacity after cardiovascular diseases and severely affects the society and families. Therefore, finding biological markers related to the diagnosis and treatment of osteoarthritis is of great significance in clinical practice.
To observe the changes and clinical value of serum inflammatory factors and miR-145 expression in patients with osteoarthritis before and after treatment.
Eighty-three patients with knee osteoarthritis (observation group) who were admitted to our hospital from April 2013 to June 2015, and 60 healthy people (control group) during the same period were selected. After 4 wk of treatment, the levels of miR-145, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 were compared between the control group and the observation group before treatment. The correlation of miR-145, TNF-α, IL-6, and IL-10 levels with visual analogue scale (VAS), Lysholm, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores was assessed by Pearson correlation analysis. The correlation of the expression of miR-145, TNF-α, IL-6, and IL-10 with Kellgren-Lawrence (K-L) grades was assessed by Spearman correlation analysis. The critical levels of miR-145, TNF-α, IL-6, and IL-10 in distinguishing different K-L grades were determined by receiver operating characteristic (ROC) curve analysis.
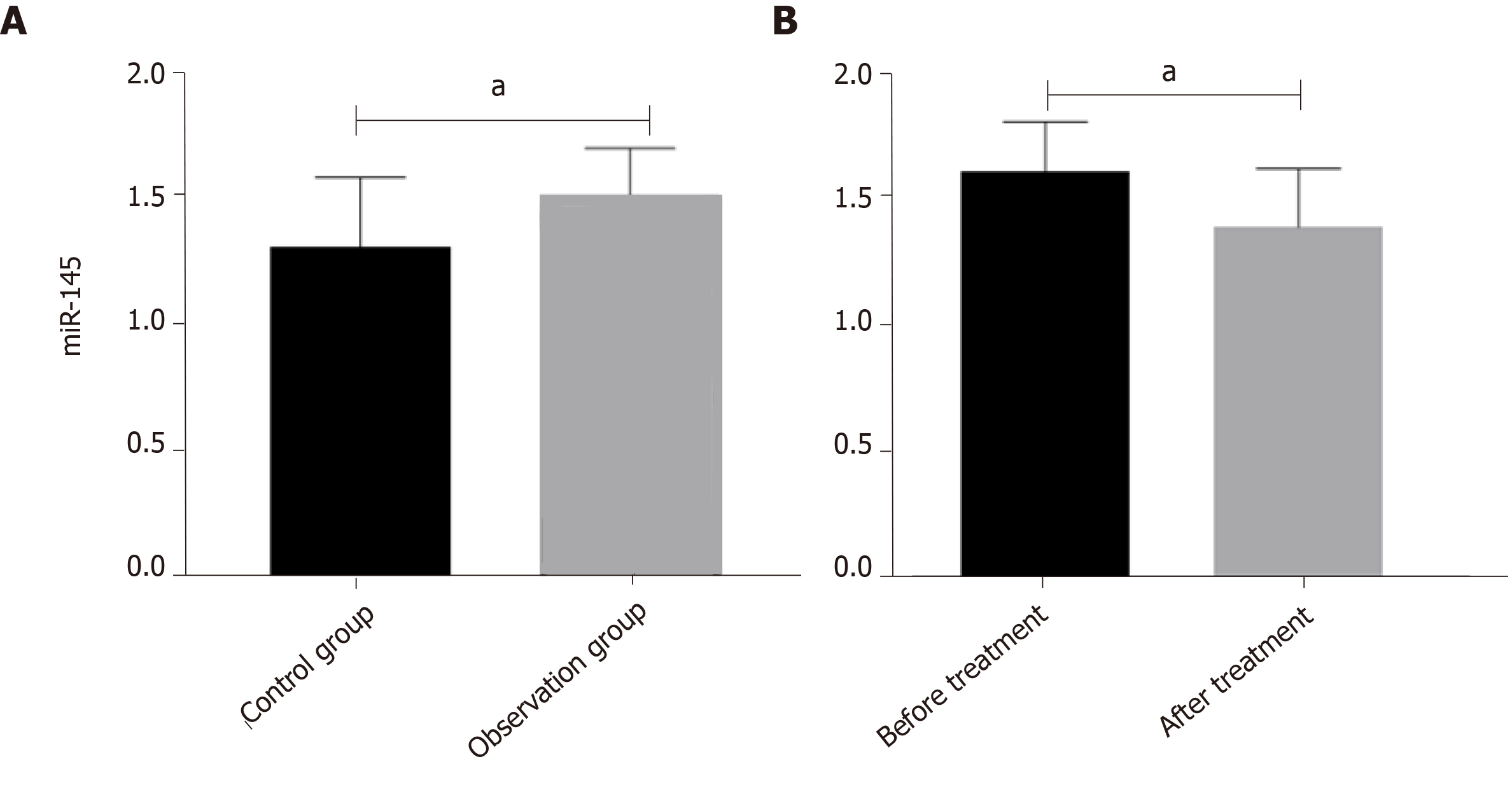
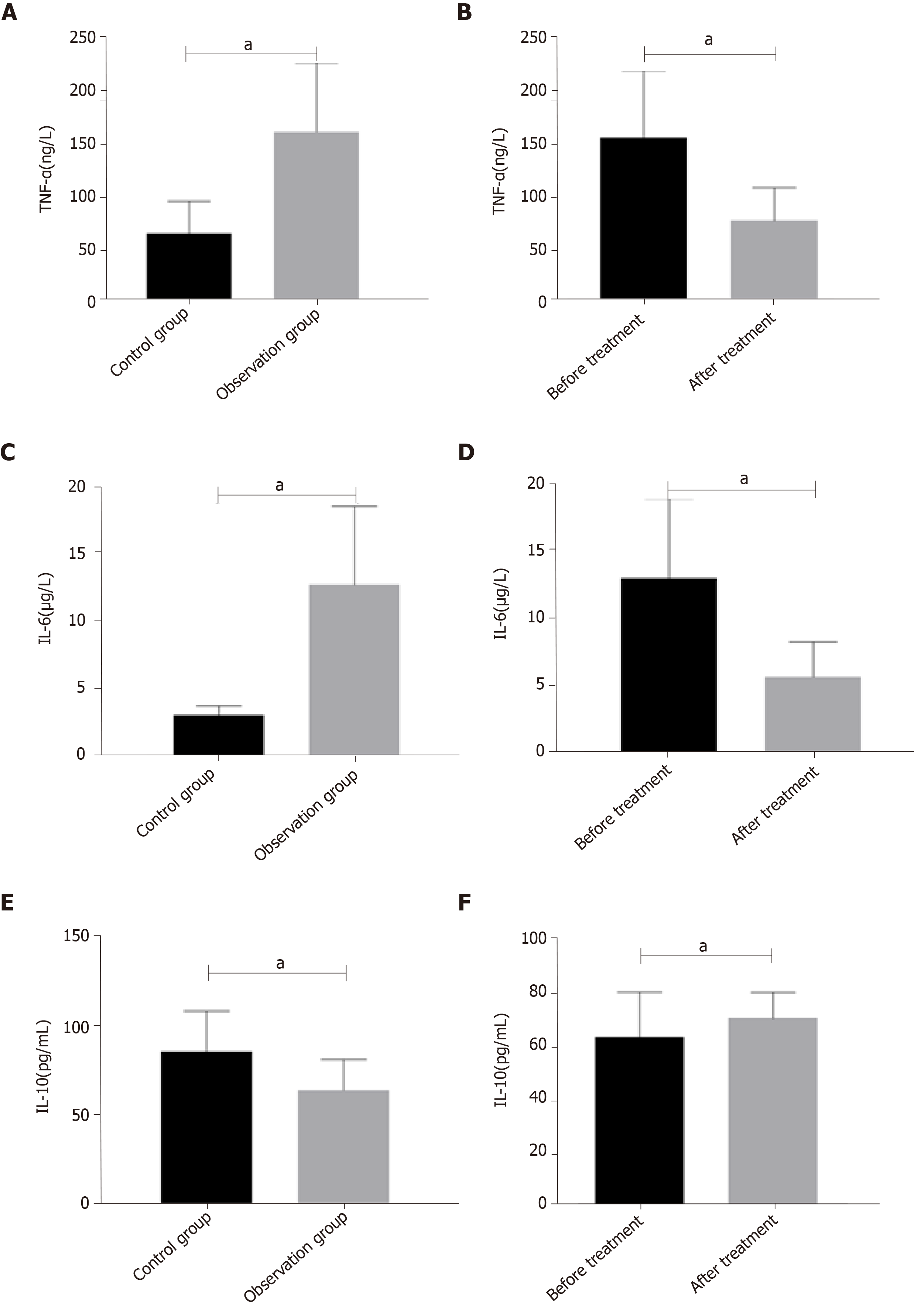
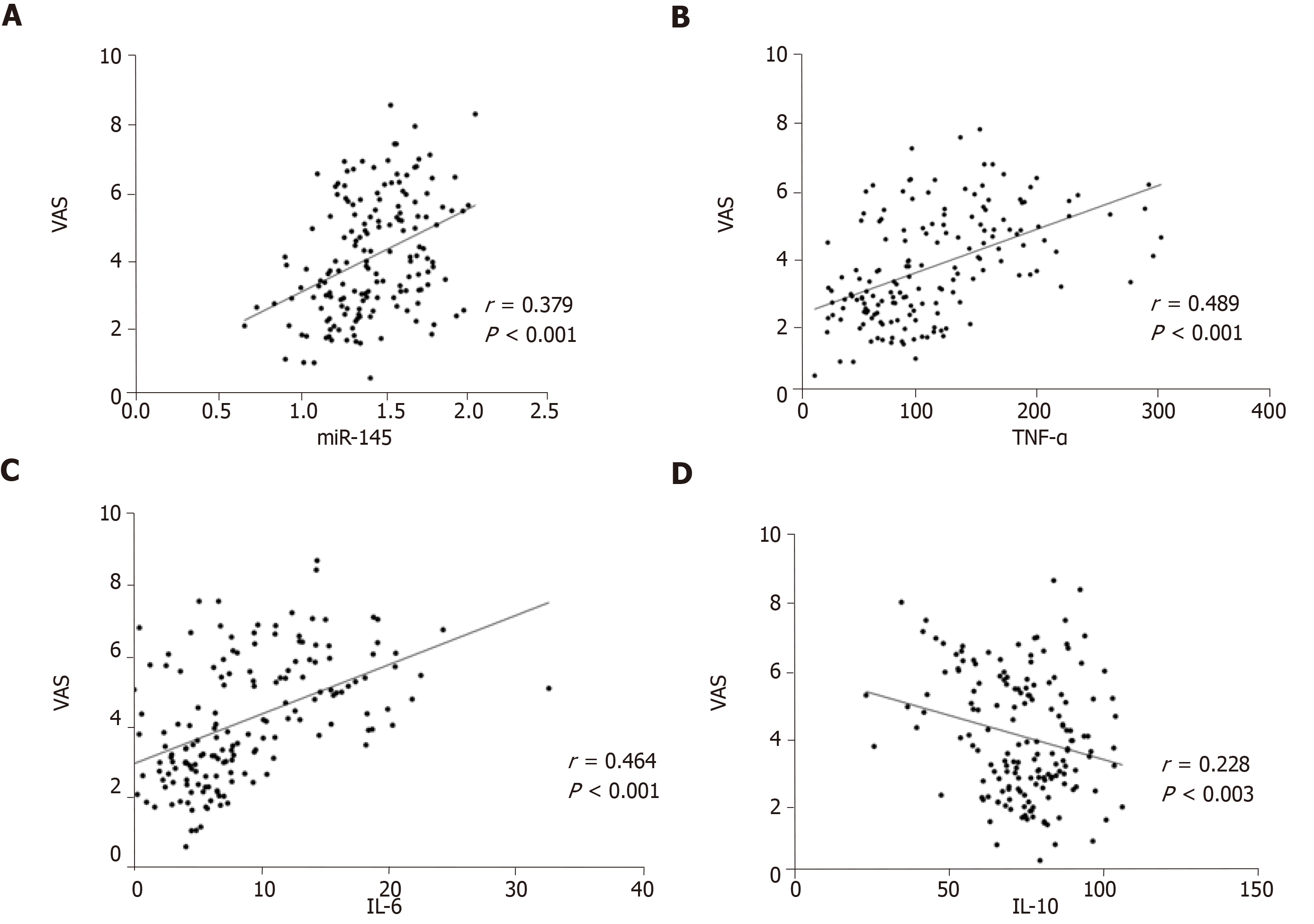
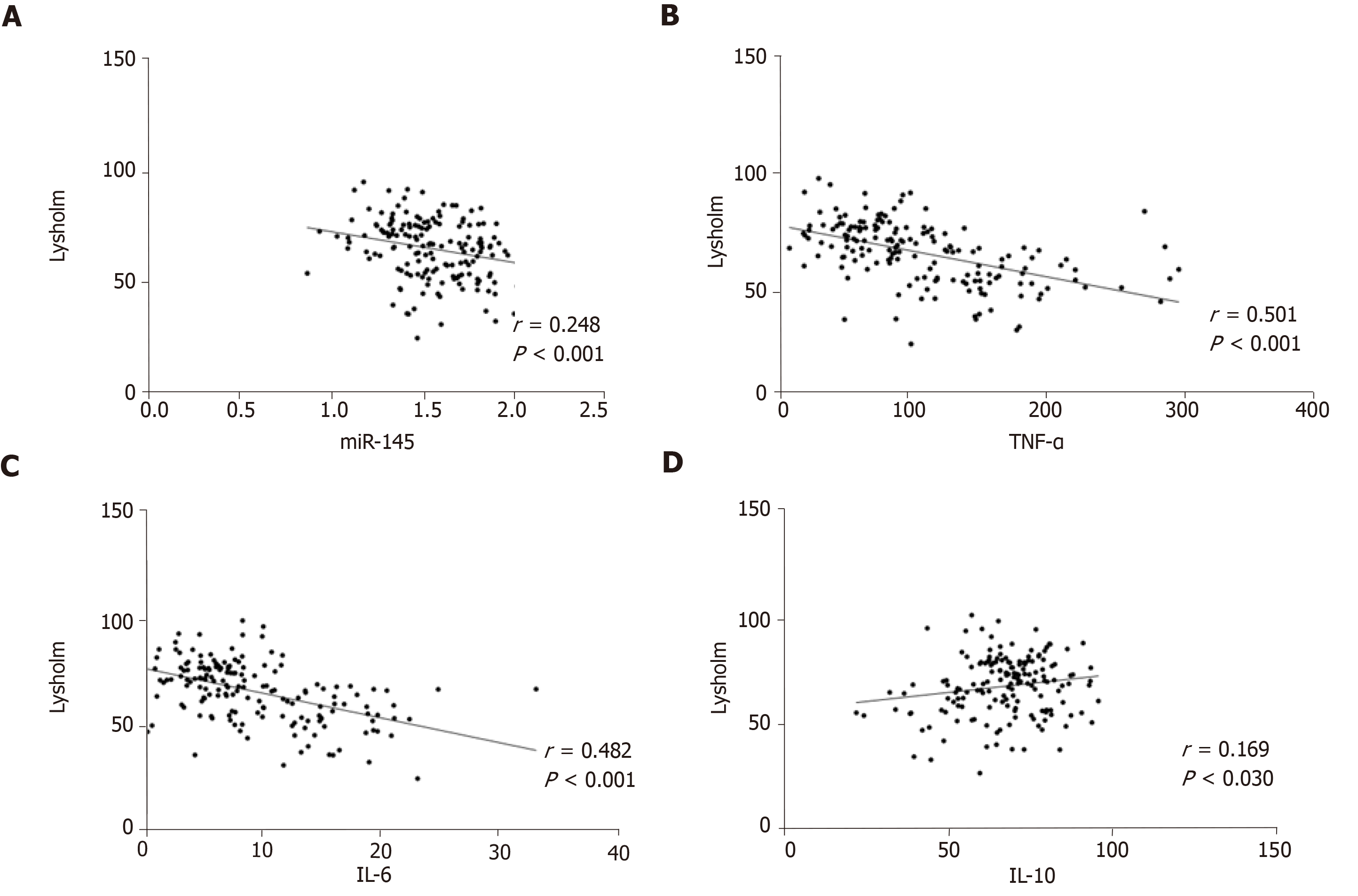
The expression level of miR-145 in the observation group was significantly higher than that in the control group before treatment (P < 0.05). After treatment, the expression level of miR-145 in the observation group was significantly lower than that before treatment (P < 0.05). The levels of TNF-α and IL-6 in the observation group were significantly higher than those in the control group (P < 0.05), and the level of IL-10 was significantly lower than that in the control group (P < 0.05). After treatment, the levels of TNF-α and IL-6 in the observation group were significantly lower than those before treatment (P < 0.05), and IL-I0 level was significantly higher than that before treatment (P < 0.05). VAS and WOMAC scores were both positively correlated with miR-145, TNF-α, and IL-6 (P < 0.05), and negatively correlated with IL-10 (P < 0.05), while Lysholm scores were negatively correlated with miR-145, TNF-α, and IL-6 (P < 0.05), and positively correlated with IL-10 (P < 0.05). K-L grades were positively correlated with miR-145, TNF-α, and IL-6 (P < 0.05), and negatively correlated with IL-10 (P < 0.05). The area under the ROC curve (AUC) and specificity of TNF-α in differentiating K-L grades I-II were the highest, which were 0.785 and 97.45%, respectively, and miR145 had the highest sensitivity of 94.59%; the AUC and sensitivity of IL-6 in differentiating K-L grades II-III were the highest, which were 0.766 and 97.30%, respectively, and TNF-α had the highest specificity of 86.68%.
MiR-145 and inflammatory factors have certain diagnostic value in osteoarthritis, and they are expected to become potential indicators for the diagnosis and evaluation of osteoarthritis in the future.
Core tip: At present, the clinical evaluation of osteoarthritis relies on VAS score, Lysholm score, and WOMAC score and other scales. These scales have a common deficiency, i.e., they are affected by a certain degree of subjective factors. Many clinical workers use Kellgren-Lawrence (K-L) grading to evaluate the therapeutic effect of patients with osteoarthritis, but the K-L grading depends on imaging, the effect of radiation on the human body and the medical expenses are high, and it takes a long time. This study explored the clinical value of miR-145 and inflammatory factors in peripheral blood, and explored the application of serological analysis in the diagnosis and treatment of osteoarthritis, so as to provide a reference for the diagnosis and treatment of osteoarthritis.
- Citation: Wang XZ, Li WX. Changes of serum inflammatory factors and miR-145 expression in patients with osteoarthritis before and after treatment and their clinical value. World J Clin Cases 2019; 7(19): 2963-2975
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/2963.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.2963
Osteoarthritis is a chronic degenerative disease characterized by cartilage erosion and lesions in the subchondral bone and other joint tissues, mostly in the hip and knee joints[1,2]. According to epidemiological statistics of osteoarthritis, the incidence of osteoarthritis in people over 65 years old can reach 50%, and the incidence of osteoarthritis in people over 80 years old is as high as 80%[3]. Osteoarthritis is the second most important cause of patients' loss of ability after cardiovascular diseases, seriously affecting the society and families[4,5].
Ultrasound is widely used in the diagnosis and efficacy evaluation of various joint diseases, which has good repeatability and is closely related to the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score. However, its operation requires experienced and skilled imaging doctors and high-resolution ultrasound equipment[6-8]. Therefore, finding osteoarthritis-related biomarkers has become a research focus in recent years.
MicroRNAs (miRNAs) are a class of non-coding small RNAs belonging to miRNA-induced silencing complexes[9]. In previous studies, it was found that the occurrence and development of osteoarthritis are accompanied by changes in various miRNAs. Santini et al[10] reported that miR-149 is down-regulated in osteoarthritis, which is associated with increased expression of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. Philipot et al[11] found that miR-24 negatively regulates the p16INK4a/MMP1 axis in osteoarthritis, which is involved in the senescence of inflammatory chondrocytes and matrix remodeling related to terminal differentiation. In recent years, it has been reported that miR-145 can regulate the function of cartilage cells and promote cartilage recovery[12]. In a related report on miRNA screening in osteoarthritis, miR-145 was found to increase in osteoarthritis[13]. These studies suggest that miR-145 plays an important role in the development of osteoarthritis. However, there are few reports on the value of miR-145 in the clinical application of osteoarthritis.
This study aimed to observe the changes and clinical value of serum inflammatory factors and miR-145 expression in patients with osteoarthritis before and after treatment, and provide a theoretical and experimental basis for clinical treatment of hip osteoarthritis.
Eighty-three patients with knee osteoarthritis (observation group; aged 42-70 years old) admitted to our hospital from April 2013 to June 2015, and 60 healthy people (control group; aged 42-70 years old) during the same period were selected. The inclusion criterion was as follows: (1) All patients in the observation group met the 2007 osteoarthritis diagnostic guidelines[14], and knee osteoarthritis was treated according to the OARSI guidelines[15]; and (2) All patients in the observation group underwent X-ray radiology diagnosis and performed Kellgren-Lawrence (K-L) radiation grading. The exclusion criteria were as follows: (1) K-L grade IV in the observation group; (2) Patients with cataclasis, other systemic inflammatory syndrome, severe endocrine dysfunction, pregnant women, cardiovascular, liver and kidney diseases, neurological or psychiatric history, chronic pain syndrome, language communication difficulties, joint bone tumors, various cancer bone metastases, recent acute stroke, autoimmune diseases, or other inflammatory arthritis; and (3) The control group had obvious clinical disease characteristics, pregnant women, psychiatric history, and language communication disorders. This study was approved by the Hospital Ethics Committee. All patients and their families signed an informed consent form.
Before treatment and after 4 wk of treatment, the levels of miR-145, TNF-α, IL-6, and IL-I0 were compared between the control group and observation group. The correlation of miR-145, TNF-α, IL-6, and IL-10 levels with visual analogue scale (VAS), Lysholm, and WOMAC scores was assessed by Pearson correlation analysis. The correlation of the expression of miR-145, TNF-α, IL-6, and IL-10 with K-L grades was assessed by Spearman correlation analysis. The critical levels of miR-145, TNF-α, IL-6, and IL-10 in distinguishing different K-L grades were determined by receiver operating characteristic (ROC) curve analysis.
Peripheral blood was collected from all patients before and after treatment, and serum was taken after centrifugation. Total RNA in serum was extracted with TRIzol (Guangzhou Labgene Biotechnology Co., Ltd.) following the manufacturer’s protocol. A micro-ultraviolet spectrophotometer DanoProp1000 (Thmorgan Biotechnology Co., Ltd.) was used to analyze the concentration and purity of the extracted RNA, and 3% agarose gel electrophoresis (gel electrophoresis kit purchased from Shanghai JingkeChemical Technology Co., Ltd.) was used to analyze the integrity of RNA. The A260/A280 value between 1.8 and 2.1 was considered to meet the experimental requirements. After RNA extraction was completed, qRT-PCR reaction was carried out. Reverse transcription was performed in a 20-μL system consisting of 4 μL of 5 × TransScript All in One SuperMix for PCR, 2 μg of total RNA, and appropriate amount of ribonuclease free distilled water. The reaction conditions were 25 °C for 10 min, 42 °C for 30 min, and inactivation of reverse transcriptase at 85 °C for 5 s. After reverse transcription, PCR amplification was carried out in a 50 μL amplification system consisting of 2 μL of cDNA template, 25 μL of 2 × TransTaq HiFi PCR SuperMix II, 1 μL of upstream primer and downstream primer, and appropriate amount of double distilled water. The cycling parameters were pre-denaturation at 95°C for 3 min, 40 cycles of 94 °C for 2 min, 94 °C for 30 s, 55 °C for 30 s, 72°C 1-2 kb/min, and extension for 5 min at 72 °C. U6 was used as the internal reference of the reaction, and the results were analyzed by the 2-△△Ct method. TransScript Two-Step RT-PCRSuperMix was purchased from Beijing TransGen Biotech, with the catalog number of AT401-0l, and the primer sequence was designed and synthesized by Hepeng (Shanghai) Biotechnology Co., Ltd. (Table 1).
| Forward primer | Reverse primer | |
| MiR-145 | 5'-GTCCAGTTTTCCCAGGAATCCCT- 3' | 5'-GCTGTCAACATACGCTACGTAACG- 3' |
| U6 | 5'-GCGCGTCGTGAAGCGTTC- 3' | 5'-GTGCAGGGTCCGAGGT- 3' |
Peripheral blood was collected from all patients before and after treatment, and serum was taken after centrifugation. The levels of TNF-α, IL-6, and IL-10 were detected by ELISA using kits purchased from Abcam (ab181421, ab178013, and ab46601) following the kit instructions.
SPSS 22.0 software (Asia Analytics Formerly SPSS, China) was used in this study for statistical analyses. Measurement data are expressed as the mean ± SD, and count data are expressed as percentages. Count data were compared by χ2 tests, and measurement data were compared by the independent sample t-test between two groups. The correlation of miR-145, TNF-α, IL-6, and IL-10 levels with VAS, Lysholm, and WOMAC scores was assessed by Pearson correlation analysis. The correlation of the expression of miR-145, TNF-α, IL-6, and IL-10 with K-L grades was assessed by Spearman correlation analysis. The critical levels of miR-145, TNF-α, IL-6, and IL-10 in distinguishing different K-L grades were determined by ROC curve analysis. Statistical significance was indicated by P < 0.05.
There were 60 patients in the control group, including 33 males (55.00%) and 27 females (45.00%), with a mean age of 54.76 ± 13.64 years old and a mean body mass index (BMI) of 23.53 ± 3.06 kg/m2. There were 83 patients in the observation group, including 52 males (62.65%) and 31 females (37.35%), with a mean age of 57.48 ± 12.49 years old and a mean BMI of 24.77 ± 4.14 kg/m2. There was no significant difference in sex ratio, age, or BMI between the two groups (P > 0.05). Other basic data of the observation group are shown in Table 2.
| Control group (n = 60) | Observation group (n = 83) | χ2/t | P-value | |
| Sex, n (%) | 0.846 | 0.358 | ||
| Male | 33 (55.00) | 52 (62.65) | ||
| Female | 27 (45.00) | 31 (37.35) | ||
| Age (yr) | 54.76 ± 13.64 | 57.48 ± 12.49 | 1.236 | 0.218 |
| BMI (kg/m2) | 23.53 ± 3.06 | 24.77 ± 4.14 | 1.964 | 0.052 |
| Course of disease (yr) | 3.25 ± 1.37 | |||
| K-L grade, n (%) | ||||
| I | 31 (37.35) | |||
| II | 37 (44.58) | |||
| II | 15 (18.07) | |||
| VAS score | ||||
| Before treatment | 5.49 ± 1.27 | |||
| After treatment | 2.53 ± 088a | |||
| Lysholm score | ||||
| Before treatment | 58.45 ± 10.62 | |||
| After treatment | 75.26 ± 9.46a | |||
| WOMAC score | ||||
| Before treatment | 50.46 ± 9.14 | |||
| After treatment | 18.86 ± 8.38a | |||
| Disease site | ||||
| Left knee | 40 (48.19) | |||
| Right knee | 31 (37.35) | |||
| Both knees | 12 (14.46) |
The expression level of miR-145 in the observation group was significantly higher than that in the control group before treatment (P < 0.05) (Figure 1A). The expression level of miR-145 in the observation group after treatment was significantly lower than that before treatment (P < 0.05) (Figure 1B).
The levels of TNF-α and IL-6 in the observation group were significantly higher than those in the control group (P < 0.05), and the level of IL-10 was significantly lower than that in the control group (P < 0.05) (Figure 2A). The levels of TNF-α and IL-6 in the observation group after treatment were significantly lower than those before treatment (P < 0.05), and IL-10 level was significantly higher than that before treatment (P < 0.05) (Figure 2B).
VAS scores were positively correlated with miR-145, TNF-α, and IL-6, and negatively correlated with IL-10 (P < 0.05) (Figure 3).
Lysholm scores were negatively correlated with miR-145, TNF-α, and IL-6 (P < 0.05), and positively correlated with IL-10 (P < 0.05) (Figure 4).
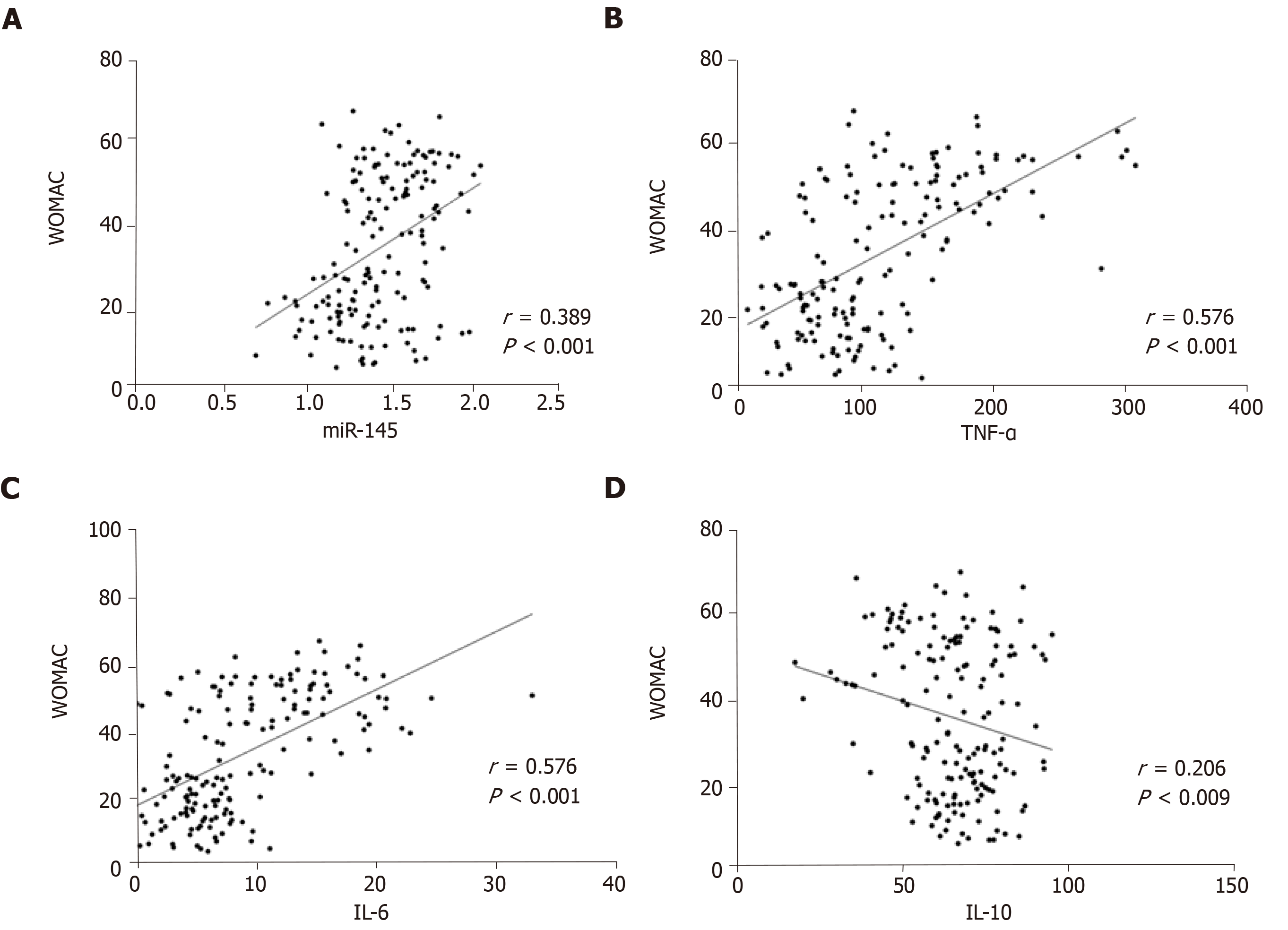
WOMAC scores were positively correlated with miR-145, TNF-α, and IL-6 (P < 0.05), and negatively correlated with IL-10 (P < 0.05) (Figure 5).
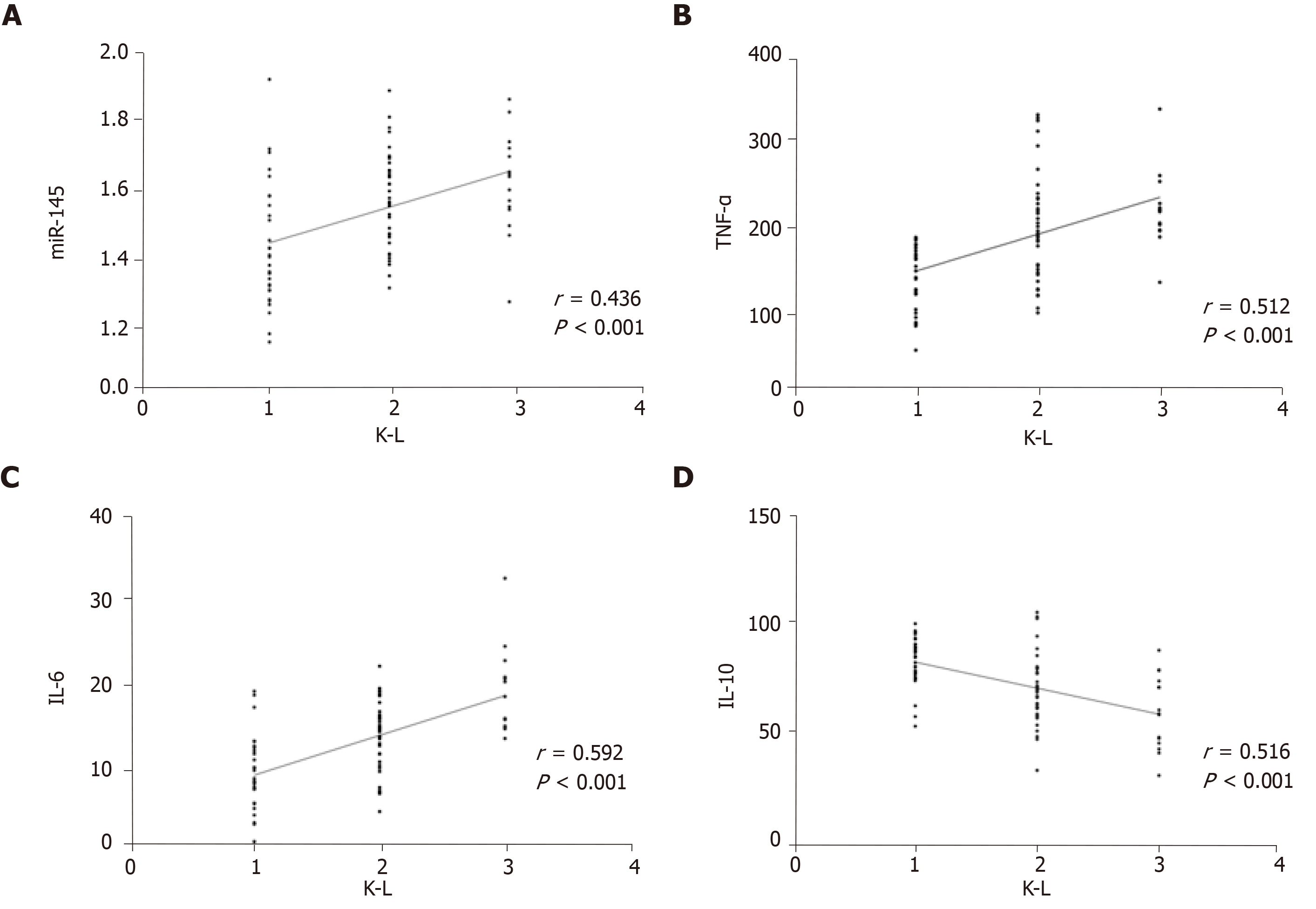
L grades were positively correlated with miR-145, TNF-α, and IL-6 (P < 0.05), and negatively correlated with IL-10 (P < 0.05) (Figure 6).
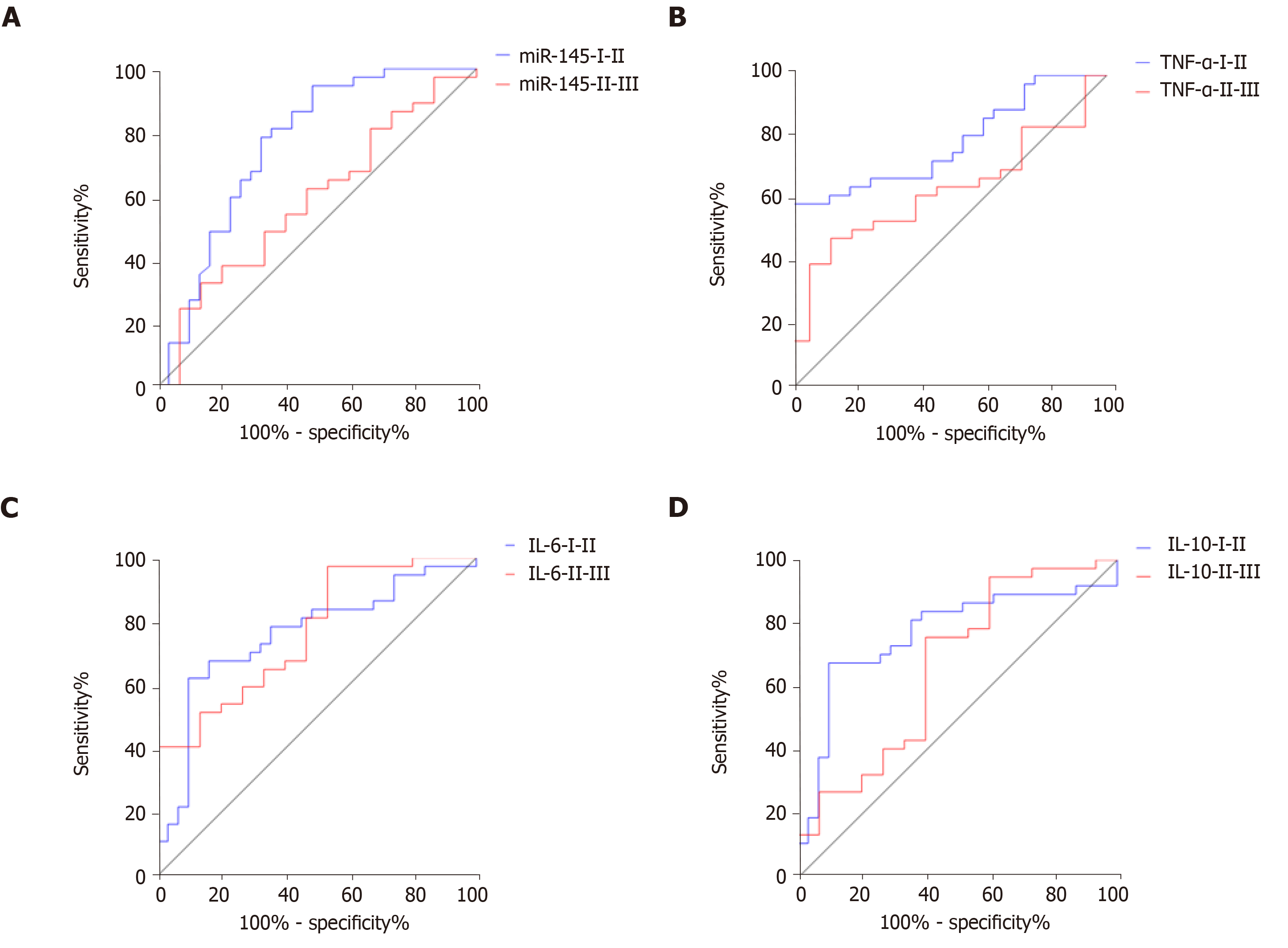
The area under the ROC area (AUC), critical level, sensitivity, and specificity of miR-145 in distinguishing K-L grades I and II were 0.762, 1.356, 94.59%, and 51.61%, respectively, and 0.587, 1.475, 32.43%, and 86.67% for grades II and III (Figure 7A). The AUC, critical level, sensitivity, and specificity of TNF-α in differentiating K-L grades I and II were 0.785, 142.00 ng/L, 59. 46%, and 97.45%, respectively, and 0.631, 167.80 ng/L, 48.65%, and 86.68% for grades II and III (Figure 7B). The AUC, critical level, sensitivity, and specificity of IL-6 in distinguishing K-L grades I and II were 0.758, 13.06 μg/L, 62. 16%, and 90.32%, respectively, and 0.766, 19.62 μg/L, 97.30%, and 46.67% for grades II and III (Figure 7C). The AUC, critical level, sensitivity, and specificity of IL-10 in differentiating K-L grades I and II were 0.767, 64.37 pg/mL, 67.57%, and 90.32%, respectively, and 0.663, 51.43 pg/mL, 75.68%, and 60.00% for grades II and III (Figure 7D) (Table 3).
| miR-145 | TNF-α | IL-6 | IL-10 | |
| I-II | ||||
| AUC | 0.762 | 0.785 | 0.758 | 0.767 |
| Critical level | 1.356 | 142.00 ng/L | 13.06 μg/L | 64.37 pg/mL |
| Sensitivity | 94.59% | 59.46% | 62.16% | 67.57% |
| Specificity | 51.61% | 97.45% | 90.32% | 90.32% |
| II-III | ||||
| AUC | 0.587 | 0.631 | 0.766 | 0.663 |
| Critical level | 1.475 | 167.80 ng/L | 19.62 μg/L | 51.43 pg/mL |
| Sensitivity | 32.43% | 48.65% | 97.30% | 75.68% |
| Specificity | 86.67% | 86.68% | 46.67% | 60.00% |
At present, the clinical evaluation of osteoarthritis relies on VAS score, Lysholm score, and WOMAC score and other scales[16-18]. These scales have a common deficiency, i.e., they are affected by a certain degree of subjective factors. Many clinical workers use K-L grading to evaluate the therapeutic effect of patients with osteoarthritis[19], but the K-L grading depends on imaging, the effect of radiation on the human body and the medical expenses are high, and it takes a long time[20]. This study explored the clinical value of miR-145 and inflammatory factors in peripheral blood, and explored the application of serological analysis in the diagnosis and treatment of osteoarthritis, so as to provide a reference for the diagnosis and treatment of osteoarthritis.
We first analyzed the changes of miR-145 and inflammatory factor levels in peripheral blood of healthy people and patients with knee osteoarthritis before and after treatment. It is clear that miR-145 is elevated in patients with knee osteoarthritis. And after treatment, the levels of inflammatory factors are consistent with previous reports[21,22]. TNF-α and IL-6 levels in peripheral blood of patients with knee osteoarthritis increased, while IL-10 levels decreased. After treatment, the levels decreased and increased accordingly. At present, there are few clinical studies on the correlation between miR-145 and osteoarthritis. From our analysis, miR-145, TNF-α, IL-6, and IL-10 had a linear correlation with VAS, Lysholm, and WOMAC scores, suggesting that these serological factors are closely related to the clinical efficacy of osteoarthritis. But the degree of correlation is mostly moderate or low, suggesting that there are still shortcomings in the use of miR-145 and inflammatory factors to evaluate the therapeutic effect. The pathogenesis of osteoarthritis is complex, especially primary osteoarthritis[23]. At present, the cause of osteoarthritis is not clear, and it may be related to the age span of our subjects and the long course of osteoarthritis[24]. Moreover, we have not further analyzed the efficacy of miR-145 and inflammatory factors in primary osteoarthritis and secondary osteoarthritis, because the specific reasons need to be further analyzed. In recent years, many scholars have verified the mechanism of action of miR-145 in osteoarthritis, such as the inhibitory effect of miR-145 targeting TNFRSF11B on proliferation and fibrosis of human osteoarthritis cartilage cells[25]. MiR-145 inhibits cartilage matrix degradation driven by TNF-α in osteoarthritis by directly inhibiting mitogen-activated protein kinase 4[26]. And researchers reported that down-regulation of miR-145 expression can promote apoptosis of cartilage cells in osteoarthritis[27], and miR-145 knockout can also inhibit the proliferation/differentiation of osteoclasts, induce osteoclast apoptosis, limit bone excessive absorption, and finally reduce osteoarthritis by up-regulating the expression of osteopontin[28,29]. These reports also suggested the complex role of miR-145 in osteoarthritis. Therefore, if miR-145 is used to evaluate the clinical efficacy of osteoarthritis, the time point of blood collection needs to be further clarified. We will design more rigorous and detailed experimental steps in the future research.
K-L grade is an important index for the diagnosis and grading of osteoarthritis. We analyzed the relationship of miR-145, TNF-α, IL-6, and IL-10 with K-L grades. K-L grades were positively correlated with miR-145, TNF-α, and IL-6, and negatively correlated with IL-10. ROC analysis showed that miR-145, TNF-a, IL-6, and IL-10 can better distinguish K-L grades I and II and grades II and III, and all the AUCs exceeded 0.5, although they had the highest discrimination value for K-L grades I and II. The above results also suggested that miR-145, TNF-α, IL-6, and IL-10 are closely related to the imaging findings in patients with osteoarthritis. In previous reports, the relationship between IL-6 and K-L grades was analyzed. Shimura et al[30] reported that there was a negative correlation between the levels of IL-6 in synovium and K-L grades in patients with knee osteoarthritis, which is completely contrary to our results. Shimura et al[31] reported that there was no significant difference in serum IL-6 levels between early stage (K-L grade 2) and advanced stage (K-L grades 3 and 4) in patients with knee osteoarthritis, but some studies have reported that IL-6 levels in peripheral blood increase with the severity of knee osteoarthritis[32]. They have not further analyzed or speculated on this phenomenon, so we can only speculate that this is related to the difference in the subjects of the study and the origin of the specimens. Therefore, more research is needed to further confirm this result.
In summary, miR-145 and inflammatory factors have certain diagnostic value in osteoarthritis, and they are expected to become potential indicators for future diagnosis and evaluation of osteoarthritis.
Osteoarthritis is the most common musculoskeletal disease in the world, which is characterized by articular cartilage degeneration, subchondral sclerosis, and narrowing of joint space. It is the sixth leading cause of disability worldwide and is expected to rise to the fourth by 2020, causing serious impact on the society and families. Therefore, finding biological markers related to the diagnosis and treatment of osteoarthritis is of great significance in clinical practice.
At present, the clinical efficacy evaluation for osteoarthritis mostly depends on VAS, Lysholm, and WOMAC scores, all of which are easily affected by subjective factors. Kellgren-Lawrence (K-L) classification is usually employed to assess the therapeutic effect of osteoarthritis patients as well, but it has the disadvantages of radiation, high cost, and being time-consuming. This study explored the application of serological analysis in diagnosis and treatment of osteoarthritis by analyzing the diagnostic and therapeutic value of miR-145 and inflammatory factors in peripheral blood of patients, so as to provide a reference for clinical diagnosis and treatment of osteoarthritis.
The study aimed to explore the application of serological analysis in the diagnosis and treatment of osteoarthritis by analyzing the diagnostic and therapeutic value of miR-145 and inflammatory factors in peripheral blood of patients, so as to provide a reference for clinical diagnosis and treatment of osteoarthritis.
Eighty-three patients with knee osteoarthritis admitted to our hospital from April 2013 to June 2015 and 60 healthy subjects were enrolled in our study. The expression of miR-145 in the peripheral blood in the two groups was detected by qRT-PCR, and the changes of miR-145, tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, as well as VAS, Lysholm, and WOMAC scores after 4 weeks of treatment were compared between the two groups. Furthermore, Pearson correlation analysis, Spearman correlation analysis, and ROC curve analysis were used to analyze the relationship of miR-145, TNF-α, IL-6, and IL-10 levels with VAS, Lysholm, and WOMAC scores to verify the application value of miR-145 and inflammatory factors in the diagnosis and treatment of osteoarthritis.
This study found that the expression of miR-145 and inflammatory factors increased in patients with knee osteoarthritis, with corresponding decrease and increase after treatment. VAS, Lysholm, and WOMAC scores were significantly improved after treatment, suggesting that the treatment was effective and the reduction of miR-145 and inflammatory factors after treatment might be related to the improvement of the patient's condition. Pearson correlation analysis showed that VAS, Lysholm, and WOMAC scores were either positively or negatively correlated with miR-145 and inflammatory factors, which verified our previous speculation. Moreover, the changes of miR-145 and inflammatory factors were related to the improvement of the patient's condition, indicating that miR-145 and inflammatory factors might be the potential efficacy predictors for osteoarthritis. This study also revealed that miR-145 and inflammatory factors were closely related to K-L classification, which suggested that miR-145 and inflammatory factors are valuable in determining the severity of osteoarthritis, and might be potential biological markers for osteoarthritis diagnosis in the future.
The levels of miR-145 and inflammatory factors (TNF-α, IL-6, and IL-10) in peripheral blood of patients with osteoarthritis are significantly higher than those in healthy individuals, and increase with the severity of the disease, suggesting that miR-145 and inflammatory factors might be objective indicators to assess the progression of osteoarthritis. After treatment, the levels of miR-145 and inflammatory factors change significantly in patients with osteoarthritis, and are closely related to therapeutic effect-related indicators (VAS, Lysholm, and WOMAC scores). Therefore, miR-145 and inflammatory factors have potential value for evaluating the therapeutic effect on osteoarthritis. This study demonstrates that miR-145 and inflammatory factors are valuable in the diagnosis and treatment of osteoarthritis, which are expected to become potential indicators for future diagnosis and efficacy evaluation.
This study reveals the potential role of miR-145 and inflammatory factors in the diagnosis and treatment of osteoarthritis. For the better use of miR-145 and inflammatory factors in clinical practice, the blood sampling time point needs further confirmation. Besides, whether miR-145 can become a target for osteoarthritis treatment needs further analysis. The mechanism of action of miR-145 on the occurrence and development of osteoarthritis needs to be verified by in vitro cell experiments and in vivo animal experiments to provide an experimental basis for identifying new targets for osteoarthritis treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd El-Razek A, Anand A, Musumeci G S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:507-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 503] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 2. | Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 3. | da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 4. | Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, McLachlan AJ, Ferreira ML. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 309] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 5. | Qin J, Barbour KE, Murphy LB, Nelson AE, Schwartz TA, Helmick CG, Allen KD, Renner JB, Baker NA, Jordan JM. Lifetime Risk of Symptomatic Hand Osteoarthritis: The Johnston County Osteoarthritis Project. Arthritis Rheumatol. 2017;69:1204-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Razek AA, Al Mahdy Al Belasy F, Ahmed WM, Haggag MA. Assessment of articular disc displacement of temporomandibular joint with ultrasound. J Ultrasound. 2014;18:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Razek AA, Fouda NS, Elmetwaley N, Elbogdady E. Sonography of the knee joint(). J Ultrasound. 2009;12:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Razek AA, El-Basyouni SR. Ultrasound of knee osteoarthritis: interobserver agreement and correlation with Western Ontario and McMaster Universities Osteoarthritis. Clin Rheumatol. 2016;35:997-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Aigner T, Söder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3:391-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Santini P, Politi L, Vedova PD, Scandurra R, Scotto d'Abusco A. The inflammatory circuitry of miR-149 as a pathological mechanism in osteoarthritis. Rheumatol Int. 2014;34:711-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Philipot D, Guérit D, Platano D, Chuchana P, Olivotto E, Espinoza F, Dorandeu A, Pers YM, Piette J, Borzi RM, Jorgensen C, Noel D, Brondello JM. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res Ther. 2014;16:R58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Martinez-Sanchez A, Dudek KA, Murphy CL. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J Biol Chem. 2012;287:916-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Nugent M. MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthritis Cartilage. 2016;24:573-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Cutolo M, Berenbaum F, Hochberg M, Punzi L, Reginster JY. Commentary on recent therapeutic guidelines for osteoarthritis. Semin Arthritis Rheum. 2015;44:611-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1893] [Cited by in F6Publishing: 1770] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 16. | Karabis A, Nikolakopoulos S, Pandhi S, Papadimitropoulou K, Nixon R, Chaves RL, Moore RA. High correlation of VAS pain scores after 2 and 6 weeks of treatment with VAS pain scores at 12 weeks in randomised controlled trials in rheumatoid arthritis and osteoarthritis: meta-analysis and implications. Arthritis Res Ther. 2016;18:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S208-S228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 796] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 18. | Raeissadat SA, Sedighipour L, Ghorbani E. Correlation of Western Ontario and Mcmaster Universities Osteoarthritis (WOMAC) and Short Form 36 (SF36) Questionnaires in Patients with Knee Osteoarthritis. Remed Open Access. 2017;2:1058. [Cited in This Article: ] |
| 19. | Kohn MD, Sassoon AA, Fernando ND. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474:1886-1893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 680] [Cited by in F6Publishing: 599] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 20. | Musumeci G, Carnazza ML, Leonardi R, Loreto C. Expression of β-defensin-4 in "an in vivo and ex vivo model" of human osteoarthritic knee meniscus. Knee Surg Sports Traumatol Arthrosc. 2012;20:216-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1966-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 303] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 22. | Silva GS, Zuravski L, Duarte MMMF, Machado MM, Oliveira LFS. Fluconazole induces genotoxicity in cultured human peripheral blood mononuclear cells via immunomodulation of TNF-α, IL-6, and IL-10: new challenges for safe therapeutic regimens. Immunopharmacol Immunotoxicol. 2019;41:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Musumeci G, Carnazza ML, Loreto C, Leonardi R, Loreto C. β-Defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochem. 2012;114:805-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Gardner OFW, Musumeci G, Neumann AJ, Eglin D, Archer CW, Alini M, Stoddart MJ. Asymmetrical seeding of MSCs into fibrin-poly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis. J Tissue Eng Regen Med. 2017;11:2912-2921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Wang GD, Zhao XW, Zhang YG, Kong Y, Niu SS, Ma LF, Zhang YM. Effects of miR-145 on the inhibition of chondrocyte proliferation and fibrosis by targeting TNFRSF11B in human osteoarthritis. Mol Med Rep. 2017;15:75-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu J, Zuo B, Zhao C, Wang C, Zhang X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8:e3140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Liu M, Zhang J, Liu W, Wang W. Salidroside protects ATDC5 cells against lipopolysaccharide-induced injury through up-regulation of microRNA-145 in osteoarthritis. Int Immunopharmacol. 2019;67:441-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Wang X, Yang M, Ruan W, Wei W, Gu D, Wang J, Guo X, Guo L, Yuan Y. miR-145-5p Increases Osteoclast Numbers In Vitro and Aggravates Bone Erosion in Collagen-Induced Arthritis by Targeting Osteoprotegerin. Med Sci Monit. 2018;24:5292-5300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Loreto C, Musumeci G, Leonardi R. Chondrocyte-like apoptosis in temporomandibular joint disc internal derangement as a repair-limiting mechanism. An in vivo study. Histol Histopathol. 2009;24:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 30. | Shimura Y, Kurosawa H, Tsuchiya M, Sawa M, Kaneko H, Liu L, Makino Y, Nojiri H, Iwase Y, Kaneko K, Ishijima M. Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin Rheumatol. 2017;36:2781-2787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Shimura Y, Kurosawa H, Sugawara Y, Tsuchiya M, Sawa M, Kaneko H, Futami I, Liu L, Sadatsuki R, Hada S, Iwase Y, Kaneko K, Ishijima M. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: a cross-sectional study. Osteoarthritis Cartilage. 2013;21:1179-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, Spector TD. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60:2037-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |