Published online Jun 26, 2019. doi: 10.12998/wjcc.v7.i12.1499
Peer-review started: February 18, 2019
First decision: April 18, 2019
Revised: April 29, 2019
Accepted: May 11, 2019
Article in press: May 11, 2019
Published online: June 26, 2019
Neuroblastoma (NB) is the most common extracranial solid tumor in children, with an incidence of approximately 1/10000. Surgical resection is an effective treatment for children with NB. Robot-assisted laparoscopic surgery is a new method and is superior to conventional laparoscopic surgery, since it has been preliminarily applied in clinical practice with a significant curative effect. This paper discusses significance and feasibility of complete resection of stage IV NB using robot-assisted laparoscopic surgery, while comparing its safety and effectiveness with conventional laparoscopic surgery.
In June 2018, a girl with stage IV retroperitoneal NB, aged 3 years and 5 mo, was admitted. Her weight was 15 kg, and her height was 100 cm. Robot-assisted, five-port laparoscopic resection of NB was performed. Starting from the middle point between the navel and the anterior superior iliac spine to the left lower abdomen, the pneumoperitoneum and observation hole (10 mm) were established using the Hasson technique. Operation arm #1 was located between the left anterior axillary line, the navel, and the costal margin (8 mm); operation arm #2 was located at the intersection of the right anterior axillary line and Pfannenstiel line (8 mm); one auxiliary hole was located between arm #2 (on the Pfannenstiel line) and the observation hole (12 mm); and another auxiliary hole (5 mm) was located slightly below the left side of the xiphoid. Along the right line of Toldt and the hepatic flexure of the transverse colon, the colon was turned to the left and below with a hook electrode. Through Kocher's incision, the duodenum and the pancreatic head were turned to the left to expose the inferior vena cava and the abdominal aorta. The vein was separated along the right external iliac, and the inferior vena cava was then lifted to expose the right renal vein from the bottom to the top. The tumor was transected horizontally below the renal vein, and it was first cut into pieces and then resected. The right renal artery and the left renal vein were also exposed, and the retrohepatic inferior vena cava was isolated. The tumor was resected along the surface of the psoas muscle, the back of the inferior vena cava, and the right side of the abdominal aorta. Finally, the lymph node metas-tases in front of the abdominal aorta and left renal vein were completely removed. The specimens were loaded into a disposable specimen retrieval bag and removed from the enlarged auxiliary hole. T-tube drainage was placed and brought out through a hole in the right lower quadrant of the abdomen. The operative time was 389 min, the time of pneumoperitoneum was 360 min, the intraoperative blood loss was approximately 200 mL, and the postoperative recovery was smooth. There were no complications, such as lymphatic fistula, diarrhea, bleeding, and paralytic ileus. Two months after discharge, there were no other complications. The literature on the application of robot-assisted laparoscopic surgery in the treatment of NB in children was reviewed
The robot has the advantages of a three-dimensional view and flexible operation, and it can operate finely along blood vessels. The successful experience of this case confirmed that robot-assisted laparoscopic surgery can skeletonize the abdominal blood vessels in the tumor and cut the tumor into pieces, indicating that robot-assisted laparoscopic surgery is feasible.
Core tip: Our paper describes the key surgical points in pediatric stage IV neuroblastoma that was completely resected using the Da Vinci robotic system. An adrenalectomy was carried out for a girl aged 3 years and 5 months by robot-assisted laparoscopic surgery at our hospital, and then systematic literature review was done to discuss the significance and feasibility of complete resection of stage IV neuroblastoma, the surgical safety and advantages, and the safety and effectiveness of robot-assisted surgery compared with traditional laparoscopic surgery.
- Citation: Chen DX, Hou YH, Jiang YN, Shao LW, Wang SJ, Wang XQ. Removal of pediatric stage IV neuroblastoma by robot-assisted laparoscopy: A case report and literature review. World J Clin Cases 2019; 7(12): 1499-1507
- URL: https://www.wjgnet.com/2307-8960/full/v7/i12/1499.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i12.1499
Neuroblastoma (NB) is a highly heterogeneous tumor. Some tumors can spontan-eously subside without treatment. However, most tumors are occult and rapidly metastasize throughout the body, ultimately becoming life-threatening. Surgical resection is an effective treatment for children with NB. Minimally invasive surgery has unique advantages in NB resection, and case reports of laparoscopic NB resection confirm its safety and feasibility[1]. Robot-assisted laparoscopic surgery is a new method and is superior to conventional laparoscopic surgery, which has been prelimi-narily applied in clinical practice, yielding significant curative effects[2]. This method can further promote the resection of pediatric tumors in a minimally invasive manner.
Robot-assisted surgery has been widely used in adult surgery, including general surgery[3-5], urinary surgery[6-8], and cardiac surgery[9-11]. Currently, robot-assisted systems, either alone or in combination with laparoscopic surgery, have been relati-vely mature in adult surgery, especially in the field of urology[12]. There are many retrospective clinical case reports comparing the safety and efficacy of robotic surgery in adult surgery through randomized controlled studies[13-15], which have confirmed its safety and feasibility.
Compared with adults, the age, physio-pathological conditions, and lesion location of children are unique and complex, rendering the advantages of robot-assisted surgery more prominent in the field of pediatric surgery. Pediatric surgeries involving the abdominal cavity, pelvic cavity, and thoracic cavity have been reported in China and abroad[16-18]. It is confirmed that with robot-assisted systems, pediatric surgery is safe and feasible.
In the field of pediatric NB, Yu et al[1] from the University of Oklahoma reported the first robot-assisted resection of pediatric NB in the Journal of Robotic Surgery in 2014. In that study, the prenatal ultrasound already suggested bilateral hydronephrosis in the patient, and a left adrenal gland mass with a size of 2.5 cm × 1.5 cm was found in the subsequent examinations. Therefore, when the patient was 15 mo old, robotic-assisted left adrenal gland mass resection and ipsilateral retroperitoneal lymph node dissection were performed. The transumbilical approach was used to perform the surgery. The robotic cannula was placed and the surgery was successfully performed using the Da Vinci robotic system. Pathological examination confirmed that the tumor was an NB (stage 2b). The patient did not have intraoperative or postoperative complications and was discharged 24 h after surgery. The patient resumed all activities within one week after the surgery, and no signs of recurrence were found during the regular postoperative follow-ups. This case shows that, compared with open surgery, robot-assisted surgery can not only successfully yield the same oncological results but also help reduce the disease recurrence rate and shorten the durations of surgery, hospitalization stay, and postoperative recovery.
Zhu et al[19] from Tongji Hospital of Huazhong University of Science and Tech-nology reported the application of a robotic surgical system in the surgical treatment of pediatric NB in 2017 in China. The outcomes of three cases of pediatric adrenal pheochromocytoma treated using the Da Vinci robot system were reported. Among the patients, one was male and the other two were female, with an average age of 5.2 years. The tumors were all on the right side, with a size of (2.0-3.6) cm × (1.0-3.6) cm. The Da Vinci robotic system was used to successfully complete the surgery, and the robotic surgery advantages of minimal trauma and quick recovery were confirmed. The study by Zhu et al[19] provided practical experience in applying robot-assisted surgery in the field of pediatric NB resection.
We successfully completed a stage IV pediatric NB resection using the Da Vinci Si robot system. The purpose of this study was to explore the feasibility and effective-ness of robotic surgery in the treatment of complicated NB in children and to summarize the preliminary experience in applying this technique.
The patient was a girl aged 3 years and 5 mo. At the end of 2017, the patient was admitted due to abdominal discomfort, and a mass in the right upper quadrant of the abdomen was found. After supraclavicular lymph node biopsy, the patient was diagnosed with stage IV retroperitoneal NB. After chemotherapy (four cycles), the tumor shrank significantly, but the primary tumor behind the inferior vena cava remained.
The abdomen was soft, without tenderness, rebound pain, or an obvious mass. The liver and spleen were impalpable, Murphy's sign was negative, and the bowel sounds were normal.
The laboratory values were the following: Serum ferratin, 1957.00 ng/mL; alanine transaminase, 31.2 U/L; aspartate transaminase, 38.9 U/L; alkaline phosphatase, 165.2 U/L; urea, 3.06 mmol/L; SCr, 33.4 µmol/L; hemoglobin, 87 g/L; red blood cell count, 2.78 × 1012/L; and white blood cell count, 9.24 × 109/L.
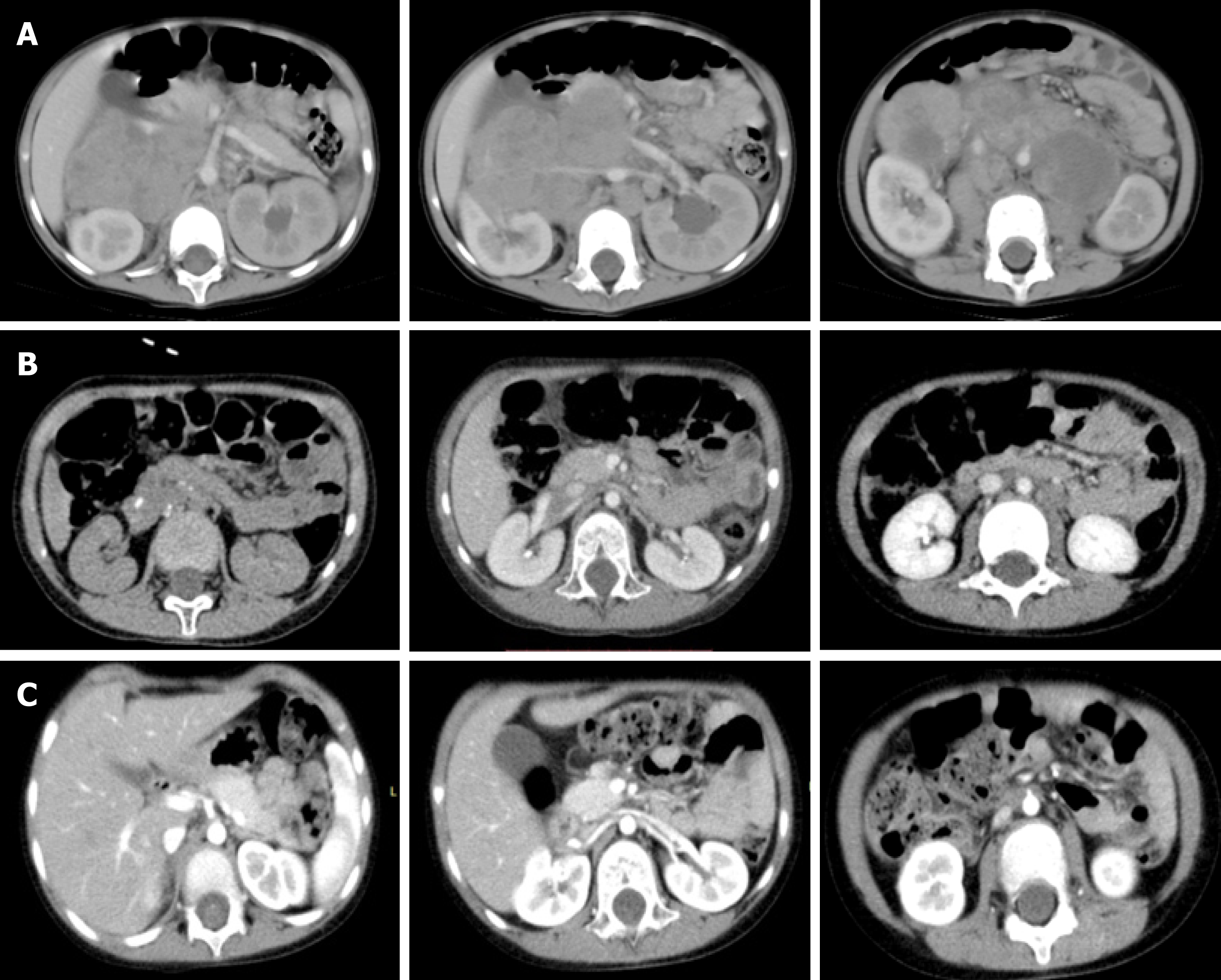
Before chemotherapy, abdominal enhanced computed tomography showed a middle line retroperitoneal neoplasm with the inferior vena cava, right renal vessels, and abdominal aorta traversing in it. After chemotherapy, the tumor shrank considerably and the above-mentioned vessels were still encased by the tumor. After surgery, the clearance of the tumor around the vessels was achieved (Figure 1).
The preoperative biopsy pathological diagnosis from the left cervical lymph nodes, combined with the pathological morphology and immune phenotype, was consistent with an undifferentiated NB metastasis. The immunohistochemical results were CD-56 (+), CD-ROM9 (+, spotty), nuclear CgA (+), CKpan (-), HMB-45 (-), Ki-67 (+, >95%), LCA (-), NSE (+, focal and weak), S-100 (-), and Syn (+).
The preoperative diagnosis was NB.
Tracheal intubation was performed under general anesthesia, followed by urinary catheterization. The catheterization of the right internal jugular vein and blood pressure monitoring of the right radial artery were performed routinely. The left lateral position at an angle of 45º was used. Stretch socks were worn on the lower extremities to prevent thrombosis. The robot was put on the patient’s back, the first assistant was located on the ventral side of the patient, and the instrument nurse was close to the patient’s feet.
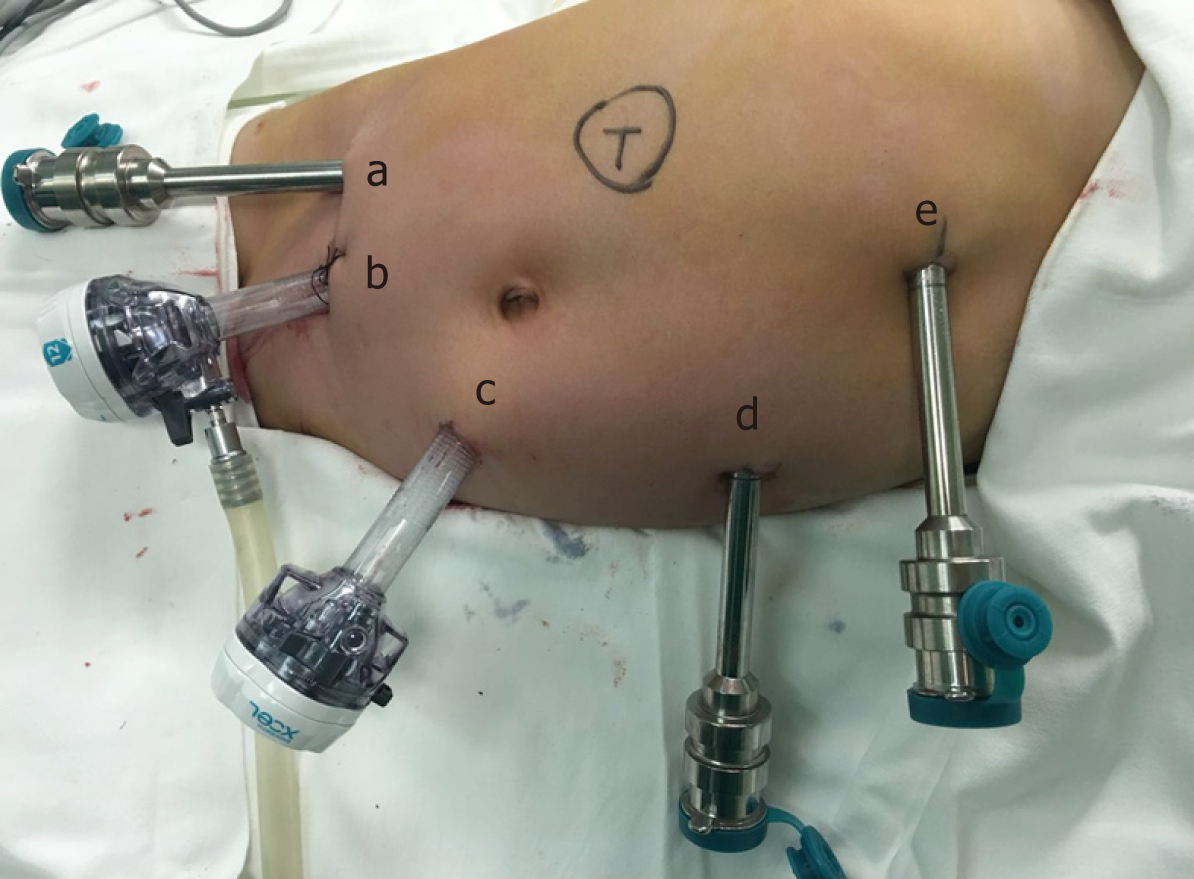
The five-port technique was used (Figure 2). Starting from the middle point between the navel and the anterior superior iliac spine to the left lower abdomen, the pneumo-peritoneum and observation hole (10 mm) were established using the Hasson technique. Operation arm #1 was located between the left anterior axillary line, the navel, and the costal margin (8 mm); operation arm #2 was located at the intersection of the right anterior axillary line and Pfannenstiel line (8 mm); one auxiliary hole was located between arm #2 (on the Pfannenstiel line) and the observation hole (12 mm); and another auxiliary hole (5 mm) (initially taken as arm 3, changing into auxiliary hole because of the narrow space leading to collision of the robotic arms) was located slightly below the left side of the xiphoid.
Along the right line of Toldt and the hepatic flexure of the transverse colon, the colon was turned to the left and below with a hook electrode. Through Kocher's incision, the duodenum and the pancreatic head were turned to the left to expose the inferior vena cava and the abdominal aorta. The vein was separated along the right external iliac, and the inferior vena cava was then lifted to expose the right renal vein from the bottom to the top. The tumor was transected horizontally below the renal vein and was first cut into pieces and then resected. The right renal artery and the left renal vein were also exposed, and the retrohepatic inferior vena cava was isolated. The tumor was resected along the surface of the psoas muscle, the back of the inferior vena cava, and the right side of the abdominal aorta. Finally, the lymph node metastases in front of the abdominal aorta and left renal vein were completely removed. The specimens were loaded into a disposable specimen retrieval bag and removed from the enlarged auxiliary hole. T-tube drainage was placed and brought out through a hole in the right lower quadrant of the abdomen. Each puncture hole was sutured using a 3-0 absorbable suture.
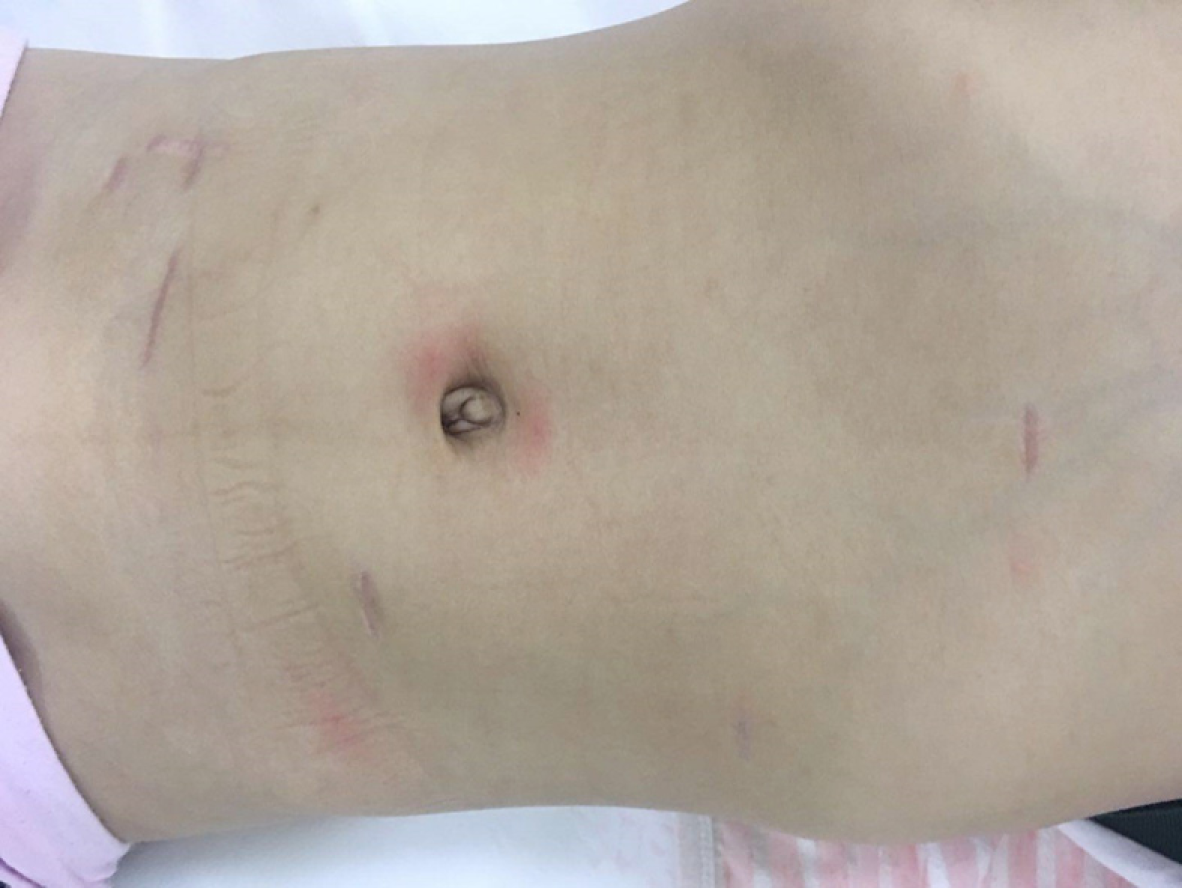
Conventional administrations of anti-inflammatory medications, fluid replacement, gastrointestinal decompression, acid suppression, and enzymatic inhibition were performed. The abdominal drainage volume and amylase level were monitored. The gastric tube was extracted on the third day after surgery, and the patient was placed on a liquid diet. After eating a normal diet, the abdominal drainage tube was removed when no lymphatic leakage or exudate was observed, and the patient was discharged (Figure 3).
The operative time was 389 min, the time of pneumoperitoneum was 360 min, and the intraoperative blood loss was approximately 200 mL.
Immediately after surgery, the blood amylase level was 1426.3 U/L (normal range, 0-150), the blood lipase level was 54.6 U/L (normal range, 23-300), the blood alanine aminotransferase level was 197.6 U/L (normal range, 0-40), and the blood aspartate aminotransferase level was 302.7 U/L (normal range, 0-40). The gastric tube was extracted on the third day after surgery, and no abdominal discomfort was found after drinking water. A liquid diet was administered to the patient on the fourth day. The blood amylase level was 64.3 U/L (normal range, 0-150), the blood lipase level was 240.6 U/L (normal range, 23-300), the blood alanine aminotransferase level was 73.7 U/L (normal range, 0-40), and the blood aspartate aminotransferase level was 49.1 U/L (normal range, 0-40) on the fourth day. The patient was given pediatric food on the seventh day. The fluid in the retroperitoneal drainage tube gradually decrea-sed; therefore, on the tenth day, the retroperitoneal drainage tube was removed, and the patient was discharged. The patient continued to receive chemotherapy after discharge. A follow-up visit was performed 2 months later. Contrast-enhanced com-puted tomography showed that no tumor recurrence was observed, and no effusion was found in the surgical area.
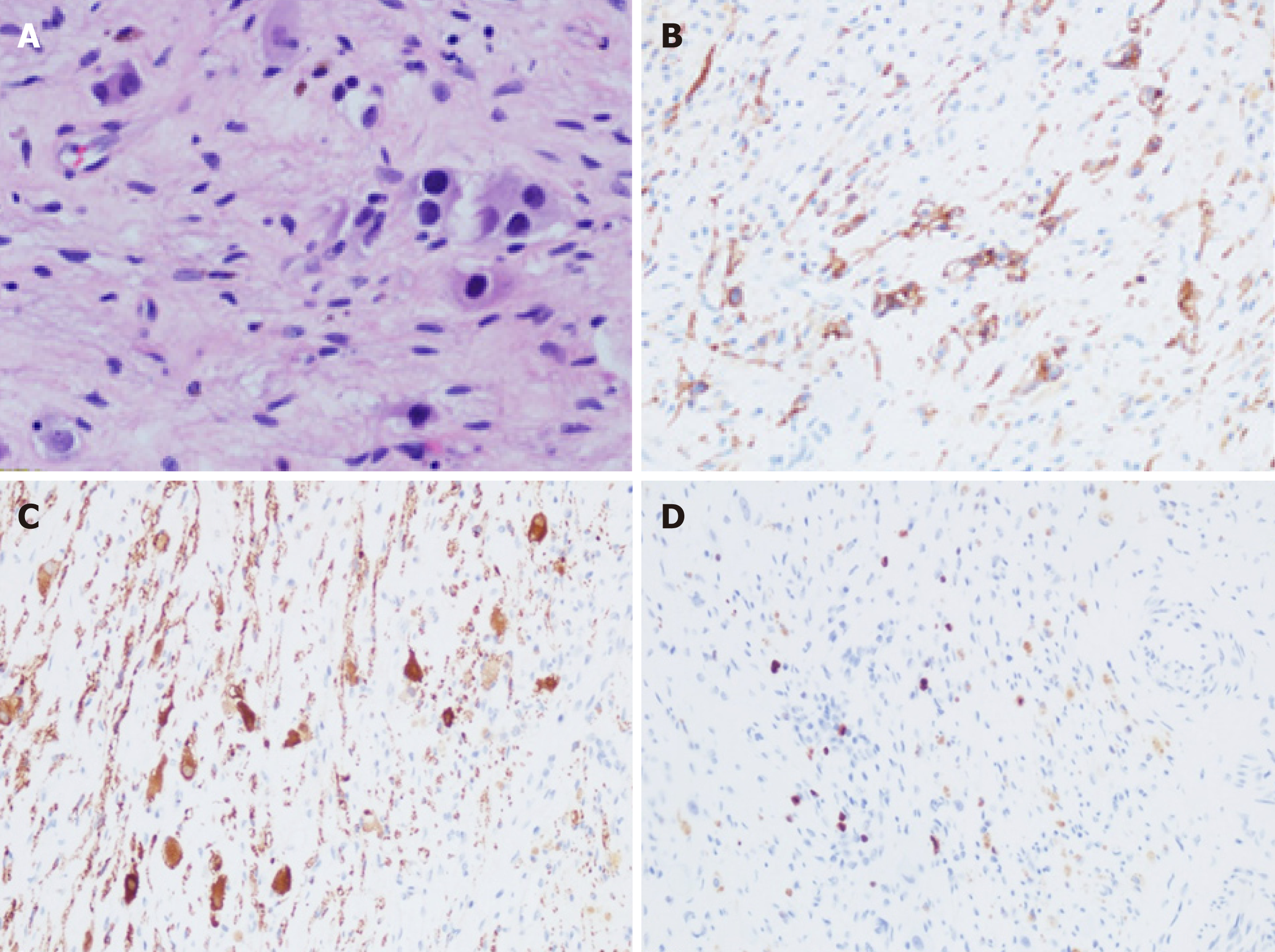
On June 29, 2018, the pathological (conventional) examination results for the right adrenal gland and retroperitoneum were obtained. Degenerated small-round cells, ganglion cells with foam cell aggregation, and hemosiderosis were scattered in the adrenal tissue and hyperplastic fibrous tissue. Locally degenerated and necrotic nodules with a large amount of foam cell aggregation and scattered calcification were found, which is consistent with postoperative changes in NB. Immunohistochemistry results were: CD56 (+), CD99 (-), NSE (+), Ki-67 (+, 2%), Syn (+), CgA (-), NF (+), S-100 (+) (Tumor metastasis in the left abdominal aorta). Necrosis and calcification of small lesions in fibrous connective tissues were present, but no tumor cells were found (Figure 4).
NB is the most common extracranial solid tumor in children, with an incidence of approximately 1/10000. It is also one of the most challenging operations in pediatric surgery. Traditional open surgery has disadvantages, such as the creation of a large incision, difficulty achieving deep exposure, severe trauma, and a long recovery period. Since the first report of laparoscopic adrenalectomy[20], laparoscopic adrena-lectomy has been frequently reported[21-23]. The application of laparoscopy in NB resection is very extensively reported in the literature; however, if the NB is closely associated with important blood vessels, the use of a laparoscope in NB resection is still challenging and has limitations. Robotic surgery is a minimally invasive surgery with greater convenience and effectiveness[24]. Robot-assisted laparoscopic resection of retroperitoneal NB is rarely reported[1].
The resection of the primary foci of stage IV NB is still a controversial issue, with some studies suggesting that it is only superior to a simple biopsy, and the prognosis is more dependent on biological characteristics rather than the number of resected primary foci[25,26] . However, most researchers still suggest the resection of over 95% of the tumor foci[27,28]. The primary foci of stage IV NB are often large and envelope important blood vessels, and different five-year survival rates after complete primary tumor resection (26%, 30%, 52%, and 65%) have been reported[29-31]. To achieve a good prognosis, the resection of 95% of the tumor tissue is the goal of tumor surgeons. However, Kiely and Sultan reported that this goal can only be achieved in 89% and 58% of patients, respectively.
La Quaglia et al[32] reported the effect of aggressive surgical resection of the tumor on the prognosis. For children with a diagnosis of stage IV NB, complete removal of the tumor is unachievable. Delayed surgery or a second surgery can improve local tumor shrinkage and the metastasis disappearance rate after preoperative chemotherapy and prolong survival. However, the study by Castel et al[26] denied the therapeutic effect of surgical resection of stage IV NB and suggested that age, N-myc gene amplification, and distant metastasis have a much greater influence on the prognosis of stage IV NB than complete resection. Therefore, the treatment of stage IV NB should be a combination of surgery, radiotherapy, chemotherapy, and other treatments. Preoperative chemotherapy is necessary to remove the NB that envelopes important peritoneal blood vessels. Through chemotherapy, tumors can shrink significantly and harden, and the blood supply and other risk factors can decrease, making the resection of unresectable tumors possible[33,34].
Separation is one of the techniques used to protect important blood vessels. We started from the relatively normal right common iliac vein, separated the inferior vena cava from bottom to top, ligated the right gonadal vein, and separated the right renal vein and left renal vein. Horizontal tumor transection was performed below the right renal vein to facilitate the exposure of the right renal artery and the right superior mesenteric artery. The inferior vena cava was lifted again, and the tumor above the right renal vein and on the right side of the abdominal aorta was resected. During the resection, the right adrenal gland covered the top of the tumor in the form of a sheet, and there was no significant thickened right middle adrenal vein. It is considered that the tumor originated from the retroperitoneal sympathetic nerve chain instead of the right adrenal gland. Finally, metastatic tumors in front of the left kidney were excised. During the separation process, we found that the stability of the hook electrode was better than that of electric scissors, and the hemostatic effect of the bipolar electrosurgical knife was good.
Piecemeal resection is a necessary technique[35]. Because the tumor envelopes the blood vessels, the vessels cannot be preserved without opening the tumor; thus, the tumor cannot be removed wholly. During NB resection in the advanced stages, en bloc resection cannot be achieved; therefore, piecemeal resection is necessary to improve the prognosis.
Maximization of the operating space should be considered. Children are typically short, and the abdominal space is small. The appropriate Trocar positions are below and to the left of the umbilicus. After placing the Trocar and the lens under the umbilicus, two auxiliary holes (left lower abdomen and left lateral xiphoid) should be arranged, since the operation is mainly performed in the right upper abdomen and behind the inferior vena cava. Arms #1 and #2 were arranged on the left side of the abdomen and the right lower abdomen, respectively, which can operate on the lesions on the right side and upper abdomen. During the operation, a cold lens was placed in the left lower abdomen, expanding the operating space. The operation was completed using the five-port approach, and no instruments collided with each other.
Robot assistance is the key to the skeletonization of blood vessels. Conventional laparoscopic surgery is difficult and cannot achieve the resection of complex tumors. During the operation, the inferior vena cava, the bilateral renal veins, and the right renal artery are skeletonized; therefore, surgery is difficult, and the risk is high. Robots can provide a three-dimensional view with 10 × magnification. Shaking can be eliminated, and hand actions can be simulated. The robots can complete clamping, suturing, knotting, and other operations and achieve results similar to those of open surgery. Robotic surgery can preserve the aesthetic characteristics of the laparoscopic surgical incision. We placed arm #2 and auxiliary hole #1 on the Pfannenstiel line, and the tumor specimens were obtained from this line, which improves the postope-rative appearance. No common complications, such as postoperative diarrhea[36], lymphatic fistula, or intestinal obstruction, were found. However, due to the long operative time, redness and swelling of the left waist skin occurred but completely disappeared after one week. Because of the isolation of the pancreas and liver, the amylase and transaminase levels also showed a transient increase but soon returned to normal. Robotic-assisted surgery not only maintains the advantages of minimally invasive surgery, such as small wounds, good aesthetics, and short operative time, hospitalization stay, and postoperative recovery time, but also addresses the limita-tions of open surgery and laparoscopic technology.
This case is the first pediatric robot-assisted laparoscopic resection of stage IV NB (according to the International Neuroblastoma Staging System criteria) at our hospital, and no serious complications during or after surgery were found. The surgical experience of this case showed that robot-assisted resection of retroperitoneal NB is feasible and can be a new approach for the treatment of advanced NB.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hayes MJ, Higgins PD S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Wang J
| 1. | Uwaydah NI, Jones A, Elkaissi M, Yu Z, Palmer BW. Pediatric robot-assisted laparoscopic radical adrenalectomy and lymph-node dissection for neuroblastoma in a 15-month-old. J Robot Surg. 2014;8:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Guan R, Chen Y, Yang K, Ma D, Gong X, Shen B, Peng C. Clinical efficacy of robot-assisted versus laparoscopic liver resection: a meta analysis. Asian J Surg. 2019;42:19-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | van der Sluis PC, van Hillegersberg R. Robot assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer. Best Pract Res Clin Gastroenterol. 2018;36-37:81-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Niu X, Yu B, Yao L, Tian J, Guo T, Ma S, Cai H. Comparison of surgical outcomes of robot-assisted laparoscopic distal pancreatectomy versus laparoscopic and open resections: A systematic review and meta-analysis. Asian J Surg. 2019;42:32-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Zhao W, Liu C, Li S, Geng D, Feng Y, Sun M. Safety and efficacy for robot-assisted versus open pancreaticoduodenectomy and distal pancreatectomy: A systematic review and meta-analysis. Surg Oncol. 2018;27:468-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Perez-Ardavin J, Sanchez-Gonzalez JV, Martinez-Sarmiento M, Monserrat-Monfort JJ, García-Olaverri J, Boronat-Tormo F, Vera-Donoso CD. Surgical Treatment of Completely Endophytic Renal Tumor: a Systematic Review. Curr Urol Rep. 2019;20:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Skolarikos A. Re: Robot-assisted Laparoscopic Prostatectomy Versus Open Radical Retropubic Prostatectomy: 24-month Outcomes from a Randomised Controlled Study. Eur Urol. 2019;75:200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ranasinghe W, de Silva D, Bandaragoda T, Adikari A, Alahakoon D, Persad R, Lawrentschuk N, Bolton D. Robotic-assisted vs. open radical prostatectomy: A machine learning framework for intelligent analysis of patient-reported outcomes from online cancer support groups. Urol Oncol. 2018;36:529.e1-529.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Canale LS, Mick S, Mihaljevic T, Nair R, Bonatti J. Robotically assisted totally endoscopic coronary artery bypass surgery. J Thorac Dis. 2013;5 Suppl 6:S641-S649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 10. | Lehr EJ. Blazing the trail for robot-assisted cardiac surgery. J Thorac Cardiovasc Surg. 2016;152:14-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Buehler AM, Ferri C, Flato UA, Fernandes JG. Robotically assisted coronary artery bypass grafting: a systematic review and meta-analysis. Int J Med Robot. 2015;11:150-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Navaratnam A, Abdul-Muhsin H, Humphreys M. Updates in Urologic Robot Assisted Surgery. F1000Res. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, Kibel AS, Hu JC. Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol. 2012;61:1239-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Pelletier JS, Gill RS, Shi X, Birch DW, Karmali S. Robotic-assisted hepatic resection: a systematic review. Int J Med Robot. 2013;9:262-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Robertson C, Close A, Fraser C, Gurung T, Jia X, Sharma P, Vale L, Ramsay C, Pickard R. Relative effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of localised prostate cancer: a systematic review and mixed treatment comparison meta-analysis. BJU Int. 2013;112:798-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Mizuno K, Kojima Y, Nishio H, Hoshi S, Sato Y, Hayashi Y. Robotic surgery in pediatric urology: Current status. Asian J Endosc Surg. 2018;11:308-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Binet A, Fourcade L, Amar S, Alzahrani K, Cook AR, Braïk K, Cros J, Longis B, Villemagne T, Lardy H, Ballouhey Q. Robot-Assisted Laparoscopic Fundoplications in Pediatric Surgery: Experience Review. Eur J Pediatr Surg. 2019;29:173-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Tomaszewski JJ, Casella DP, Turner RM 2nd, Casale P, Ost MC. Pediatric laparoscopic and robot-assisted laparoscopic surgery: technical considerations. J Endourol. 2012;26:602-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Zhu TQ, Liu SB, Zhang W, Yuan JY. Preliminary experience of robotic-assisted laparoscopic adrenalectomy in children. Zhonghua Xiaoer Waike Zazhi. 2017;38:775-777. [DOI] [Cited in This Article: ] |
| 20. | Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327:1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 905] [Cited by in F6Publishing: 840] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Carvalho JA, Nunes PT, Antunes H, Parada B, Retroz E, Tavares-da-Silva E, Paiva I, Figueiredo AJ. Transumbilical laparoendoscopic single-site adrenalectomy: A feasible and safe alternative to standard laparoscopy. Arch Ital Urol Androl. 2019;91:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mohammed A, Amine H, Atiq SE, Mohammed B, Ouadii M, Khalid M, Khalid AT, Abdelmalek O. Applicability and outcome of laparoscopic adrenalectomy for large tumours. Pan Afr Med J. 2018;31:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Wind P, Genser L, Zarzavadjian Le Bian A. The Modified Semi-lateral Transmesocolic Approach for Laparoscopic Left Adrenalectomy. World J Surg. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Zhou X, Zhang H, Feng M, Zhao J, Fu Y. New remote centre of motion mechanism for robot-assisted minimally invasive surgery. Biomed Eng Online. 2018;17:170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Luo YB, Cui XC, Yang L, Zhang D, Wang JX. Advances in the Surgical Treatment of Neuroblastoma. Chin Med J (Engl). 2018;131:2332-2337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Castel V, Tovar JA, Costa E, Cuadros J, Ruiz A, Rollan V, Ruiz-Jimenez JI, Perez-Hernández R, Cañete A. The role of surgery in stage IV neuroblastoma. J Pediatr Surg. 2002;37:1574-1578. [PubMed] [Cited in This Article: ] |
| 27. | Yeung F, Chung PH, Tam PK, Wong KK. Is complete resection of high-risk stage IV neuroblastoma associated with better survival? J Pediatr Surg. 2015;50:2107-2111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Zwaveling S, Tytgat GA, van der Zee DC, Wijnen MH, Heij HA. Is complete surgical resection of stage 4 neuroblastoma a prerequisite for optimal survival or may >95 % tumour resection suffice? Pediatr Surg Int. 2012;28:953-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Adkins ES, Sawin R, Gerbing RB, London WB, Matthay KK, Haase GM. Efficacy of complete resection for high-risk neuroblastoma: a Children's Cancer Group study. J Pediatr Surg. 2004;39:931-936. [PubMed] [Cited in This Article: ] |
| 30. | Sultan I, Ghandour K, Al-Jumaily U, Hashem S, Rodriguez-Galindo C. Local control of the primary tumour in metastatic neuroblastoma. Eur J Cancer. 2009;45:1728-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Koh CC, Sheu JC, Liang DC, Chen SH, Liu HC. Complete surgical resection plus chemotherapy prolongs survival in children with stage 4 neuroblastoma. Pediatr Surg Int. 2005;21:69-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | La Quaglia MP, Kushner BH, Su W, Heller G, Kramer K, Abramson S, Rosen N, Wolden S, Cheung NK. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004;39:412-7; discussion 412-7. [PubMed] [Cited in This Article: ] |
| 33. | Irtan S, Brisse HJ, Minard-Colin V, Schleiermacher G, Galmiche-Rolland L, Le Cossec C, Elie C, Canale S, Michon J, Valteau-Couanet D, Sarnacki S. Image-defined risk factor assessment of neurogenic tumors after neoadjuvant chemotherapy is useful for predicting intra-operative risk factors and the completeness of resection. Pediatr Blood Cancer. 2015;62:1543-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Avanzini S, Pio L, Erminio G, Granata C, Holmes K, Gambart M, Buffa P, Castel V, Valteau Couanet D, Garaventa A, Pistorio A, Cecchetto G, Martucciello G, Mattioli G, Sarnacki S. Image-defined risk factors in unresectable neuroblastoma: SIOPEN study on incidence, chemotherapy-induced variation, and impact on surgical outcomes. Pediatr Blood Cancer. 2017;64:e26605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Kiely E. A technique for excision of abdominal and pelvic neuroblastomas. Ann R Coll Surg Engl. 2007;89:342-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Han W, Wang HM. Refractory diarrhea: A paraneoplastic syndrome of neuroblastoma. World J Gastroenterol. 2015;21:7929-7932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |