Published online Jun 26, 2019. doi: 10.12998/wjcc.v7.i12.1492
Peer-review started: February 11, 2019
First decision: March 9, 2019
Revised: April 24, 2019
Accepted: May 2, 2019
Article in press: May 2, 2019
Published online: June 26, 2019
Cardiovascular side effects occur frequently during anti-cancer treatment, and there is a growing concern that they may lead to premature morbidity and death.
A 32-year-old woman was diagnosed with breast cancer. After comprehensive treatment with neoadjuvant chemotherapy, surgery, postoperative adjuvant chemotherapy, postoperative adjuvant radiotherapy, and endocrine therapy, her breast cancer was cured. However, heart failure associated with anti-cancer treatment presented, most probably related to chemotherapy containing anthracycline. After active treatment, her cardiac function returned to normal. Unfortunately, follow-up visits revealed a second primary malignancy, lymphoma. After multiple courses of chemotherapy combined with targeted therapy, her lymphoma acquired complete remission and no cardiotoxicity was observed again. Heart failure related to breast treatment may be reversible.
Using alternatives to anthracycline in patients with lymphoma who are at risk of cardiac failure may preserve cardiac function.
Core tip: Anti-cancer treatment is frequently associated with the development of cardiovascular side effects. A 32-year-old woman diagnosed and treated for breast cancer was cured after comprehensive treatment. Unfortunately, the patient later presented with heart failure associated with anti-cancer treatment involving the use of anthracycline. Her cardiac function returned to normal after active treatment but a second primary malignancy, lymphoma, was detected in subsequent visits. Following multiple courses of chemotherapy combined with targeted therapy, there was complete remission of the acquired lymphoma with no re-occurrence of cardiotoxicity. Thus, heart failure related to breast cancer treatment may be reversible. Furthermore, anthracycline should be avoided in patients at risk of cardiac failure having lymphoma to preserve cardiac function.
- Citation: Han S, An T, Liu WP, Song YQ, Zhu J. Secondary lymphoma develops in the setting of heart failure when treating breast cancer: A case report. World J Clin Cases 2019; 7(12): 1492-1498
- URL: https://www.wjgnet.com/2307-8960/full/v7/i12/1492.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i12.1492
Advances in anti-cancer treatment have greatly improved the survival rate of breast cancer patients, but morbidity and mortality due to side effects remain a concern[1]. Cardiovascular diseases (CVDs) are the most frequent side effects that may lead to premature morbidity and death among cancer survivors[2]. Among anticancer agents, anthracyclines are probably the most well-known class of cardiotoxic drugs capable of causing myocardial dysfunction and heart failure[3].
Besides unpleasant effects from chemotherapy, another challenge affecting the growing population of cancer survivors is the development of a second primary malignancy[4]. The development of a second primary malignancy is under multifac-torial influence not least the late effects of chemotherapy and radiotherapy[5].
A 34-year-old Chinese woman presented with chest tightness and shortness of breath.
A right breast tumor was found during a routine physical examination 2 years prior to presentation. The diagnosis made was stage IIIA (T3N1M0) breast invasive ductal carcinoma, with ipsilateral axillary lymph node metastasis, ER (+), PR (+), and Her-2 (-). Thereafter, the patient first underwent four cycles of neoadjuvant chemotherapy using the following regimen: cyclophosphamide 600 mg/m2 1.0 g D1, epirubicin 100 mg/m2 160 mg D1, and fluorouracil 600 mg/m2 1.0 g D1, q21d. After this course of therapy, the patient was assessed as having partial remission (PR). Subsequently, a modified radical mastectomy was performed. Based on her postoperative pathological stage ypT1cN3, the patient further received another four cycles of adjuvant chemo-therapy comprising paclitaxel 90 mg/m2 150 mg D1, 120 mg D8 and D15, q21d. Postoperative adjuvant radiotherapy (50 Gy/30f) was also introduced. The irradiation field included the right chest wall and supraclavicular region. Since the patient tested positive for hormone receptor, the patient accepted being given adjuvant endocrine therapy consisting of goserelin acetate 3.6 mg once a month and anastrozole 1 mg qd. At the one-year follow-up visit, no recurrence or metastasis of breast cancer was ob-served.
The patient had good health.
The patient had no relevant personal or family history.
She was unable to lie down. Blood pressure was 119/86 mmHg. Heart rate was 78 beats per minute. Dry and wet rales could be heard in both lungs.
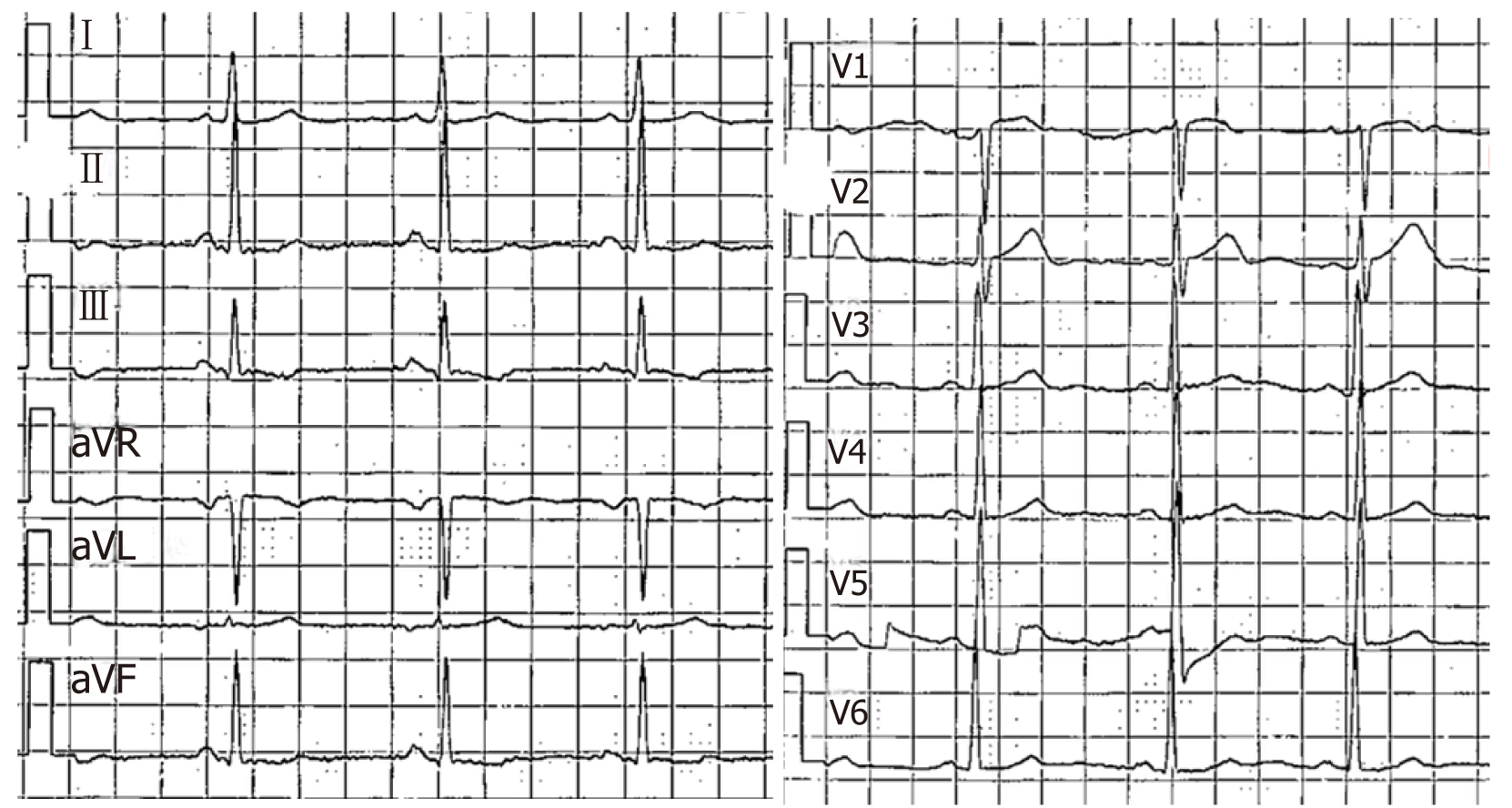
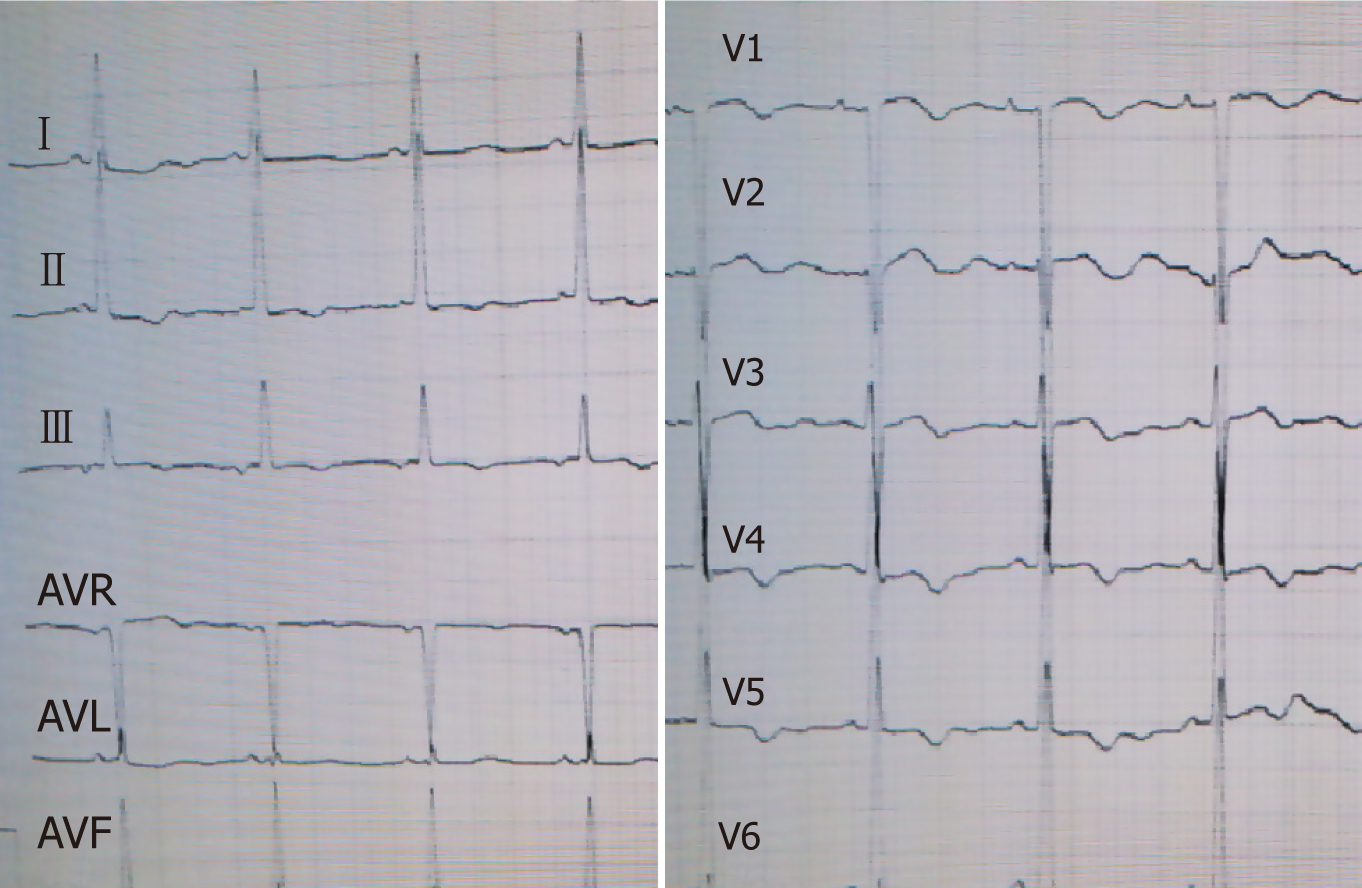
Laboratory results indicated negative myocardial enzymes and a brain natriuretic peptide level exceeding 2000 pg/mL. Her electrocardiogram showed T-wave inver-sion in chest leads V1-V6 (Figures 1 and 2).
Echocardiography reported a left ventricular ejection fraction (LVEF) of 30%-38%. Cardiac contrast-enhanced magnetic resonance imaging showed that the left ventricle was markedly dilated (left ventricular end diastolic diameter was 60 mm), the left ventricular systolic function was diffused, and the left ventricular myocardial wall thickening rate was reduced. Moreover, the LVEF was 33%.
The patient was diagnosed as having acute left heart failure, which was considered as a morbidity of previous anti-cancer therapy.
After treatment with diuretics, nitrates, angiotensin converting enzyme inhibitor, and digoxin, the patient’s symptoms and signs gradually improved. Finally, the patient reported complete resolution of symptoms. She adhered to a treatment protocol consisting of digoxin, trimetazidine, and spironolactone for 3 years. Echocardio-graphy was reviewed at 12 mo after the diagnosis of heart failure, which indicated that the anteroposterior diameter of the left atrium was 30 mm, left ventricular end diastolic diameter was 45 mm, and LVEF had returned to normal (76%).
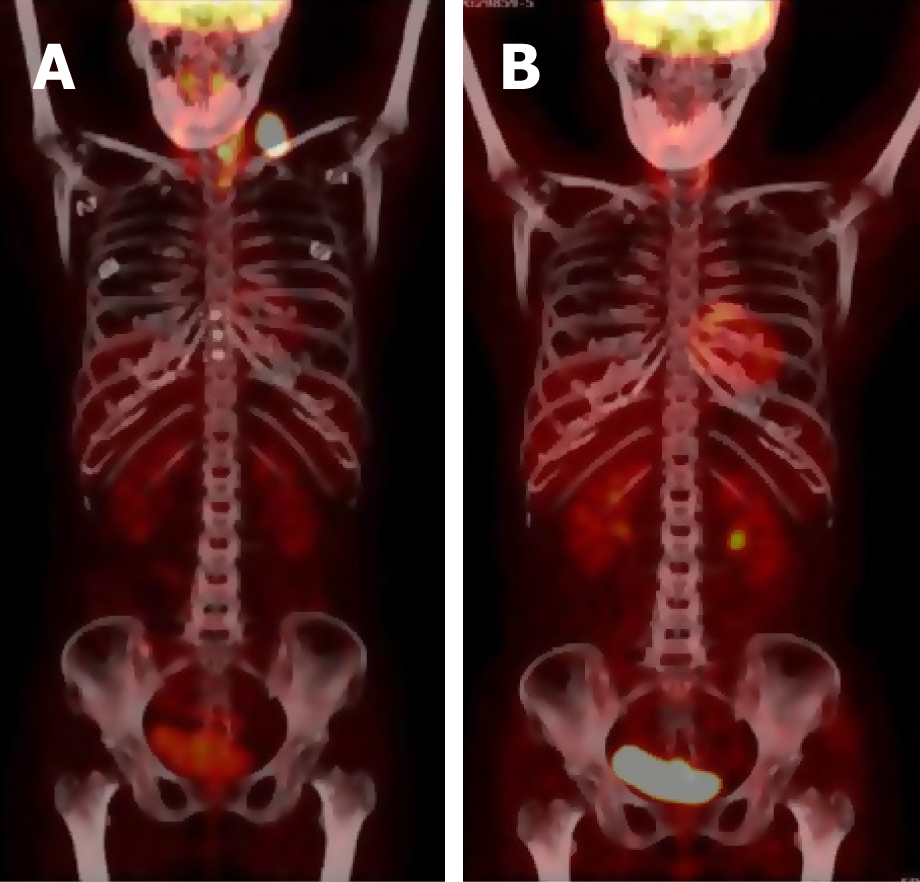
One year later, a left supraclavicular lymph node enlargement was found in the patient (Figure 3A). Lymph node biopsy indicated diffuse large B cell lymphoma (DLBCL) leading to a diagnosis of stage IIA involving the neck, left clavicle area, posterior left lobe of thyroid, and superior mediastinal lymph nodes. After fully assessing her cardiac function and the cardiac toxicity risk of chemotherapy, the patient was placed on a treatment regimen of rituximab combined with chemo-regimen. Considering her heart condition, the CHOP regimen was adjusted to CEOP (cyclophosphamide, vincristine, prednisone, and etoposide instead of doxorubicin). Thus, anthracycline was avoided. The patient received eight cycles of chemotherapy plus targeted therapy.
After four cycles of R-CEOP, complete remission (CR) was achieved and maintained continuously (Figure 3B). The major adverse effect was myelosuppression (leukocyte reduction). There was no obvious cardiotoxicity.
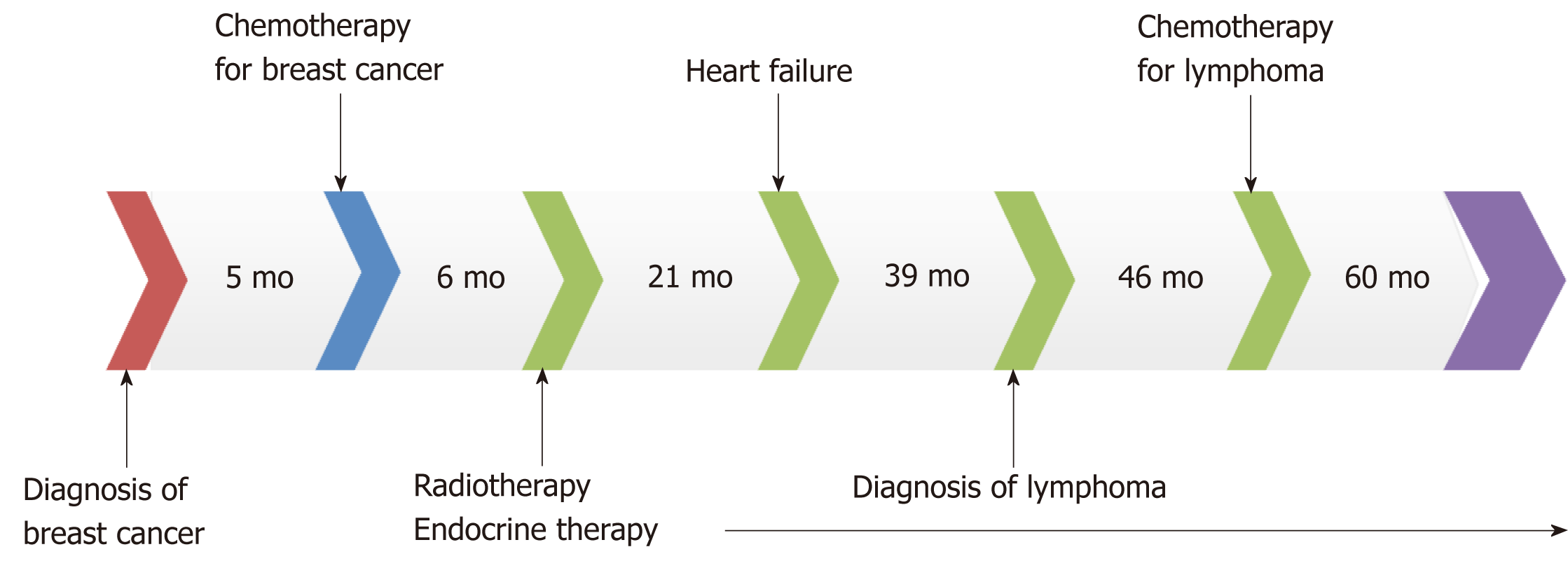
Follow-up visits continued for another 3 years. No recurrence of breast cancer or lymphoma was observed and cardiac function remained normal. The patient discontinued use of cardiac drugs but maintained endocrine therapy for breast cancer. The whole course of the patient’s treatment is shown in Figure 4.
The application of anthracyclines is a landmark in the development of medical oncology. Even today, this class of drugs are still widely used in the treatment of many solid tumors and hematological malignancies. However, the main dose limitation of these drugs is cardiac toxicities, which restrict their clinical use. The cardiotoxicity of anthracyclines may be acute, delayed, or chronic. Acute toxicities, predominantly appearing as supraventricular arrhythmia, transient LV dysfunction, and ECG changes, develop immediately after infusion and are usually reversible. The changes in the electrophysiologic properties of the heart may lead to QT prolongation. Though rare, this condition is dangerous because QT prolongation can lead to life-threatening arrhythmias such as torsades de pointes[6]. Direct activation of the delayed rectifier potassium current and inhibition of the expression of potassium current channels may be potential mechanisms for the observed cardiac toxicity[7]. Besides arrhythmia, anthracyclines are well known for their potential to cause cardiac insufficiency and heart failure. A higher cumulative dose of anthracycline poses a higher risk of cardiac toxicity[8]. For example, when the cumulative dose of doxorubi-cin reaches 400 mg/m2, the incidence of congestive heart failure is 5%, which increases to 48% when the cumulative amount reaches 700 mg/m2[3]. Therefore, in clinical practice, the cumulative dose of doxorubicin is generally kept below 450 mg/m2. Nonetheless, there is no absolute safe dose. As we have reported in this case, the patient received an epirubicin total dose of only 400 mg/m2. After comprehensive anti-cancer treatment, including chemotherapy, surgery, radiotherapy, and endocrine therapy, the patient presented with serious heart failure. This condition was directly associated with anti-cancer therapy and anthracycline was undoubtedly the most important contributor. The risk factors of anthracycline-induced cardiotoxicity include cumulative doses, gender (female), age (older than 65 years or less than 18 years), renal insufficiency, mediastinal radiotherapy history, and other associated drugs with cardiac toxicity (including cyclophosphamide, trastuzumab, and paclitaxel), and potential CVDs[9-11]. As mentioned earlier, the main cardiac toxicities of anthracycline are myocardial dysfunction and heart failure. The left ventricular dysfunction caused by anthracycline is partly due to the production of oxygen free radicals and the increased oxidative stress. As a result, myocardial damage occurs, partly due to the inhibition of topoisomerase II beta by the oxidation-reduction cycle of the quinone group, which indirectly leads to mitochondrial abnormalities. Anthracyclines also inhibit iron metabolism and lead to iron accumulation in cardiac myocytes; therefore, iron balance may also play a role in myocardial injury[12]. A consequence of exposure to anthracyclines is cardiomyocyte apoptosis or necrosis. This patient’s cardiotoxicity occurred more than one year after the treatment for breast cancer and, hence, is a delayed cardiotoxicity. It is generally believed that anthracycline-induced cardiac toxicity is irreversible[13]. However, some studies have shown that it is reversible in the first 3-6 mo after the decrease in ejection fraction[14,15]. In this case, the patient’s heart function gradually returned to normal after active treatment.
The complexity of this case is that the patient developed a second primary malig-nancy, lymphoma. Developing a secondary malignancy is not rare but, a comorbidity of heart failure presents a great challenge in the treatment of lymphoma. For DLBCL, the classic regimen is CHOP, which contains cardiotoxic drugs. So, it is important to fully assess the cardiac function and the cardiac toxicity risk before initiating chemotherapy. In this case, anthracycline was replaced by etoposide, which may have lower efficacy but no significant cardiac toxicity. Fortunately, this patient acquired persistent CR without experiencing further cardiac toxicity. There is no standard treatment for DLBCL in elderly patients or patients with underlying diseases, such as cardiac dysfunction, which restricts the use of anthracycline. If there are contraindica-tions in the use of anthracycline, other drugs such as gemcitabine, etoposide, and bendamustine can be explored as alternatives. Although most published large-scale clinical data show that chemotherapy without anthracycline had a negative effect on the long-term survival of aggressive non-Hodgkin lymphoma, if anthracycline use poses potentially life-threatening cardiac toxicity, it may well be in the patient’s best interest to discontinue its use[16].
The adjuvant treatment for early breast cancer is associated with improved survival but at the expense of increased cardiotoxicity risk and cardiac dysfunction. It seems that concomitant therapy with angiotensin receptor blocker and/or beta-blocker can alleviate the decline in LVEF associated with adjuvant anthracycline-containing regimens with or without trastuzumab and radiation[17]. For a long time, the clinical use of doxorubicin is limited by its cardiotoxic effects that can lead to heart failure. However, earlier work focused on the direct impact of doxorubicin on cardiomyo-cytes. Recent studies have evaluated the effects on the endothelium, because doxorubicin-induced endothelial damage can trigger the development and progression of cardiomyopathy by decreasing the release and activity of key endothe-lial factors and inducing endothelial cell death. Thus, the endothelium represents a novel target for improving the detection, management, and prevention of doxorubicin-induced cardiomyopathy[18].
The process of anti-cancer treatment should be adjusted according to the risks of cardiac toxicities, medical history, and previous treatment[19]. So, similar to the reported case, if we diligently weigh the pros and cons, choose regimens carefully, monitor cardiac toxicity closely, and even take some preventive measures, patients would benefit more without encountering high risks.
Comprehensive treatment of breast cancer may induce cardiac toxicity such as heart failure. However, heart failure related to anti-cancer treatment may be reversible. A second primary tumor is not rare for breast cancer survivors. When a secondary lymphoma develops in the setting of heart failure when treating breast cancer, anthracycline should be avoided to preserve cardiac function. Etoposide may be chosen instead of anthracycline, with equal effects.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Altarabsheh SE, Barik R, Petix NR, Teragawa H S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Wang J
| 1. | Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1973] [Cited by in F6Publishing: 2026] [Article Influence: 168.8] [Reference Citation Analysis (1)] |
| 2. | Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-2879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1494] [Cited by in F6Publishing: 1491] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 4. | Donin N, Filson C, Drakaki A, Tan HJ, Castillo A, Kwan L, Litwin M, Chamie K. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, Ron E. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 6. | Arbel Y, Swartzon M, Justo D. QT prolongation and Torsades de Pointes in patients previously treated with anthracyclines. Anticancer Drugs. 2007;18:493-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Milberg P, Fleischer D, Stypmann J, Osada N, Mönnig G, Engelen MA, Bruch C, Breithardt G, Haverkamp W, Eckardt L. Reduced repolarization reserve due to anthracycline therapy facilitates torsade de pointes induced by IKr blockers. Basic Res Cardiol. 2007;102:42-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231-2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 853] [Cited by in F6Publishing: 823] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 9. | Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981-1988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 997] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 10. | Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, Border WL, Feijen EA, Green DM, Meacham LR, Meeske KA, Mulrooney DA, Ness KK, Oeffinger KC, Sklar CA, Stovall M, van der Pal HJ, Weathers RE, Robison LL, Yasui Y. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33:394-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP, Sami A, Glaspy J, Juhos E, Wardley A, Fornander T, Hainsworth J, Coleman R, Modiano MR, Vinholes J, Pinter T, Rodríguez-Lescure A, Colwell B, Whitlock P, Provencher L, Laing K, Walde D, Price C, Hugh JC, Childs BH, Bassi K, Lindsay MA, Wilson V, Rupin M, Houé V, Vogel C; TRIO/BCIRG 001 investigators. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14:72-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Kwok JC, Richardson DR. Anthracyclines induce accumulation of iron in ferritin in myocardial and neoplastic cells: inhibition of the ferritin iron mobilization pathway. Mol Pharmacol. 2003;63:849-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 740] [Cited by in F6Publishing: 744] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 14. | Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1170] [Cited by in F6Publishing: 1073] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 15. | Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474-2481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 663] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 16. | Lin RJ, Behera M, Diefenbach CS, Flowers CR. Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood. 2017;130:2180-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland Å, Storås TH, Hagve TA, Røsjø H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 18. | Luu AZ, Chowdhury B, Al-Omran M, Teoh H, Hess DA, Verma S. Role of Endothelium in Doxorubicin-Induced Cardiomyopathy. JACC Basic Transl Sci. 2018;3:861-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768-2801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1409] [Cited by in F6Publishing: 1498] [Article Influence: 187.3] [Reference Citation Analysis (0)] |