Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.1964
- This article has been corrected.
- See: World J Clin Cases. Nov 6, 2020; 8(21): 5494-5495
Peer-review started: April 15, 2019
First decision: May 16, 2019
Revised: June 16, 2019
Accepted: June 26, 2019
Article in press: June 27, 2019
Published online: August 6, 2019
The clinical significance of intratumoral human epidermal growth factor receptor 2 (HER2) heterogeneity is unclear for HER2-positive gastric cancer, although it has been reported to be a significant prognosticator for HER2-positive breast cancer, which has received trastuzumab-based chemotherapy.
To clarify the clinical significance of intratumoral HER2 heterogeneity for HER2-positive gastric cancer, which has received trastuzumab-based chemotherapy.
Patients with HER2-positive unresectable or metastatic gastric cancer who received trastuzumab-based chemotherapy as a first line treatment were included. The patients were classified into two groups according to their intratumoral HER2 heterogeneity status examined by immunohistochemistry (IHC) on endoscopic biopsy specimens before treatment, and their clinical response to chemotherapy and survival were compared.
A total of 88 patients were included in this study, and HER2 heterogeneity was observed in 23 (26%) patients (Hetero group). The overall response rate was significantly better in patients without HER2 heterogeneity (Homo group) (Homo vs Hetero: 79.5% vs 35.7%, P = 0.002). Progression-free survival of trastuzumab-based chemotherapy was significantly better in the Homo group (median, 7.9 vs 2.5 mo, HR: 1.905, 95%CI: 1.109-3.268). Overall survival was also significantly better in the Homo group (median survival time, 25.7 vs 12.5 mo, HR: 2.430, 95%CI: 1.389-4.273). Multivariate analysis revealed IHC HER2 heterogeneity as one of the independent poor prognostic factors (HR: 3.115, 95%CI: 1.610-6.024).
IHC of HER2 heterogeneity is the pivotal predictor for trastuzumab-based chemotherapy. Thus, HER2 heterogeneity should be considered during the assessment of HER2 expression.
Core tip: Although intratumoral human epidermal growth factor receptor 2 (HER2) heterogeneity has been reported as an important predictor of trastuzumab-based chemotherapy for HER2-positive breast cancer, the clinical significance of HER2 heterogeneity for gastric cancer had been unclear. We defined intratumoral HER2 heterogeneity as biopsy specimens were taken from two or more different portions of the tumor that showed different HER2 positivity by immunohistochemistry, and HER2 heterogeneity based on this definition was a pivotal poor predictor of tumor shrinkage and poor prognosticator. Thus, intratumoral HER2 heterogeneity should be included in the assessment of HER2 positivity.
- Citation: Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, Kinoshita T. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases 2019; 7(15): 1964-1977
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/1964.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.1964
Human epidermal growth factor receptor 2 (HER2) was introduced as a predictive biomarker for the treatment of gastric cancer along with trastuzumab[1], and trastuzumab was subsequently recommended to be administered for HER2-positive gastric cancer as a molecular target drug[2,3]. Assessment of HER2 expression was performed by immunohistochemistry (IHC) and in-situ hybridization (ISH), where positive HER2 expression was defined as 3+ on IHC or 2+ on IHC with ISH positive[4]. The strong HER2 intensity of IHC 3+ was reported as a better prognostic factor for HER2-positive gastric cancer treated with trastuzumab-based chemotherapy[1].
The frequency of intratumoral HER2 heterogeneity was reported as 45%-79% by IHC and 23%-54% by ISH for HER2-positive gastric cancers[5-8], which were more frequent than that with HER2-positive breast cancers[9]. Although intratumoral HER2 heterogeneity was reported to be one of the poor predictors for treatment response and poor prognosticator for patients with HER2-positive breast cancer who received trastuzumab-based chemotherapy[10,11], the clinical significance of intratumoral HER2 heterogeneity for HER2 positive gastric cancer treated with trastuzumab has not been well investigated. Recently, Wakatsuki et al[12] reported that intratumoral HER2 heterogeneity had a negative survival benefit for patients with surgically resected HER2-positive gastric cancer. However, HER2 assessment before treatment is usually based on the endoscopic biopsy specimen for metastatic gastric cancer, and the clinical significance of intratumoral HER2 heterogeneity for biopsy specimen is still unknown.
The aim of this study was to clarify the clinical significance of intratumoral HER2 heterogeneity for HER2 positive gastric cancer treated with trastuzumab-based chemotherapy by evaluation of biopsy specimens.
Patients with histologically confirmed HER2-positive metastatic or unresectable adenocarcinoma of the stomach or gastroesophageal junction cancer who received trastuzumab-based chemotherapy as first-line treatment at our hospital between July 2011 and December 2017 were included in this study. The patients were classified into two groups according to intratumoral HER2 heterogeneity, and their clinico-pathological findings, clinical responses, progression-free survival (PFS) and overall survival (OS) were compared. Furthermore, the predictive factor for clinical response and prognostic factor were analyzed using multivariable analyses. We extracted clinicopathological findings such as age, sex, tumor diameter, tumor location, macroscopic tumor type, tumor markers, Eastern Cooperative Oncology Group performance score, TNM stage, metastatic site, chemotherapy regimen, histological type of endoscopic biopsy specimen, HER2 positivity, survival outcomes from our prospectively collected database and medical records. Biopsied specimens stained by IHC were reviewed and assessed for their intratumoral HER2 heterogeneity by two pathologists (A.K. and T.K.).
Patients who received gastrectomy prior to chemotherapy and patients who received chemotherapy without trastuzumab were excluded. Clinical response was evaluated according to new response evaluation criteria in solid tumors (RECIST guideline ver. 1.1) for patients with measurable metastatic lesions[13]. The onset of PFS was defined as the start of chemotherapy, and tumor progression was evaluated by imaging techniques and physical examination. Tumor progression dates for patients who received radical gastrectomy after good clinical response to chemotherapy were defined as the date of first recurrence after surgery. Tumor staging of gastric and EGJ type III tumor followed the Union for International Cancer Control TNM classification of 7th edition for gastric cancer, and tumor staging of EGJ type II tumor followed that for esophageal cancer[14]. Tumor histology was assessed according to the Japanese Classification of Gastric Carcinoma[15], with well and moderately differentiated adenocarcinoma and papillary adenocarcinoma classified as differentiated type, and poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma classified as undifferentiated type.
Chemotherapy regimens were capecitabine or 5-fluorouracil plus cisplatin with trastuzumab (XPT/FPT) before September 2015 and S-1 plus oxaliplatin with trastuzumab (SOXT) after October 2015. XPT or FPT regimens were followed a ToGA study regimen[1]. SOXT regimen was followed according to the G-SOX study regimen in combination with trastuzumab; oxaliplatin was administered intravenously 100 mg/m2 on day 1, while S-1 was administered orally 80 mg/m2 for 14 d followed by a 7-d rest. This schedule was repeated every 3 wk. Trastuzumab was given by intravenous infusion at a dose of 8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg every 3 wk until disease progression[16].
All endoscopic biopsy samples were fixed in neutral buffered 10% formalin. Formalin-fixed paraffin-embedded tumor samples were examined for HER2 using IHC and fluorescence in situ hybridization (FISH) when the HER2 score was 2+.
HER2 IHC analyses were performed using the PATHWAY anti-HER-2/neu (4B5) rabbit monoclonal primary antibody (Ventana Medical Systems, Tucson, AZ, United States). IHC HER2 scoring by biopsy specimen was performed as described in the ToGA study[1]. In brief, tumor cell clusters of at least five positive cells with complete basolateral or lateral membranous reactivity was considered HER2-positive for endoscopic biopsy samples irrespective of percentage of tumor cells stained. Tumor cells with strong membranous reactivity were scored 3+, and those with moderate reactivity were scored 2+. HER2 FISH analyses were performed at SRL (Tokyo, Japan) using the Path Vysion HER2 DNA Probe kit (Vysis, Downers Grove, IL, United States). When the ratio of HER2 signals to chromosome 17 centromere signals was 2.0 or greater, the gene was considered amplified (i.e., FISH-positive). HER2 positivity was defined as IHC score 3+ or IHC score 2+ and FISH positivity, because these criteria were considered to be indications for using trastuzumab by a subset analysis of the ToGA trial[1,3].
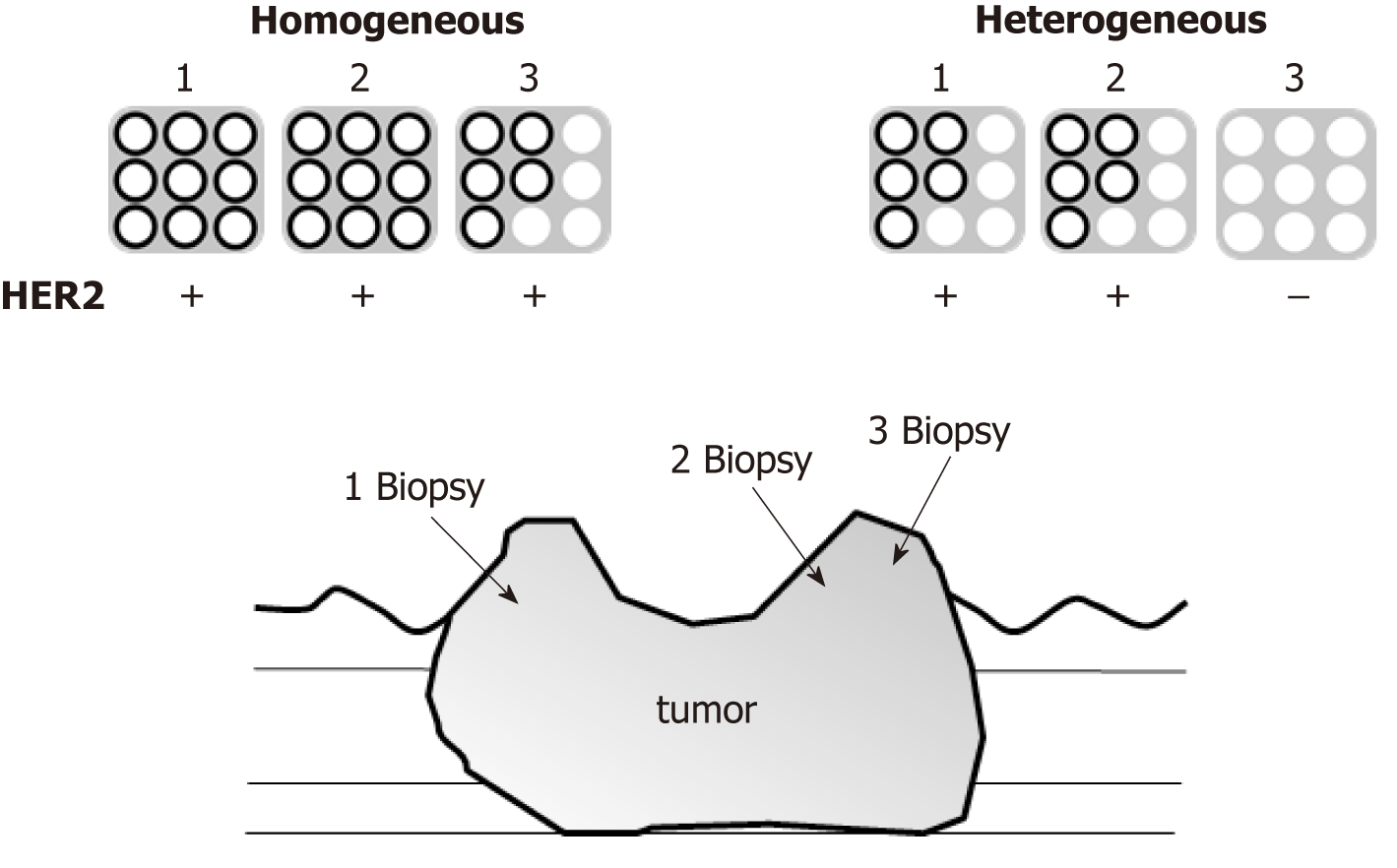
Assessment of intratumoral HER2 heterogeneity was conducted on patients who underwent assessment of HER2 positivity from two or more different portions of the same tumor, and three or more biopsy specimens were obtained from each portion. Those patients whose HER2 assessment was performed from only one portion were excluded for further examination. Intratumoral HER2 homogeneity was defined as every portion being HER2-positive by IHC, and if any portion of the tumor was HER2-negative, the tumor was defined as intratumoral HER2 heterogeneity (Figure 1).
The baseline characteristics of each group were compared using the χ2 test or the Fisher’s exact test for categorical data, and P < 0.05 was considered statistically significant. The median OS rate and PFS rate were estimated by the Kaplan-Meier method. Independent prognostic factors for OS and PFS were evaluated by univariate and multivariable analysis of Cox proportional hazard model and presented as hazard ratio and 95% confidence interval (CI). Evaluated factors in multivariable analysis were those that were significant by univariate analysis. Data were censored on May 31, 2019. All statistical analyses were performed using SPSS Statistics 20 (SPSS Inc., Chicago, IL, United States).
This study was approved by the Institutional Review Board of the National Cancer Center, Japan (IRB file No. 2017-164, approval date: Oct. 11, 2017).
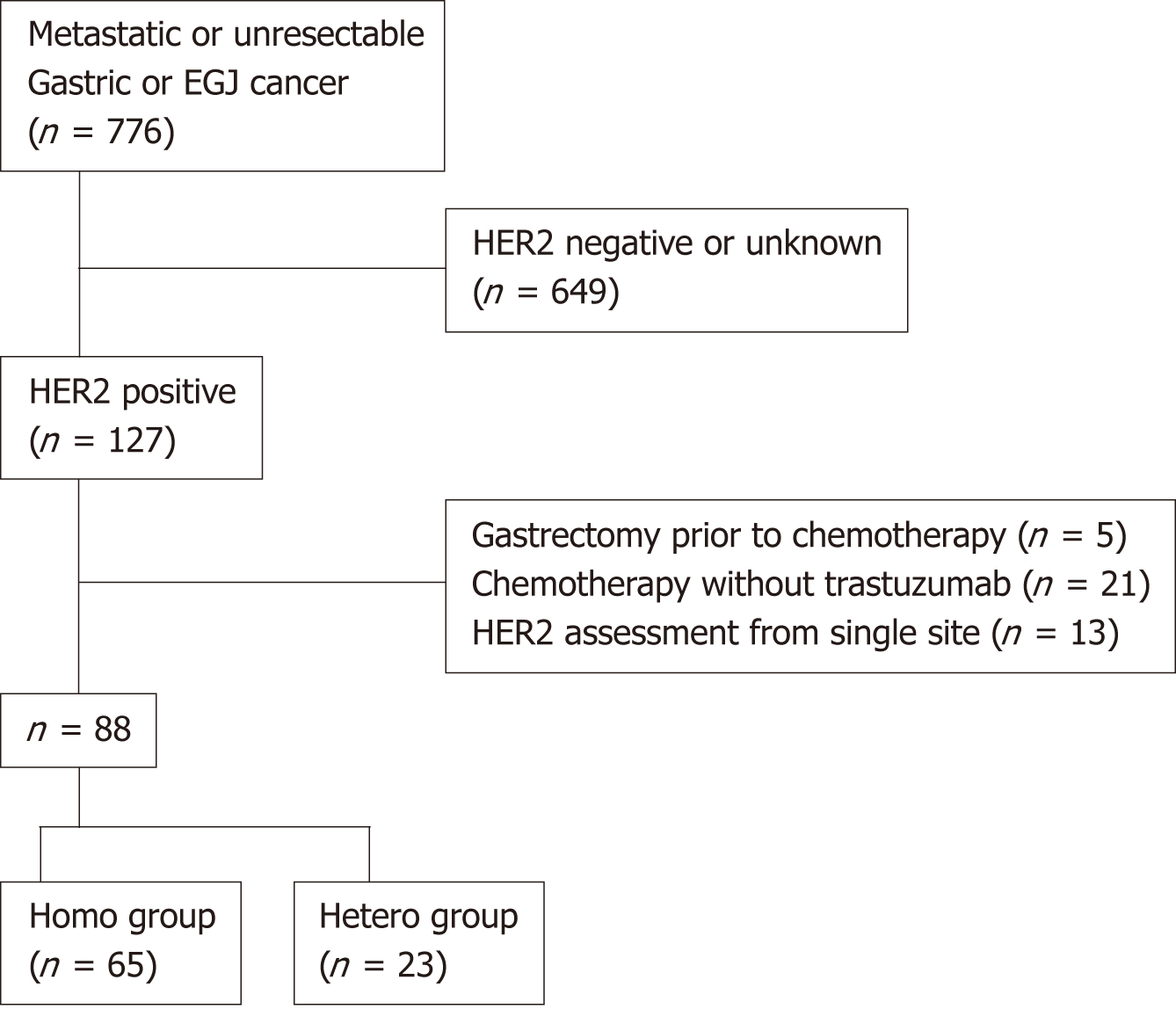
A total of 776 patients with metastatic or unresectable adenocarcinoma of the stomach or gastroesophageal junction were treated in this study period, and HER2 positivity was observed in 127 cases (16.3%). Of these, patients who received upfront gastrectomy before chemotherapy (n = 5) or chemotherapy without trastuzumab (n = 21), and patients who underwent HER2 assessment from only one portion of the tumor (n = 13) were excluded (Figure 2). Finally, a group of 88 patients were evaluated for intratumoral HER2 heterogeneity, in which HER2 homogeneity was observed in 65 (Homo group) and HER2 heterogeneity was observed in 23 (Hetero group) patients.
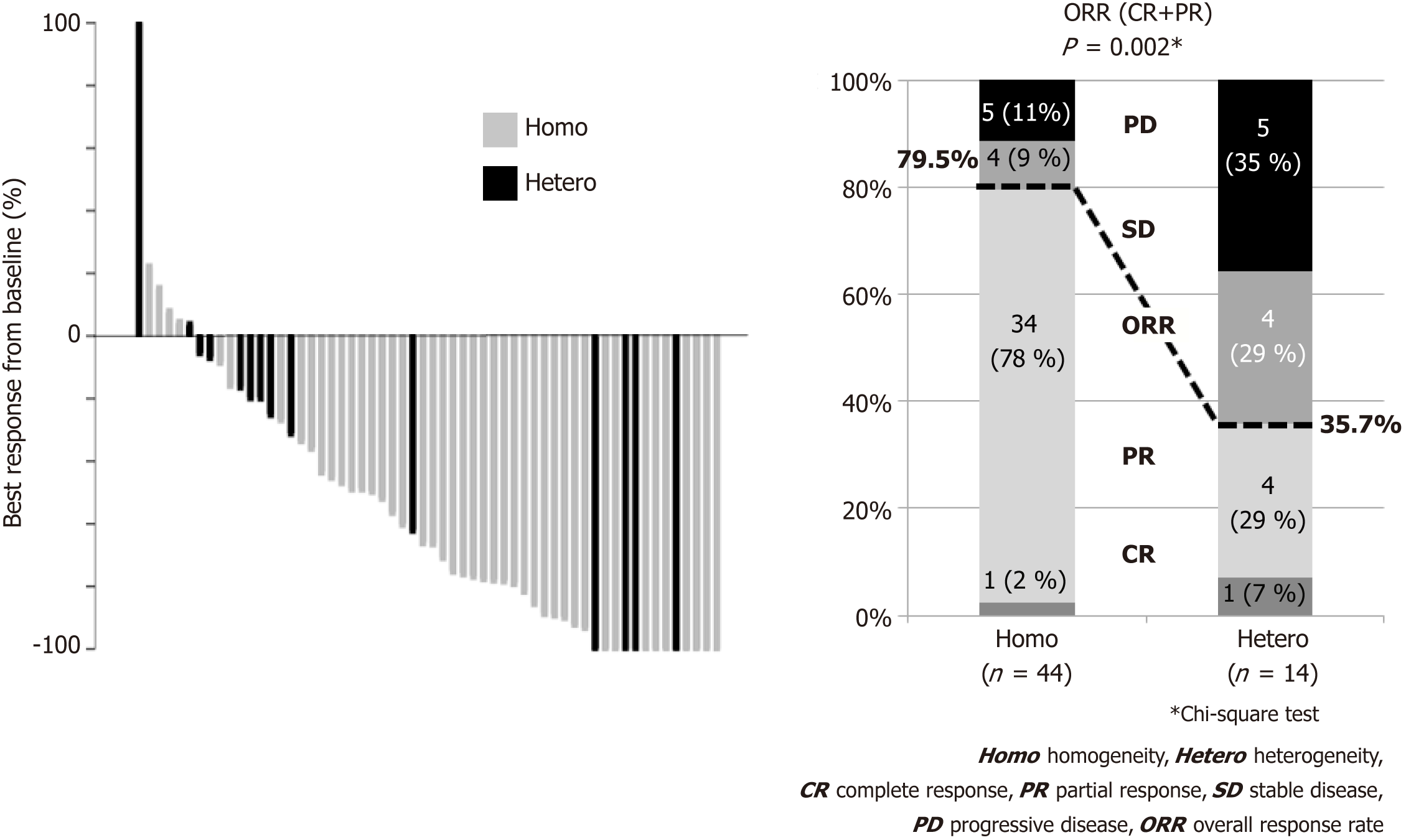
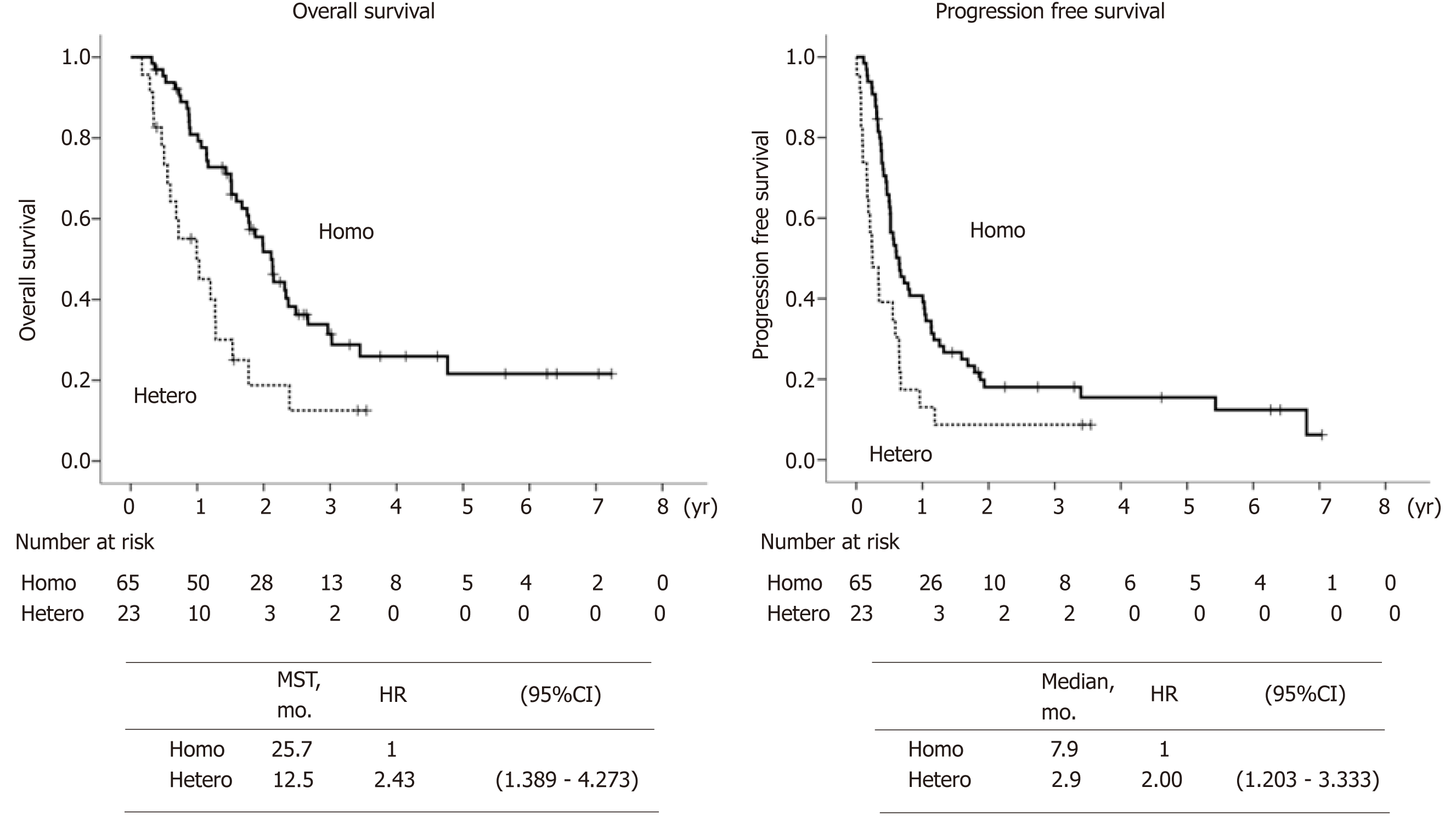
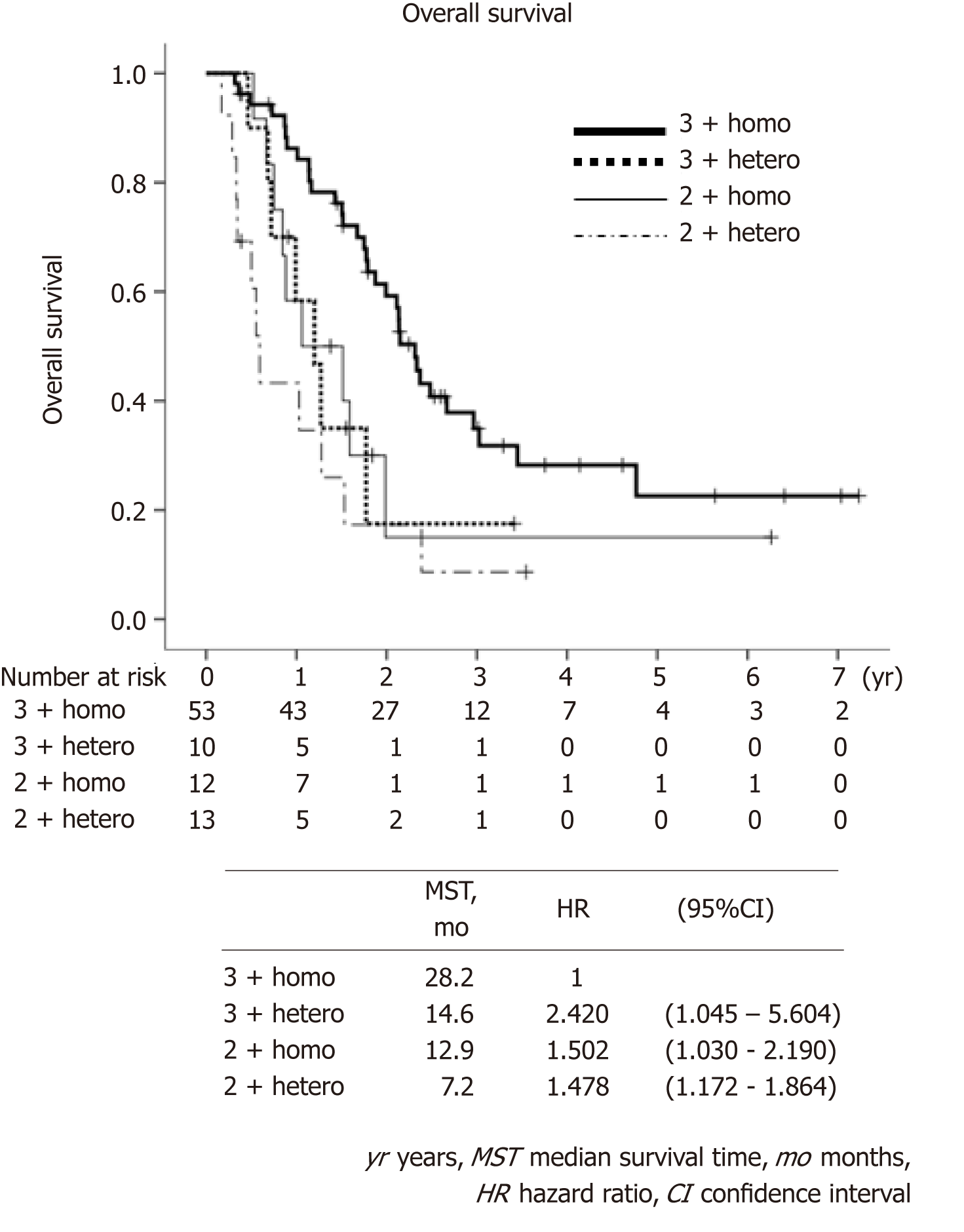
Patients’ backgrounds are shown in Table 1. Intratumoral HER2 heterogeneity was significantly more frequently observed in macroscopically type 3 and type 4 patients than other types, and in patients with IHC 2+ compared to IHC 3+. Other background characteristics such as age, sex, performance status, TNM stage, metastatic site and chemotherapeutic regimens were not significantly different between the groups. Besides, the number of biopsy sites for HER2 assessment was not significantly different, i.e., those numbers more than three was 46% for Homo group and 57% for Hetero group (P = 0.393). Waterfall plot for tumor shrinkage and clinical responses for the patients with measurable metastatic lesions (n = 58) of both groups are shown in Figure 3. Overall response rate (complete response and partial response) was 79.5% in the Homo group, which was considerably better than that in the Hetero group (35.7%, P = 0.002). Kaplan-Meier curves of OS and PFS in both groups are shown in Figure 4. At the time of the analysis, the median follow-up was 18.5 mo (range, 4.7-88.0 mo). The median survival time (MST) in the Hetero group was 12.5 mo, which was considerably worse than that in the Homo group (25.7 mo, HR; 2.430, 95%CI: 1.389-4.273). Median PFS time in the Hetero group was 2.9 mo, which was also significantly worse than that in the Homo group (7.9 mo, HR: 2.000, 95%CI: 1.203-3.333). Multivariate analysis revealed IHC HER2 heterogeneity as one of the independent poor prognostic factors for OS (HR: 3.115, 95%CI: 1.610-6.024) and PFS (HR: 2.123, 95%CI: 1.225-3.676) (Table 2). Undifferentiated histological type (HR: 2.612, 95%CI: 1.388-4.916), number of non-curative factors (HR: 2.252, 95%CI: 1.113-4.553), clinical nodal status (HR: 2.119, 95%CI: 1.165-3.855), hepatic metastasis (HR: 2.084, 95%CI: 1.076-4.036) and HER2 score (2+) (HR: 2.008, 95%CI: 1.094-3.690) were also extracted as independent poor prognostic factors for OS. We compared survival results by the combination of each HER2 score (2+ or 3+) and HER2 heterogeneity (homogeneity or heterogeneity). MST for the Homo group with HER2 3+ was longest (28.2 mo), followed by Hetero group with 3+ (14.6 mo) and Homo group with 2+ (12.9 mo). Hetero group with 2+ had the worst prognosis (MST: 7.2 mo) (Figure 5).
| Homo, n = 65 | Hetero, n = 23 | P-value | |
| Age in yr | 69 (42-81) | 67.0 (45-82) | |
| < 70 | 34 (52%) | 15 (65%) | 0.284 |
| ≥ 70 | 31 (48%) | 8 (35%) | |
| Sex | |||
| Male | 43 (66%) | 18 (78%) | 0.279 |
| Female | 22 (34%) | 5 (22%) | |
| ECOG performance status | |||
| 0 | 61 (94%) | 20 (87%) | 0.152 |
| 1 | 2 (3%) | 3 (13%) | (0 vs ≥ 1) |
| 2 | 2 (3%) | 0 | |
| Macroscopic type | |||
| 1 | 7 (11%) | 1 (5%) | 0.008 |
| 2 | 28 (43%) | 4 (17%) | (type 1/2 vs 3/4) |
| 3 | 23 (35%) | 16 (69%) | |
| 4 | 7 (11%) | 2 (9%) | |
| Tumor location | |||
| Upper | 27 (42%) | 11 (48%) | 0.601 |
| Middle / lower | 38 (58%) | 12 (52%) | |
| Histology | |||
| Well differentiated | 48 (74%) | 14 (61%) | 0.31 |
| Poorly differentiated | 15 (23%) | 9 (39%) | |
| Other/unknown | 2 (3%) | 0 | |
| T status | |||
| cT2 | 2 (3%) | 0 | 0.267 |
| cT3 | 17 (26%) | 4 (17%) | (cT2/3 vs cT4) |
| cT4 | 46 (71%) | 19 (83%) | |
| N status | |||
| cN0 | 5 (8%) | 2 (9%) | 0.748 |
| cN1 | 5 (8%) | 1 (4%) | (≤cN2 vs cN3) |
| cN2 | 32 (49%) | 11 (48%) | |
| cN3 | 23 (35%) | 9 (39%) | |
| Number of non-curative factor | |||
| 0 | 1 (1%) | 1 (4%) | 0.404 |
| 1 | 32 (49%) | 13 (57%) | (≤1 vs >1) |
| 2 | 29 (45%) | 9 (39%) | |
| >2 | 3 (5%) | 0 | |
| Non-curative factor | |||
| Lymph node | 34 (52%) | 10 (43%) | |
| Peritoneal | 22 (34%) | 6 (26%) | |
| Hepatic | 27 (42%) | 12 (52%) | |
| Other | 9 (14%) | 1 (5%) | |
| Number of HER2 assessment | |||
| 2 | 35 (54%) | 10 (43%) | 0.393 |
| 3 | 27 (42%) | 13 (57%) | |
| >3 | 3 (4%) | 0 | |
| HER2 score | |||
| 3+ | 53 (82%) | 10 (43%) | 0.001 |
| 2+ | 12 (18%) | 13 (57%) | |
| Upfront chemotherapy | |||
| SOXT | 33 (50%) | 12 (52%) | |
| XPT | 25 (37%) | 7 (30%) | |
| SPT | 3 (5%) | 2 (9%) | |
| FPT | 1 (2%) | 2 (9%) | |
| FLT | 1 (2%) | ||
| PTXT | 1 (2%) | ||
| DCST | 1 (2%) | ||
| Covariates | Overall survival | Progression-free survival | ||||||
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | |||||
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Age, ≥ 80 vs < 80 | 1.31 (0.473-3.625) | 0.604 | 1.138 (0.492-2.633) | 0.762 | ||||
| Sex, female vs male | 1.057 (0.621-1.798) | 0.838 | 1.005 (0.615-1.642) | 0.985 | ||||
| Macroscopic type, type 3,4 vs 1,2 | 1.72 (1.027-2.881) | 0.039 | 1.641 (1.037-2.598) | 0.034 | ||||
| Clinical tumor depth, cT4 vs ≤ cT3 | 1.838(0.992-3.405) | 0.053 | 1.408 (0.826-2.401) | 0.209 | ||||
| Clinical nodal status, cN3 vs ≤ cN2 | 2.026 (1.184-3.466) | 0.01 | 2.119 (1.165-3.855) | 0.014 | 1.691 (1.056-2.708) | 0.029 | 1.622 (0.951-2.764) | 0.076 |
| Histology, undiff. vs diff. | 1.884 (1.090-3.255) | 0.023 | 2.612 (1.388-4.916) | 0.003 | 1.643 (1.031-2.618) | 0.037 | 1.902 (1.117-3.237) | 0.018 |
| HER2 score, 2+ vs 3+ | 2.331 (1.353-4.016) | 0.002 | 2.008 (1.094-3.690) | 0.024 | 1.828 (1.110-3.012) | 0.018 | 1.612 (0.940-2.770) | 0.083 |
| HER2 heterogeneity, hetero vs homo | 2.439 (1.389-4.274) | 0.002 | 3.115 (1.610-6.024) | 0.001 | 2 (1.203-3.333) | 0.008 | 2.123 (1.225-3.676) | 0.007 |
| No. of non-curative factors, ≥ 2 vs 1 | 1.904 (1.138-3.186) | 0.014 | 2.252 (1.113-4.553) | 0.024 | 1.875 (1.185-2.965) | 0.007 | 1.871 (1.023-3.424) | 0.042 |
| M1, lymph node | 0.687 (0.410-1.150) | 0.154 | 0.568 (0.358-0.902) | 0.017 | ||||
| M1, peritoneal | 1.207 (0.699-2.086) | 0.5 | 1.327 (0.828-2.127) | 0.24 | ||||
| M1, hepatic | 1.902 (1.141-3.173) | 0.014 | 2.084 (1.076-4.036) | 0.029 | 1.974 (1.244-3.132) | 0.004 | 2.053 (1.151-3.664) | 0.015 |
In this study, we examined the effect of intratumoral HER2 heterogeneity by IHC for HER2-positive advanced gastric cancer treated with trastuzumab-based chemotherapy on their therapeutic responses and survival. Intratumoral HER2 heterogeneity by IHC evaluated by biopsy specimen before treatment was one of the independent poor predictive factors for tumor shrinkage and a poor prognosticator.
The definition of intratumoral HER2 heterogeneity for HER2-positive gastric cancer has not been well established. The cut-off value between HER2 homogeneity and heterogeneity of HER2 positive tumor cells varies from 50% to 100% for surgically resected specimens[5,6,12,17] and 30% to 100% for endoscopic biopsy specimens[7,8,18]. The clinical significance of intratumoral HER2 heterogeneity for HER2-positive gastric cancer treated with trastuzumab has not been well investigated, and there have been only a few studies conducted to date. Wakatsuki et al[12] defined the cut-off value of HER2 heterogeneity as 100% and reported that HER2 homogeneity group had significantly longer PFS (HR: 0.11, 95%CI: 0.03-0.41) and OS (HR: 0.18, 95%CI: 0.06-0.72). However, their study included only 28 patients who received trastuzumab-based chemotherapy after upfront gastrectomy for resectable gastric cancer, not metastatic cancer.
In post hoc exploratory analyses of the ToGA trial, the proportion of HER2-positive tumor cells in more than 30% of biopsy specimens could not be extracted as a predictive factor for good response to trastuzumab-based chemotherapy[18]. Yagi et al[8] recently reported that intratumoral HER2 heterogeneity was a poor predictor for trastuzumab-based chemotherapy and a poor prognosticator with the cut-off value of 100% from the median number of four biopsy specimens. Our current study selected patients in whom HER2 positivity could be assessed from more than two different portions of the tumor, and we defined HER2 heterogeneity as any of those tumor portions being negative for HER2. The number of biopsy specimens examined for each patient in our current study was more than six (i.e., each of the three fragments were obtained from two or more different portions). Although Yagi et al[8] adopted the overall proportion of HER2-positive tumor cells to evaluate intratumoral HER2 heterogeneity, we employed the standard criteria of HER2 positivity generally performed in clinical diagnosis, i.e., tumor cell cluster of at least five positive cells with complete basolateral or lateral membranous reactivity. According to our simple definition of intratumoral HER2 heterogeneity, the treatment outcomes were definitively different between the HER2 homogeneity group and heterogeneity group.
The stain intensity for IHC was reported as a significant prognostic factor for HER2-positive gastric cancer in the ToGA trial. The OS was significantly longer in IHC 3+ patients than IHC 2+ patients (MST: 17.9 and 12.3 mo)[1]. Assessment of HER2 positivity for endoscopic biopsy specimen in the ToGA trial was established without consideration of intratumor HER2 heterogeneity, which is now commonly adopted worldwide[4]. Prognostic significance of stain intensity for IHC was also explored in combination with heterogeneity for IHC in our current study. MST of Homo-IHC 3+ patients was 28.2 mo, which was much better than MST of IHC 3+ patients (17.9 mo) in the ToGA trial. Hetero-IHC 3+ and Homo-IHC 2+ patients had comparable survival (14.6 and 12.9 mo), while Hetero-IHC 2+ patients had poorer survival (7.2 mo) compared to IHC 2+ patients (12.3 mo) in the ToGA trial. These results suggest that intratumoral HER2 heterogeneity should be included in the assessment of HER2 positivity in addition to the stain intensity for IHC, and patients with Homo-IHC 3+ might be the most responsive to further anti-HER2 agents. The survival benefits for anti-HER2 drugs such as lapatinib or pertuzumab in combination with trastuzumab and adjuvant therapy of trastuzumab or trastuzumab beyond progression have been demonstrated for breast cancers[19-22]. However, the efficacy of those anti-HER2 agents has not been shown for gastric cancers, and intratumoral HER2 heterogeneity may be one of the reasons for the difference of these results between breast and gastric cancers[23,24].
Although the intrinsic mechanism underlying the correlation between HER2 heterogeneity and poor efficacy of trastuzumab-based chemotherapy is still unclear, chemo-resistance can be one of the main reasons for the treatment failure. Fabi et al[25] reported that the discordances of HER2 positivity between primary and metastatic lesions may be a possible cause of chemo-resistance of trastuzumab for metastatic breast cancers. Park et al[26] reported these discordances between primary and metastatic lesions could also be observed in HER2-positive gastric cancers, and HER2 heterogeneity of primary lesions existed in such cases with discordances of HER2 positivity. Another explanation for the poor efficacy of trastuzumab might be genomic alterations. Pietrantonio et al[27] reported that chemo-resistance against trastuzumab was more frequently observed in patients with genomic alternations including EGFR/MET/KRAS/PI3K/PTEN mutations than those without. They stated such genomic mutations were correlated with IHC 2+, i.e., the existence of HER2 heterogeneity.
Our study has several limitations. First, concordance of intratumoral HER2 heteroge-neity between endoscopic biopsy specimen and whole tumor tissues was not investigated in the current study. The concordance of HER2 positivity between biopsy specimens and surgically resected specimens was reported to be 80%-91% for IHC 3+ and 25%-57% for IHC 2+ patients[28-30], thus the interpretation of HER2 heterogeneity for IHC 2+ patients must be made with care. In breast cancer, similar results have been reported comparing core needle biopsy specimens and surgically resected specimens[31], and repeat testing or reflex testing using an alternative assay (IHC or ISH) are recommended for IHC equivocal cases[32]. Secondly, there was no common standard of adequate numbers and portions for the assessment of intratumoral HER2 heterogeneity. In this study, the assessment of intratumoral HER2 heterogeneity was performed from at least two portions of the tumor. Ohno et al[33] reported that HER2 positivity was different in different tumor regions, i.e., the frequency of HER2 positivity in the biopsy specimen from the superficial spreading portion, ulcer mound, and mass portion was as high as 90% to 100%, while these from ulcer bed was only 45% due to the presence of concomitant necrotic tissues and associated inflammation[34]. These results suggest that the assessment of HER2 heterogeneity should be based on biopsy specimens taken from the appropriate portion of the tumor. Thirdly, this study did not include the intratumoral heterogeneity of HER2 gene amplification. Intratumoral heterogeneity of HER2 gene amplification has been reported to be a significant prognostic factors for metastatic or resectable advanced breast cancers[9,11]. The significance of intratumoral heterogeneity of HER2 gene amplification should also be clarified in further investigations.
In conclusion, intratumoral HER2 heterogeneity by IHC was a significant predictor of clinical response and a poor prognosticator for trastuzumab-based chemotherapy. Thus, intratumoral HER2 heterogeneity would be useful to further stratify patients with HER2-positive gastric cancer, and thus should be taken into account in future clinical trials.
Human epidermal growth factor receptor 2 (HER2) was introduced as a predictive biomarker for the treatment of gastric cancer along with trastuzumab, and the strong HER2 intensity of immunohistochemistry (IHC) 3+ was reported as a better prognostic factor for HER2-positive gastric cancer treated with trastuzumab-based chemotherapy. Intratumoral HER2 heterogeneity for HER2-positive gastric cancers was reported to be more frequent than in HER2-positive breast cancers. Although intratumoral HER2 heterogeneity was reported to be one of the poor predictors for the treatment response and poor prognosticator for patients with HER2-positive breast cancer who received trastuzumab-based chemotherapy, the clinical significance of intratumoral HER2 heterogeneity for HER2-positive gastric cancer treated with trastuzumab has not been well investigated. Furthermore, even the definitions of HER2 heterogeneity for HER2-positive gastric cancer have not been established. In this study, we established the definition of intratumoral HER2 heterogeneity and clarified the clinical significance of HER2 heterogeneity for HER2-positive gastric cancer.
HER2 heterogeneity may be a pivotal predictor for the efficacy of trastuzumab-based chemotherapy. Authors have previously reported that intratumoral HER2 heterogeneity had a negative survival benefit for patients with surgically resected HER2-positive gastric cancer. However, HER2 assessment before treatment is usually based on endoscopic biopsy specimen for metastatic gastric cancer, and the clinical significance of intratumoral HER2 heterogeneity for biopsy specimen is still unknown. If these clinical significances are clarified, HER2 heterogeneity might be introduced to the assessment of HER2 positivity for gastric cancer in the future.
The aim of this study was to clarify the clinical significance of intratumoral HER2 heterogeneity for HER2-positive gastric cancer treated with trastuzumab-based chemotherapy by evaluation of biopsy specimens.
Patients with HER2-positive metastatic or unresectable adenocarcinoma of the stomach or gastroesophageal junction who received trastuzumab-based chemotherapy as first-line treatment at our hospital were included. The patients were classified into two groups (Homo- and Hetero- group) according to intratumoral HER2 heterogeneity, and their clinicopathological findings, clinical responses, progression-free survival (PFS) and overall survival (OS) were compared. Furthermore, the predictive factor for clinical response and prognostic factor were analyzed using multivariable analyses. Assessment of intratumoral HER2 heterogeneity was conducted on patients who underwent assessment of HER2 positivity from two or more different portions of the same tumor, and three or more biopsy specimens were obtained from each portion. Those patients whose HER2 assessment was performed from only one portion were excluded. We defined intratumoral HER2 homogeneity as every portion of the tumor being HER2-positive by IHC, and any portion of the tumor staining negative for HER2 was defined as intratumoral HER2 heterogeneity.
A total of 776 patients with metastatic or unresectable adenocarcinoma of the stomach or gastroesophageal junction were treated in the study period, and HER2 positivity was observed in 127 patients (16.3%). Intratumoral HER2 heterogeneity was significantly more frequently observed in macroscopically type 3 and type 4 patients than other types, and patients with IHC 2+ than IHC 3+. Tumor shrinkage and clinical responses for the patients with measurable metastatic lesions were evaluated, and overall response rate (complete response and partial response) was considerably better in the Homo group than in the Hetero group. Median survival time in the Hetero group was 12.5 mo, which was considerably worse than that in the Homo group (25.7 mo, HR; 2.430, 95%CI: 1.389-4.273). Median PFS time in the Hetero group was 2.9 mo, which was also significantly worse than that in the Homo group (7.9 mo, HR: 2.000, 95%CI: 1.203-3.333). Multivariate analysis revealed IHC HER2 heterogeneity as one of the independent poor prognostic factors for OS (HR: 3.115, 95%CI: 1.610-6.024) and PFS (HR: 2.123, 95%CI: 1.225-3.676).
In this study, we examined the effect of intratumoral HER2 heterogeneity by IHC for HER2-positive advanced gastric cancer treated with trastuzumab-based chemotherapy on their therapeutic responses and survival. Intratumoral HER2 heterogeneity by IHC evaluated by biopsy specimen before treatment was one of the independent poor predictive factors for tumor shrinkage and a poor prognosticator. Our current study selected patients in whom HER2 positivity could be assessed from more than two different portions of the tumor, and we newly defined HER2 heterogeneity as any of those portions of the tumor that did not show HER2 positivity. We employed the standard criteria of HER2 positivity generally performed in clinical diagnosis, i.e., tumor cell cluster of at least five positive cells with complete basolateral or lateral membranous reactivity. According to our simple definition of intratumoral HER2 heterogeneity, the treatment outcomes were definitively different between the HER2 homogeneity group and heterogeneity group. Assessment of HER2 positivity for endoscopic biopsy specimen in the ToGA trial was established without consideration of intratumor HER2 heterogeneity. Our current study suggests that the combination of heterogeneity and IHC might be beneficial to predict the efficacy of trastuzumab-based chemotherapy.
HER2 heterogeneity is a pivotal predictor for the efficacy of HER2-positive gastric cancers. However, there are still some problems that need to be solved in the future. First, concordance of intratumoral HER2 heterogeneity between endoscopic biopsy specimen and whole tumor tissues has not been investigated in the current study. Secondly, there was no common standard of adequate numbers and portions for the assessment of intratumoral HER2 heterogeneity. Thirdly, this study did not include the intratumoral heterogeneity of HER2 gene amplification. Intratumoral heterogeneity of HER2 gene amplification has been reported to be one of the significant prognostic factors for metastatic or resectable advanced breast cancers. The significance of intratumoral heterogeneity of HER2 gene amplification should also be clarified by further investigations. The survival benefits for anti-HER2 drugs such as lapatinib or pertuzumab in combination with trastuzumab and adjuvant therapy of trastuzumab or trastuzumab beyond progression were demonstrated for breast cancers. However, the efficacy of those anti-HER2 agents had not been proven for gastric cancers, and intratumoral HER2 heterogeneity may be considered as one of the reasons for the difference of these results between breast and gastric cancers. If more detailed clinical significance of intratumoral HER2 heterogeneity is solved in the future, anti-HER2 drugs other than trastuzumab can be adopted, and treatment outcomes of HER2-positive gastric cancers would be improved.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen S, Cao ZF, Cao J S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4831] [Article Influence: 345.1] [Reference Citation Analysis (0)] |
| 2. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1793] [Article Influence: 256.1] [Reference Citation Analysis (0)] |
| 3. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [PubMed] [Cited in This Article: ] |
| 4. | Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB, Carrato A, Gulley ML, Jain D, Kakar S, Mackay HJ, Streutker C, Tang L, Troxell M, Ajani JA. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:446-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 5. | Lee HE, Park KU, Yoo SB, Nam SK, Park DJ, Kim HH, Lee HS. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN, Takiguchi S, Mori M, Doki Y. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Ahn S, Ahn S, Van Vrancken M, Lee M, Ha SY, Lee H, Min BH, Lee JH, Kim JJ, Choi S, Jung SH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, Kim KM. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget. 2015;6:38372-38380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Yagi S, Wakatsuki T, Yamamoto N, Chin K, Takahari D, Ogura M, Ichimura T, Nakayama I, Osumi H, Shinozaki E, Suenaga M, Fujisaki J, Ishikawa Y, Yamaguchi K, Namikawa K, Horiuchi Y. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer. 2019;22:518-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Lee HJ, Kim JY, Park SY, Park IA, Song IH, Yu JH, Ahn JH, Gong G. Clinicopathologic Significance of the Intratumoral Heterogeneity of HER2 Gene Amplification in HER2-Positive Breast Cancer Patients Treated With Adjuvant Trastuzumab. Am J Clin Pathol. 2015;144:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Park SY. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25:938-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 11. | Lee HJ, Seo AN, Kim EJ, Jang MH, Suh KJ, Ryu HS, Kim YJ, Kim JH, Im SA, Gong G, Jung KH, Park IA, Park SY. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014;142:755-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Wakatsuki T, Yamamoto N, Sano T, Chin K, Kawachi H, Takahari D, Ogura M, Ichimura T, Nakayama I, Osumi H, Matsushima T, Suenaga M, Shinozaki E, Hiki N, Ishikawa Y, Yamaguchi K. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol. 2018;53:1186-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15860] [Cited by in F6Publishing: 18963] [Article Influence: 1264.2] [Reference Citation Analysis (1)] |
| 14. | Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors, 7th ed. Wiley-Blackwell, A John Wiley & Sons, Ltd. 2009;. [Cited in This Article: ] |
| 15. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 16. | Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, Muro K, Komatsu Y, Tsuburaya A. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol. 2010;21:1001-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Motoshima S, Yonemoto K, Kamei H, Morita M, Yamaguchi R. Prognostic implications of HER2 heterogeneity in gastric cancer. Oncotarget. 2018;9:9262-9272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 19. | Johnston SRD, Hegg R, Im SA, Park IH, Burdaeva O, Kurteva G, Press MF, Tjulandin S, Iwata H, Simon SD, Kenny S, Sarp S, Izquierdo MA, Williams LS, Gradishar WJ. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: ALTERNATIVE. J Clin Oncol. 2018;36:741-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortés J; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1303] [Cited by in F6Publishing: 1362] [Article Influence: 151.3] [Reference Citation Analysis (0)] |
| 21. | Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Magazzù D, Heinzmann D, Steinseifer J, Valagussa P, Baselga J. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 334] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 22. | von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, Baumann KH, Clemens MR, Duerr R, Uleer C, Andersson M, Stein RC, Nekljudova V, Loibl S. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27:1999-2006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 505] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 23. | Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K, Kang YK. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 292] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 24. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, Miwa H, Qin SK, Chung IJ, Yeh KH, Feng JF, Mukaiyama A, Kobayashi M, Ohtsu A, Bang YJ. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 450] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 25. | Fabi A, Di Benedetto A, Metro G, Perracchio L, Nisticò C, Di Filippo F, Ercolani C, Ferretti G, Melucci E, Buglioni S, Sperduti I, Papaldo P, Cognetti F, Mottolese M. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res. 2011;17:2055-2064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, Lee JH, Lee GH, Kang YK. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Pietrantonio F, Fucà G, Morano F, Gloghini A, Corso S, Aprile G, Perrone F, De Vita F, Tamborini E, Tomasello G, Gualeni AV, Ongaro E, Busico A, Giommoni E, Volpi CC, Laterza MM, Corallo S, Prisciandaro M, Antista M, Pellegrinelli A, Castagnoli L, Pupa SM, Pruneri G, de Braud F, Giordano S, Cremolini C, Di Bartolomeo M. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: the AMNESIA Case-Control Study. Clin Cancer Res. 2018;24:1082-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Yoshida H, Yamamoto N, Taniguchi H, Oda I, Katai H, Kushima R, Tsuda H. Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch. 2014;465:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Fazlollahi L, Remotti HE, Iuga A, Yang HM, Lagana SM, Sepulveda AR. HER2 Heterogeneity in Gastroesophageal Cancer Detected by Testing Biopsy and Resection Specimens. Arch Pathol Lab Med. 2018;142:516-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Kanayama K, Imai H, Yoneda M, Hirokawa YS, Shiraishi T. Significant intratumoral heterogeneity of human epidermal growth factor receptor 2 status in gastric cancer: A comparative study of immunohistochemistry, FISH, and dual-color in situ hybridization. Cancer Sci. 2016;107:536-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Tsuda H, Kurosumi M, Umemura S, Yamamoto S, Kobayashi T, Osamura RY. HER2 testing on core needle biopsy specimens from primary breast cancers: interobserver reproducibility and concordance with surgically resected specimens. BMC Cancer. 2010;10:534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2910] [Cited by in F6Publishing: 2802] [Article Influence: 254.7] [Reference Citation Analysis (0)] |
| 33. | Oono Y, Kuwata T, Takashima K, Yoda Y, Ikematsu H, Shitara K, Kinoshita T, Yano T. Clinicopathological features and endoscopic findings of HER2-positive gastric cancer. Surg Endosc. 2018;32:3964-3971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Gullo I, Grillo F, Molinaro L, Fassan M, De Silvestri A, Tinelli C, Rugge M, Fiocca R, Mastracci L. Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc Int Open. 2015;3:E165-E170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |