Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1660
Peer-review started: March 4, 2019
First decision: April 18, 2019
Revised: April 26, 2019
Accepted: May 1, 2019
Article in press: May 1, 2019
Published online: July 6, 2019
Thrombocytopenia associated with acute kidney injury is a challenging disorder. Thrombotic microangiopathy (TMA) is a potentially life- or organ-threatening syndrome that can be induced by several disorders or medical interventions. There is overlap between the clinical presentation and pathophysiology of thrombotic thrombocytopenia purpura and hemolytic uremic syndrome (HUS), and to a lesser extent, disseminated intravascular coagulation (DIC). We describe a case to illustrate the potential diagnostic difficulty, especially at initial presentation.
We reported a case of a 44-year-old woman that presented with diarrhea, thrombocytopenia, schistocytes, elevated serum lactate dehydrogenase (LDH) level and acute kidney injury. While the clinical presentation resembled that of Shiga toxin–induced HUS, the disease course was more consistent with gastrointestinal infection-related DIC. To aid in the accurate diagnosis of TMA and other associated disorders, we have undertaken a review and provided a clear interpretation of some typical biomarkers including schistocytes, LDH and platelet count, coagulation profile and more specific indexes of ADAMTS13, complement profile, and the isolation of Shiga toxin-producing Escherichia coli (commonly referred to as STEC).
The use and correct interpretation of classical indexes of schistocyte, LDH, and platelet count is vital in diagnosing TMA and associated disorders. Understanding the characteristics of these biomarkers in the context of thrombocytopenia purpura, HUS and DIC will facilitate the accurate diagnosis and early initiation of appropriate treatment.
Core tip: Thrombotic microangiopathy is a severe and challenging disorder. There is overlap between the clinical presentation and pathophysiology of thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, and disseminated intravascular coagulation. Upon literature review, we use a case study to illustrate the characteristics and utility of classical clinical indexes of schistocytes, lactate dehydrogenase, platelet count and coagulation profile in parallel with more specific investigations of ADAMTS13, complement profile, and isolation of Shiga toxin-producing Escherichia coli, in an attempt to facilitate the early recognition and diagnosis of thrombotic microangiopathy.
- Citation: Li XY, Mai YF, Huang J, Pai P. Gastrointestinal infection-related disseminated intravascular coagulation mimicking Shiga toxin-mediated hemolytic uremic syndrome - implications of classical clinical indexes in making the diagnosis: A case report and literature review. World J Clin Cases 2019; 7(13): 1660-1670
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1660.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1660
The term Thrombotic Microangiopathy (TMA) was first proposed by Symmers in 1952 to describe generalized microvascular thrombi in thrombotic thrombocytopenia purpura (TTP)[1]. In 1955, Gasser first documented similar pathological changes in the kidneys in hemolytic uremic syndrome (HUS)[2]. TMA was synonymous with micro-angiopathic hemolytic anemia (MAHA) in that mechanical fragmentation of erythrocytes occurred as they passed through the thrombotic meshwork and strictured microvasculature[1]. MAHA diagnosis relies on the presence of peripheral schistocytes, elevated serum lactate dehydrogenase (LDH)[3], along with other markers of hemolysis (e.g., indirect bilirubin and haptoglobins)[3,4]. Nowadays, TMA is used to describe histological abnormalities of arterioles and capillaries characterized by thrombosis with endothelial impairment and mural damage[3,5]. The clinical hallmarks of TMA include consumptive thrombocytopenia and MAHA with varying degrees of ischemic injury and organ dysfunction[3,5]. The prototypes of TMA are TTP and HUS. Low platelet (PLT) counts makes disseminated intravascular coagulation (DIC) disorder part of a differential diagnosis. TMA may be induced by several disorders or medical conditions, such as DIC[6], systemic infection and sepsis, systemic rheumatological diseases (e.g., systemic lupus erythematosus, small vessel vasculitis, and antiphospholipid syndrome), malignancies, pregnancy, malignant hypertension, coagulopathy, and may follow solid organ or hematopoietic stem cell transplantation and drug therapy[4].
Both TMA and DIC related disorders are a matter of clinical emergency. Hence, timely diagnosis and early initiation of targeted therapy is essential for the survival of patients and the affected organs[7]. However, the complexity of pathological pathways and clinical overlaps of TMA prototypes create diagnostic uncertainties. In practice, physicians rely on clinical phenotype and biomarkers to diagnose TMA and its underlying conditions. However, these markers are limited by specificity or sensitivity and need to be interpreted carefully. Herein, we report a case of infection-related DIC in a woman who presented with diarrhea, thrombocytopenia, elevated LDH, schistocytes and acute kidney injury, mimicking Shiga toxin-HUS (ST-HUS). In this report, we review and discuss the utility and drawbacks of several classical clinical indexes used in the discrimination of TTP-HUS and DIC.
Vomiting, diarrhea and abdominal pain for 2 h.
A previously healthy 44-year-old female presented to the Accident and Emergency Department with a 2 h duration of diarrhea, vomiting, painful lower abdominal cramping and dizziness. The woman was working as a cleaner. According to the patient, she had eaten an unpeeled apple a few hours before. The diarrhea was descri-bed as watery but non-bloody. There was no chill, rigor or fever.
The patient denied any significant past medical history and had no recent travel. She denied exposure to any animals or consumption of any under-cooked vegetables or meats. There was no significant family history. She reported no pregnancy related disorders or history of drug allergy.
The patient was fully oriented at presentation with a temperature of 37.1˚C. There was no rash. Her respiratory rate was 23 breaths per minute and oxygen saturation was 98% on air. Her pulse was 48 beats per minute; her lying blood pressure was 69/41 mmHg. Her lungs were clear. There was no heart murmur. The abdomen showed only mild epigastric tenderness. There were no masses or hepatosplenomegaly. There were no abnormal neurology signs.
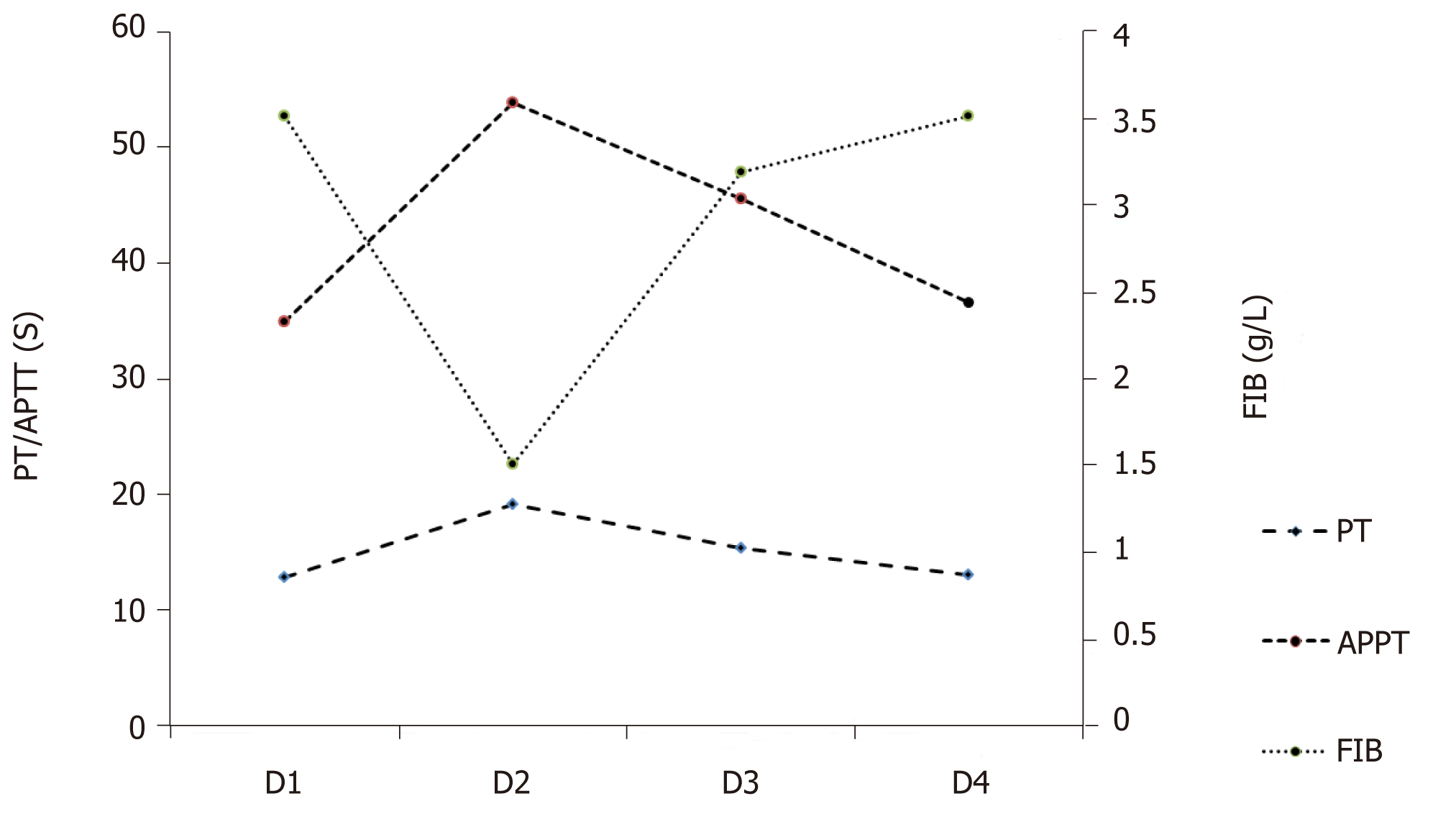
Her initial blood tests at the Emergency Department showed a white blood cell (WBC) of 2.67 × 109/L (normal 4 × 109/L - 10 × 109/L); neutrophils, 73.4%; lymphocytes, 26.6%; hemoglobin (HB), 134 g/L (normal 115-150 g/L); PLT count 31 × 109/L (normal 150 × 109/ L - 350 × 109/L) (Table 1). The serum creatinine was 74.5 μmol/L (normal 46-92 μmol/L); blood urea nitrogen (BUN), 5.6 mmol/L (normal 2.5-6.1 mol/L); serum amylase, 74 U/L (normal 30-110 U/L); total bilirubin, 50.9 μmol/L (normal 3-22 μmol/L), indirect bilirubin, 47.3 μmol/L (normal 0-19 μmol/L). The coagulation test showed normal prothrombin time (PT) of 12.9 s (normal 11-14.5 s; international normalized ratio (INR), 1.0 (normal 0.8-1.2); activated partial thromboplastin time (APTT) was 34.9 s (normal 26-40 s); fibrinogen (Fib), 2.53 g/L (2-4 g/L); thrombin time (TT), 17.8 s (14-21 s) (Table 2, Figure 1). She was given oxygen, and intravenous 0.9% saline and dopamine were commenced at 10 μg per kg of body weight per minute. Her blood pressure rose to 97/58 mmHg after an hour. She was transferred to the Department of gastrointestinal disease with a primary diagnosis of acute gastroenteritis and early shock. The thrombocytopenia was thought to be related to severe infection.
| Item | Value | Reference interval | |
| Day 1 | Day 2 | ||
| WBC, × 109/L | 2.67 | 23.53 | 3.89-9.93 |
| Neutrophil, % | 73.4 | 98 | 44.0-72.0 |
| LYM, % | 26.6 | 1.1 | 20.0-45.0 |
| EOS, % | 0 | 0 | 2.0-10.0 |
| HGB, g/L | 134 | 120 | 115-148 |
| PLT, × 109/L | 31 | 20 | 162-341 |
| Item | Value | Reference interval | |||
| Day 1 | Day 2 | Day 3 | Day 4 | ||
| PT, s | 12.9 | 19.2 | 15.4 | 13.1 | 11-14.5 |
| PT-INR | 1.0 | 1.64 | 1.25 | 1.02 | 0.8-1.2 |
| APTT, s | 34.9 | 53.9 | 45.6 | 36.6 | 26-40 |
| FIB, g/L | 2.53 | 1.51 | 3.19 | 3.51 | 2-4 |
| TT, s | 17.8 | 25 | 16.3 | 16.1 | 14-21 |
| D-dimer, µg/mL | - | - | > 20 | - | 0.0-0.5 |
Following admission, she was given 2.0 g intravenous ceftriaxone and fluid. The next day, her abdominal pain and the watery diarrhea had decreased. A repeat blood routine test showed: WBC, 23.52 × 109/L; neutrophils, 98%; lymphocytes, 1.1%; eosinophils, 0%; monocytes, 0.9%; HB, 120 g/L; PLT, 20 × 109/L (Table 1). Urinalysis showed protein 1+, WBC 86/μL (normal, 0-23/μL), red blood cell (RBC) 96/μL (normal, 0-18/μL) (Table 3). The stool microscopy and culture and occult blood tests were negative. Repeat clotting study showed a prolonged PT of 19.2 s; INR, 1.64; APTT, 53.9 s; and Fib lowered to 1.51 g/L; TT to 25 s (Table 2, Figure 1). The C-reactive protein was 55.13 g/L (normal < 10 mg/L); serum procalcitonin (PCT), 73 ng/mL (normal < 0.05-0.1 ng/mL); amylase, 92 U/L; lipase, 77 U/L; alanine aminotransferase, 38 IU/L (normal 9-52 IU/L); aspartate aminotransferase, 54 (normal 14-36 IU/L). The stool rotavirus antigen and Clostridium difficile toxin A/B tests were negative (Table 4). Despite a 24-h urine volume of two liters, her serum creatinine levels had increased to 145 μmol/L.
| Item | Value | Reference interval |
| PRO | 1+ | Negative |
| WBC, /µL | 86 | 0-22 |
| RBC, /µL | 95.7 | 0-18 |
| URO | 2+ | Negative |
| ERY | 2+ | Negative |
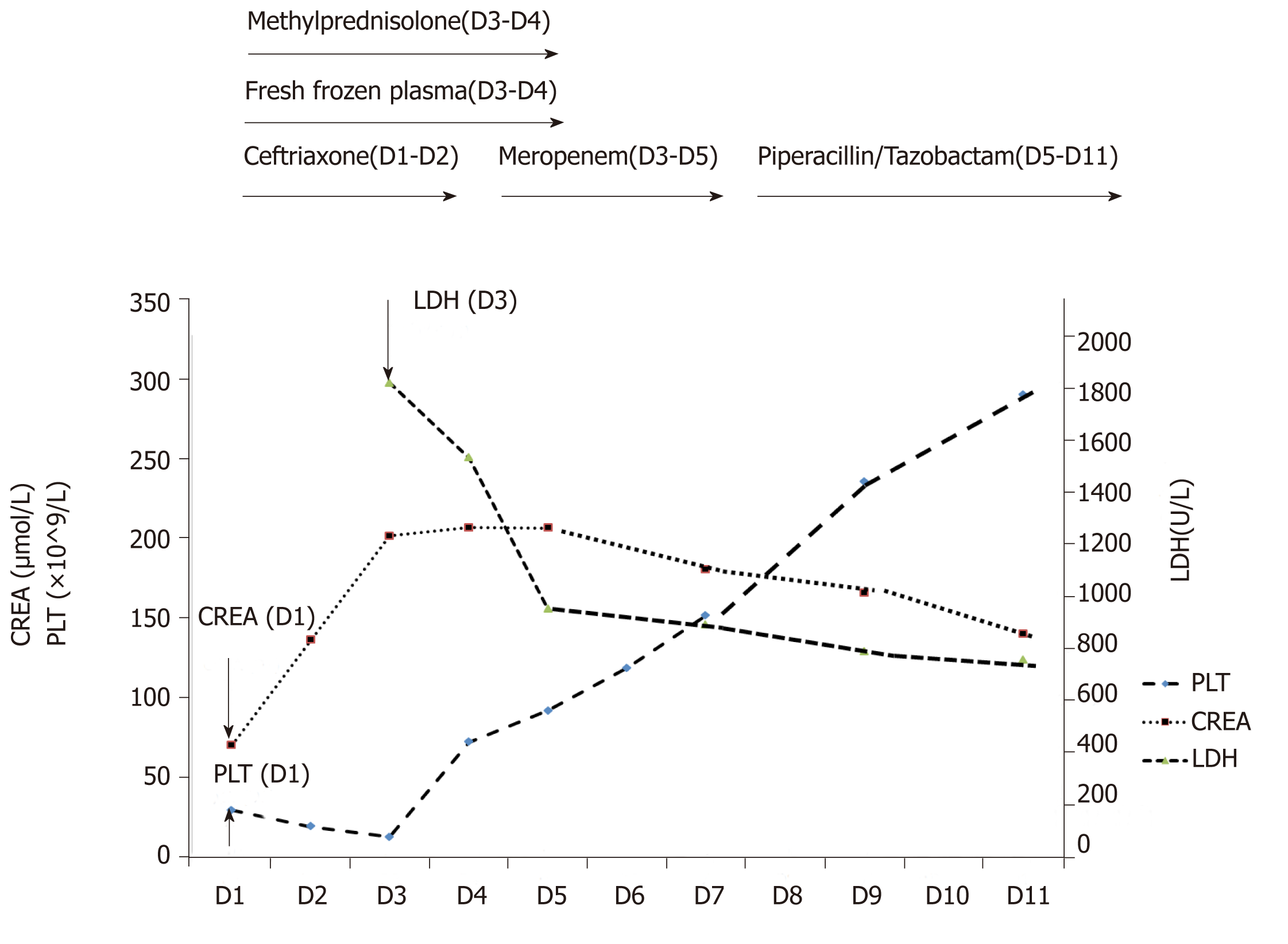
Intravenous ceftriaxone was continued at a dose of 2.0 g daily. On day 3, the diarrhea had reduced to four or five times per day. Total 24-h urine output was 1 L. The creatinine levels further increased to 215.7 μmol/L (Figure 2), BUN to 6.7 mmol/L. The peripheral blood count showed WBC, 14.59-13.81 × 109/L; neutrophils 0.88-89; PLT, 15 × 109/L. Peripheral blood smear showed a schistocyte count of 0.6%; serum LDH level was 1818 U/L (normal 313-618 U/L) (Figure 2). The clotting profile had improved: PT, 15.4 s; INR 1.25; APTT, 45.6 s; Fib 3.19 g/L; TT 16.3 s. D-dimer level > 20 mg/L (normal 0-0.5 mg/L) (Table 2). Plasma troponin I level was 0.082 ng/mL (normal 0-0.034 ng/mL); serum ferritin, 1417.3 ng/mL (normal 11-306.8 ng/mL); PCT, 40.3 ng/mL. Two stool cultures (taken on day 2 and 3) were negative for Escherichia coli O157:H7, Shigella, Salmonella, Vibrio, Aeromonas, Plesiomonas or Campylobacter. Two blood and urine cultures (taken on day 2 and 3) were negative for bacteria and fungus (Table 4).
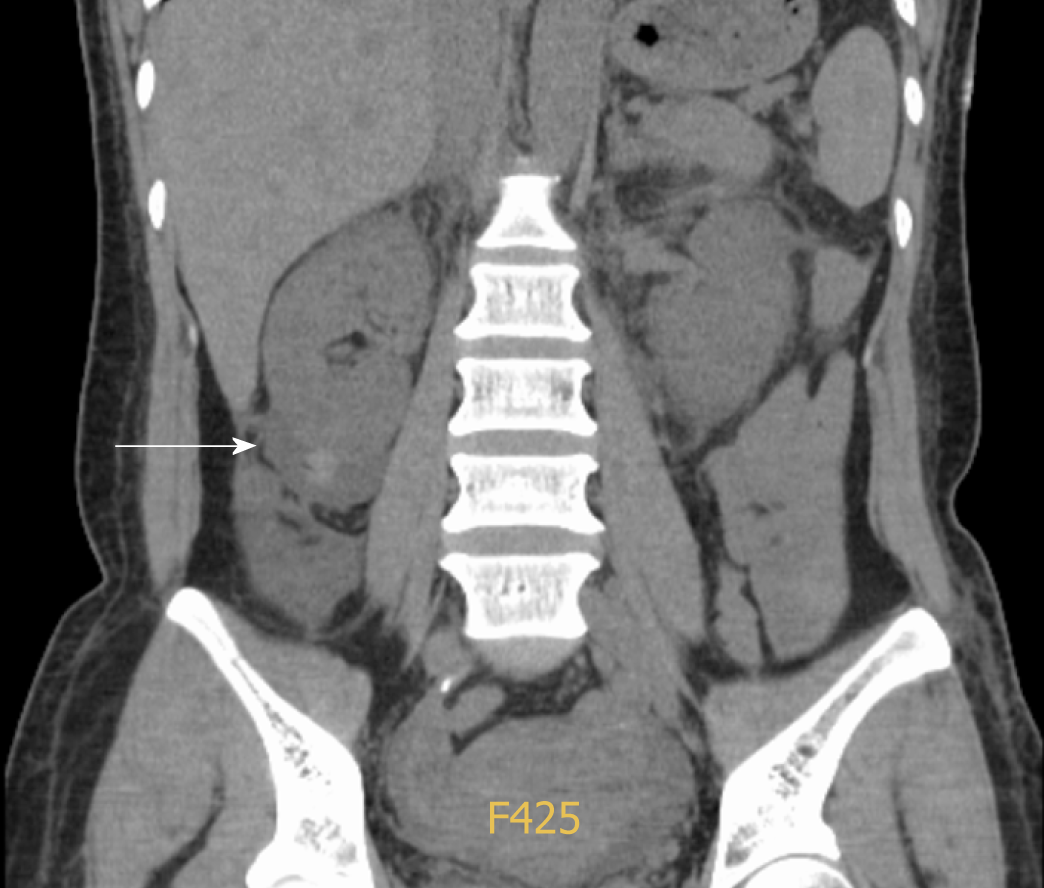
Ultrasonography of the urinary system on day 2 revealed symmetrical bilateral kidneys with increased parenchymal echogenicity and a low echogenic area of 57 mm by 21 in the lower right kidney, suggestive of effusion or hematoma. A subsequent whole abdominal computed tomography scan showed signs of upper abdomen peritonitis, mild ascites and a peri-renal hematoma around the lower right kidney (Figure 3).
Acute gastroenteritis complicated by DIC.
The antimicrobial therapy was increased to 1 g meropenem every 12 h starting on day 3 for 3 days, followed by Piperacillin/Tazobactam from day 5 to 11 (Figure 2). A dose of 40 mg methylprednisolone daily was given intravenously for sepsis from day 3 to 4. On day 3, she was also given 400 mL fresh frozen plasma (commonly referred to as FFP) and ten units concentrated PLT (3-4 × 1011) to correct the coagulopathy and to control the right peri-renal hemorrhage. On day 4, the PLT was 77 × 109/L; WBC 14.39 × 109/L; Hb 111 g/L. Another 200 mL FFP was given along with 20 mg methylpred-nisolone. On day 5, her diarrhea had resolved. The serum creatinine levels had plateaued at 220 μmol/L; PLT, 98 × 109/L; LDH lowered to 952 U/L (Figure 2). Serum complement 3 (C3) and C4, and immunoglobulins G, A and M were within normal ranges. Serum anti-nuclear antibody, anti-cardiolipin antibodies, lupus anticoagulant, anti-beta 2 glycoprotein I, and extractable nuclear antigens were all normal or negative. On day 6, PLT was 127 × 109/L; WBC was 7.8 × 109/L. By day 11, serum LDH had decreased to 786 U/L and serum creatinine fell to 148.9 μmol/L (Figure 2).
The patient was feeling better and was discharged on day 11 after her condition further stabilized. At her 3 mo follow-up visit, her serum creatinine level was 78 μmol/L.
This previously healthy woman presented with the acute onset of abdominal cram-ping and diarrhea. Physical examination at presentation revealed hypotension and mild epigastric tenderness. The initial presentation was thought to be related to acute gastroenteritis, systemic inflammation syndrome and hypovolemic shock. The subsequent development with worsening thrombocytopenia, schistocytes, elevated serum LDH, and acute kidney injury suggested a diagnosis of HUS, but the presence of leukocytosis, coagulopathy, hypofibrinogenemia and spontaneous visceral bleeding indicated possible infection related-DIC. Differentiation between these diseases is crucial for proper clinical management.
Schistocyte may be induced by mechanical fragmentation of RBCs when passing through strictured microvasculature. The Mayo Clinic issued a consensus statement that the “mere presence of schistocytes is adequate (for a diagnosis of TMA) in the appropriate clinical context”[4]. But, correct interpretation of this marker can only be achieved if its significance and limitation under different clinical settings are under-stood.
Schistocytes are fragments of RBCs with the morphology of helmet cells; small, irregular, triangular, or crescent-shaped cells, pointed projections, and without central pallor[8]. Their presence is suggestive of but not specific to TMA[9]. In fact, a low rate of schistocytes at 0.05% ± 0.03% (range 0%-0.27%)[10], or ≤ 0.2%[11] may be observed in healthy donors. Further, it might be seen in disorders of erythrocyte cytoskeletal abnormalities or hemoglobinopathies, as well as a range of other conditions, chronic renal disease[10], renal failure[12], sepsis (0.87% ± 0.67%)[12], and mechanical heart valves (0.18% ± 0.15%[10] or 0.43% ± 0.32%[12]). It is noted that patients with TTP and HUS had a relatively higher schistocyte rate of 8.35% ± 2.74%[10] and 3.5% ± 1.88%[12], respec-tively. Although schistocytes can also be detected in the setting of DIC, it is seldom > 1%. In a retrospective study of 35 patients (mostly in intensive care units), schistocytes were present in 30 subjects (85.7%), among which 20 patients (57.1%) had schistocytes < 0.5%, 6 (17.1%) patients had schistocytes between 0.5%-1%, and only 4 (11.4%) had schistocyte counts ≥ 1%; The four DIC patients with schistocytes ≥ 1% had concurrent diseases of leukemia, pregnancy, and severe infection[11]. The International Council for Standardization in Hematology recommends that ≥ 1% schistocytes in the absence of other moderate dysmorphic RBC is an important criterion for TMA[8]. It is also important to note that automated schistocyte analysis is unreliable[12,13]; the detection of schistocytes needs to be performed manually, and is thus subject to observer bias[10,12]. Occasionally, at the early presentation of TMA, schistocytes may not be detected for up to 2-3 d on serial peripheral blood smear[14]. Rarely, schistocytes have not been detected during TMA recurrence[15].
LDH is a commonly used TMA biomarker[3], but its level is variable between patients. LDH catalyzes the reversible transformation of pyruvate to lactate under anaerobic conditions. Normal tissues produce five distinct function-related LDH isoenzymes with different electrophoretic mobility[16]. LDH1 and LDH2 are primarily found in RBCs, heart muscle and the kidneys; LDH3 is highest in the lungs; LDH4 and LDH5 are highest in skeletal muscle and liver[17]. Serum LDH increases in response to tissue injury, hemolysis, necrosis, hypoxia, and myocardial infarction. LDH is released from ruptured RBCs, and ineffective erythropoiesis has been regarded as an index of hemolysis. The routine determination of serum LDH includes all of the five isoenz-ymes. Interestingly, LDH isoenzymatic distributional study showed LDH1 and LDH2 (erythrocytic origin) were not disproportionately elevated in 9 out of 10 TTP patients, suggesting the increased serum LDH observed in TTP patients is released from a variety of ischemic tissues rather than by intravascular hemolysis alone[18]. In the context of TTP, LDH levels are unlikely to be > 2500 IU/L, and a higher level should raise the possibility of other hematological disorders, such as B12 deficiency[19]. Rarely, LDH levels may not exceed the normal range in TTP. Among the 72 TTP patients reported in a series at Johns Hopkins University, the median serum LDH concentra-tion was 1184 IU/L with an interquartile range of 152 to 5950 IU/L[20]. In an Oklahoma TTP registry comprising 261 patients, serum LDH ranged from 114 to 12587 IU/L[21]. In the TTP cohort from Washington University with 36 patients, serum LDH levels were between 328 and 28000 IU/L; 32 out of the 36 patients had LDH < 2000 IU/L, and 35 out of the 36 patients had LDH < 3000 IU/L[22]. As a biomarker, LDH is often used to monitor disease activity of HUS or TTP and treatment response. In practice, once the LDH is normalized or near normal for 2 consecutive days, therapeutic plasma exchange (TPE) for TTP or HUS may cease[23,24]. However, LDH normalization has been shown to lag behind PLT recovery by an average of 9 d, so the initial LDH levels might not be used to predict response to TPE[20].
Thrombocytopenia is commonly present in TTP and HUS. A depressed PLT may be caused by infections, hemodilution, increased consumption, decreased production, increased sequestration, drugs, and immune-mediated destruction. Thrombocy-topenia is defined as PLT count below the lower limit of normal range (i.e. < 150 × 109/L for adults). The severity of thrombocytopenia can be further subdivided into mild (100-150 × 109/L), moderate (50-99 × 109/L), and severe (< 50 × 109/L)[25]. Thrombocytopenia is generally prominent in TTP (< 30 × 109/L) due to extensive PLT-rich thrombi formation[6]. While significant thrombocytopenia (15-50 × 109/L) is typical of TMA[4], a normal PLT count at initial presentation does not exclude the diagnosis[6]. There have been reports that PLT may fall within the normal range at TMA onset or during early recurrence of the disease[4]. An abrupt decrease or a decreasing trend of PLT reflects progressive PLT consumption[26]. Similarly, PLT may be used to monitor disease activity. It has been suggested that the restoration of PLT (above 150 × 109/L) signifies clinical remission and that TPE therapy may be discontinued[24].
According to the Scientific Subcommittee on DIC of the International Society of Thrombosis and Hemostasis, DIC is “an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different causes. DIC can originate from and cause damage to the microvasculature, if sufficiently severe, can produce organ dysfunction”[27]. Using this definition, it is clear that there is an overlap between DIC and TMA both clinically and pathologically. Some authors even consider DIC as a common cause of TMA[6]. In contrary to DIC, the coagulation profile is essentially normal in TTP-HUS and other TMA diseases. Normal values of PT, APTT and Fib distinguish TTP-HUS from DIC in the presence of schistocytes and high LDH levels. In comparison with DIC control, a very low PLT count (< 20 × 109/L) and a PT within 5 s of the upper limit are specific to TTP-HUS[28]. This is because the microthrombi plugged in TTP-HUS are PLT-rich[29,30], whereas in DIC, the microthrombi formed in small vasculature are rich in fibrin[31].
ADAMTS13 (A Disintegrin And Metalloproteinase with Thrombospondin type 1 motif, member 13) is a specific marker for TMA. In addition, it is well known that TTP may result from severe functional deficiency of the VWF-cleaving protease ADAMTS13, which leads to accumulation of ultra-large VWF-multimers along the lumen of small blood vessels, resulting in extensive PLT/VWF-rich intravascular thrombus formation. ADAMTS13 assay includes activity, functional inhibitor (based on plasma mixing studies) and anti-ADAMTS13-IgG[6]. The proteolytic activity of ADAMTS13 is considered “normal” if it is above 50% activity of the normal control (tested with pooled local blood samples). A high certainty of TTP (90% specificity) can only be made if the ADAMTS activity is < 10%[32]. Such a low cut-off value (< 10% of normal activity) is applied because most ADAMTS13 molecules are bound to CD36 (an integral membrane also known as PLT glycoprotein 4) on the endothelial cell surface. Therefore, the in vivo ADAMTS13 activity exceeds the circulatory value determined via peripheral blood specimen[3]. In practice, these assays are usually available in specialized centers and laboratories and are not easily available.
Nowadays, HUS is generally divided into ST-HUS, secondary-HUS and atypical HUS (aHUS)[33]. The majority of ST-HUS cases are caused by STEC or Shigella infections. Isolation of STEC and identification of alternative complement pathway defects may be used to pinpoint a definite illness underlying TMA syndrome. Approximately 6%-9% of STEC infections are complicated by ST-HUS. The triad of acute kidney injury, MAHA, and non-immune thrombocytopenia typically begin 5 to 10 d after the onset of diarrhea[34]. The diagnosis of ST-HUS from atypical HUS may be challenging. Stool culture for STEC may take days, and Shiga toxin stool PCR assay is not widely used[35]. A panel approach is required to identify complement disorders utilizing serological, genetic and flow cytometric analyses (CD46). However, apart from C3, C4 and CH50, the determination of AP components (e.g., FH, FB, FI, CFHR1-5 and anti-FH) is not easily available. Serological detection of components of AP is neither sensitive nor specific[36]. So far, genetic screening for dysregulated complement genes, mutation or deletion can be found in only approximately 50%-80% of complement-mediated TMA[4,37]. ST-HUS is best managed by supportive care with symptomatic treatment. Interestingly, antibiotic use is thought to increase the release of Shiga toxin and has been associated with subsequent development of HUS in some studies[38,39]. Where acquired or hereditary TTP is suspected, treatment is necessary with TPE or FFP replacement, whereas eculizumab (complement inhibition) may be considered for cases of aHUS.
With reference to our case, the patient presented with diarrhea and vomiting without neurological deficit, in the background of severe thrombocytopenia, acute kidney injury, elevated LDH level and slightly increased schistocytes (0.6%). There was also an elevated indirect bilirubin level indicative of intravascular hemolysis. While the initial presentation was consistent with the clinical “triad” of ST-HUS, there were points to support DIC as a cause. In a typical ST-HUS case, abdominal pain and diarrhea often begin several days after contaminated food has been ingested, and thrombocytopenia and renal failure commonly occur after gastrointestinal symptoms are resolved[40], but this was not the case here. The schistocytes were < 1%, which makes the diagnosis of TMA less likely. The negative stool culture of STEC/Shigella and the presence of coagulopathy further argue against ST-HUS as the cause. According to the International Society on Thrombosis and Hemostasis (ISTH) diagnostic criteria of DIC can be made if the ISTH score ≥ 5 is in the context of gastrointestinal infection. Our patient scored 7 with reference to the ISTH-DIC score (PLT ≤ 50 × 109/L, 2 points; 3 s < PT < 6 s, 2 points; a marked increased D-dimer, 3 points). The presence of low rate of schistocytes, a high LDH level, acute kidney injury and perirenal hematoma fit well into a DIC diagnosis.
In our case report, we made a diagnosis of infection-associated DIC, which allows timely treatment. Correct understanding and careful interpretation of various biomarkers including schistocytes, LDH, and PLT can enable clinicians to differentiate TMA from other related diseases. However, clinicians need to be aware that each of these biomarkers bears its own limitations in specificity and sensitivity.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hilmi I S-Editor: Dou Y L-Editor: Filipodia E-Editor: Wang J
| 1. | Symmers WS. Thrombotic microangiopathic haemolytic anaemia (thrombotic microangiopathy). Br Med J. 1952;2:897-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 140] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Gasser C, Gautier E, Steck A, Siebenmann RE, Oechslin R. Hemolytic-uremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemia. Schweiz Med Wochenschr. 1955;85:905-909. [PubMed] [Cited in This Article: ] |
| 3. | Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 968] [Cited by in F6Publishing: 877] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 4. | Go RS, Winters JL, Leung N, Murray DL, Willrich MA, Abraham RS, Amer H, Hogan WJ, Marshall AL, Sethi S, Tran CL, Chen D, Pruthi RK, Ashrani AA, Fervenza FC, Cramer CH 2nd, Rodriguez V, Wolanskyj AP, Thomé SD, Hook CC; Mayo Clinic Complement Alternative Pathway-Thrombotic Microangiopathy Disease-Oriented Group. Thrombotic Microangiopathy Care Pathway: A Consensus Statement for the Mayo Clinic Complement Alternative Pathway-Thrombotic Microangiopathy (CAP-TMA) Disease-Oriented Group. Mayo Clin Proc. 2016;91:1189-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Barbour T, Johnson S, Cohney S, Hughes P. Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transplant. 2012;27:2673-2685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Scully M, Cataland S, Coppo P, de la Rubia J, Friedman KD, Kremer Hovinga J, Lämmle B, Matsumoto M, Pavenski K, Sadler E, Sarode R, Wu H; International Working Group for Thrombotic Thrombocytopenic Purpura. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 7. | George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 693] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 8. | Zini G, d'Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, McFadden S, Vives-Corrons JL, Yutaka N, Lesesve JF; International Council for Standardization in Haematology (ICSH). ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol. 2012;34:107-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Huh HJ, Chung JW, Chae SL. Microscopic schistocyte determination according to International Council for Standardization in Hematology recommendations in various diseases. Int J Lab Hematol. 2013;35:542-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Burns ER, Lou Y, Pathak A. Morphologic diagnosis of thrombotic thrombocytopenic purpura. Am J Hematol. 2004;75:18-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Lesesve JF, Martin M, Banasiak C, André-Kerneïs E, Bardet V, Lusina D, Kharbach A, Geneviève F, Lecompte T. Schistocytes in disseminated intravascular coagulation. Int J Lab Hematol. 2014;36:439-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Schapkaitz E, Mezgebe MH. The Clinical Significance of Schistocytes: A Prospective Evaluation of the International Council for Standardization in Hematology Schistocyte Guidelines. Turk J Haematol. 2017;34:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Lesesve JF, El Adssi H, Watine J, Oosterhuis W, Régnier F. Evaluation of ICSH schistocyte measurement guidelines in France. Int J Lab Hematol. 2013;35:601-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Allford SL, Hunt BJ, Rose P, Machin SJ; Haemostasis and Thrombosis Task Force, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. Br J Haematol. 2003;120:556-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Daram SR, Philipneri M, Puri N, Bastani B. Thrombotic thrombocytopenic purpura without schistocytes on the peripheral blood smear. South Med J. 2005;98:392-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Neely CL, Wajima T, Kraus AP, Diggs LW, Barreras L. Lactic acid dehydrogenase activity and plasma hemoglobin elevations in sickle cell disease. Am J Clin Pathol. 1969;52:167-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kopperschläger G, Kirchberger J. Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. J Chromatogr B Biomed Appl. 1996;684:25-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Cohen JA, Brecher ME, Bandarenko N. Cellular source of serum lactate dehydrogenase elevation in patients with thrombotic thrombocytopenic purpura. J Clin Apher. 1998;13:16-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Walter K, Vaughn J, Martin D. Therapeutic dilemma in the management of a patient with the clinical picture of TTP and severe B12 deficiency. BMC Hematol. 2015;15:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhan H, Streiff MB, King KE, Segal JB. Thrombotic thrombocytopenic purpura at the Johns Hopkins Hospital from 1992 to 2008: clinical outcomes and risk factors for relapse. Transfusion. 2010;50:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115:1500-11; quiz 1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 22. | Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043-4049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Haas M, Leko-Mohr Z, Lang T, Jansen M, Knöbl P, Hörl WH, Druml W. The LDH ratio as a marker for response to plasma exchange in HUS/TTP of the adult. Clin Nephrol. 2002;57:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31:149-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 25. | Williamson DR, Albert M, Heels-Ansdell D, Arnold DM, Lauzier F, Zarychanski R, Crowther M, Warkentin TE, Dodek P, Cade J, Lesur O, Lim W, Fowler R, Lamontagne F, Langevin S, Freitag A, Muscedere J, Friedrich JO, Geerts W, Burry L, Alhashemi J, Cook D; PROTECT collaborators, the Canadian Critical Care Trials Group, and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144:1207-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 712] [Cited by in F6Publishing: 757] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 27. | Taylor FB, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1370] [Cited by in F6Publishing: 1302] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 28. | Park YA, Waldrum MR, Marques MB. Platelet count and prothrombin time help distinguish thrombotic thrombocytopenic purpura-hemolytic uremic syndrome from disseminated intravascular coagulation in adults. Am J Clin Pathol. 2010;133:460-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Hosler GA, Cusumano AM, Hutchins GM. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol Lab Med. 2003;127:834-839. [PubMed] [Cited in This Article: ] |
| 30. | Tsai HM, Chandler WL, Sarode R, Hoffman R, Jelacic S, Habeeb RL, Watkins SL, Wong CS, Williams GD, Tarr PI. von Willebrand factor and von Willebrand factor-cleaving metalloprotease activity in Escherichia coli O157:H7-associated hemolytic uremic syndrome. Pediatr Res. 2001;49:653-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Kojima M, Shimamura K, Mori N, Oka K, Nakazawa M. A histological study on microthrombi in autopsy cases of DIC. Bibl Haematol. 1983;95-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Lotta LA, Wu HM, Musallam KM, Peyvandi F. The emerging concept of residual ADAMTS13 activity in ADAMTS13-deficient thrombotic thrombocytopenic purpura. Blood Rev. 2013;27:71-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, Coppo R, Emma F, Johnson S, Karpman D, Landau D, Langman CB, Lapeyraque AL, Licht C, Nester C, Pecoraro C, Riedl M, van de Kar NC, Van de Walle J, Vivarelli M, Frémeaux-Bacchi V; HUS International. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 34. | Su C, Brandt LJ. Escherichia coli O157:H7 infection in humans. Ann Intern Med. 1995;123:698-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 211] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Qin X, Klein EJ, Galanakis E, Thomas AA, Stapp JR, Rich S, Buccat AM, Tarr PI. Real-Time PCR Assay for Detection and Differentiation of Shiga Toxin-Producing Escherichia coli from Clinical Samples. J Clin Microbiol. 2015;53:2148-2153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, Tripodo C, Bettoni S, Donadelli R, Valoti E, Tedesco F, Amore A, Coppo R, Ruggenenti P, Gotti E, Remuzzi G. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 37. | Besbas N, Gulhan B, Soylemezoglu O, Ozcakar ZB, Korkmaz E, Hayran M, Ozaltin F. Turkish pediatric atypical hemolytic uremic syndrome registry: initial analysis of 146 patients. BMC Nephrol. 2017;18:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Walterspiel JN, Ashkenazi S, Morrow AL, Cleary TG. Effect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin I. Infection. 1992;20:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin Infect Dis. 2012;55:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 40. | Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 680] [Article Influence: 35.8] [Reference Citation Analysis (0)] |