Published online Aug 26, 2024. doi: 10.12998/wjcc.v12.i24.5513
Revised: May 30, 2024
Accepted: June 20, 2024
Published online: August 26, 2024
Processing time: 72 Days and 18.3 Hours
Hypothermia during laparoscopic surgery in patients with multiple trauma is a significant concern owing to its potential complications. Machine learning models offer a promising approach to predict the occurrence of intraoperative hypother
To investigate the value of machine learning model to predict hypothermia during laparoscopic surgery in patients with multiple trauma.
This retrospective study enrolled 220 patients who were admitted with multiple injuries between June 2018 and December 2023. Of these, 154 patients were allocated to a training set and the remaining 66 were allocated to a validation set in a 7:3 ratio. In the training set, 53 cases experienced intraoperative hypothermia and 101 did not. Logistic regression analysis was used to construct a predictive model of intraoperative hypothermia in patients with polytrauma undergoing laparoscopic surgery. The area under the curve (AUC), sensitivity, and specificity were calculated.
Comparison of the hypothermia and non-hypothermia groups found significant differences in sex, age, baseline temperature, intraoperative temperature, duration of anesthesia, duration of surgery, intraoperative fluid infusion, crystalloid infusion, colloid infusion, and pneumoperitoneum volume (P < 0.05). Differences between other characteristics were not significant (P > 0.05). The results of the logistic regression analysis showed that age, baseline temperature, intraoperative temperature, duration of anesthesia, and duration of surgery were independent influencing factors for intraoperative hypothermia during laparoscopic surgery (P < 0.05). Calibration curve analysis showed good consistency between the predicted occurrence of intraoperative hypothermia and the actual occurrence (P > 0.05). The predictive model had AUCs of 0.850 and 0.829 for the training and validation sets, respectively.
Machine learning effectively predicted intraoperative hypothermia in polytrauma patients undergoing laparoscopic surgery, which improved surgical safety and patient recovery.
Core Tip: Intraoperative hypothermia is a significant concern during laparoscopic surgery in patients with multiple trauma. This study investigated the value of a machine learning model in predicting hypothermia in this patient population. The results showed that machine learning effectively predicted intraoperative hypothermia, providing a valuable tool to improve surgical safety and patient recovery. Age, baseline temperature, intraoperative temperature, duration of anesthesia, and duration of surgery were identified as independent factors influencing hypothermia. The predictive model had good accuracy and consistency in both the training and validation sets.
- Citation: Zhu K, Zhang ZX, Zhang M. Application value of machine learning models in predicting intraoperative hypothermia in laparoscopic surgery for polytrauma patients. World J Clin Cases 2024; 12(24): 5513-5522
- URL: https://www.wjgnet.com/2307-8960/full/v12/i24/5513.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i24.5513
Polytrauma refers to a condition where a patient suffers multiple injuries simultaneously, which can lead to severe illness, difficulty in treatment, and high surgical risks[1]. Laparoscopic surgery has become an important means of treating patients with polytrauma. However, laparoscopic surgery poses significant risks for such patients. Intraoperative hypothermia often occurs in patients with polytrauma, which can pose a significant risk to the patient's surgical safety and postoperative recovery[2]. Hypothermia is defined as a body temperature that falls below the normal range of 36.5 °C to 37.5 °C in adults. A temperature below that range is considered hypothermic. Intraoperative hypothermia refers to a situation where a patient's body temperature drops below normal levels during surgery. Although intraoperative hypo
This retrospective study analyzed the clinical data of 220 patients with multiple injuries who were admitted to our hospital between June 2018 and December 2023. Of these, 154 were allocated to a training set and 66 to a validation set in a 7:3 ratio. In the training set, 53 cases experienced intraoperative hypothermia and 101 did not.
Patients who were 18-80 years of age who underwent elective surgery under general anesthesia and had clear consciousness and the ability to communicate normally were included.
Patients younger than 18 or older than 80 years of age; with severe cardiovascular, liver, kidney, and respiratory system disease; severe bleeding tendency; liver and kidney dysfunction, abnormal routine blood indicators; abdominal tumors or other malignant disease; preoperative infection or other preoperative complications; not able to communicate normally, with unclear consciousness, or cognitive impairment were excluded.
Study variables: Both predictive and outcome variables were included in the analysis: (1) The predictive variables were patient demographic and clinical data: sex, age, baseline temperature, body mass index (BMI), diabetes, hypertension, smoking history, alcohol consumption, intraoperative temperature, American society of anesthesiologists classification, anesthesia method, anesthesia duration, operation duration, intraoperative infusion of fluids, crystalloid infusion, colloid infusion, pneumoperitoneum flow rate, intraoperative blood loss, hemoglobin, platelet count, blood glucose, alanine aminotransferase, and aspartate aminotransferase; and (2) The outcome variable was hypothermia, and the core temperature was measured from the beginning of anesthesia until the end of surgery by an anesthesia monitor[6]. After the inducing general anesthesia, the temperature-sensitive probe of the monitor was placed in the patient's nasopharynx with a depth of the distance from the nostril to the mandibular angle on same side. The nasopharyngeal temperature was recorded every 15 min after the start of anesthesia, at the start of surgery, and until the end of surgery. The operating room temperature was maintained at 22-24 °C, and a patient temperature of < 36 °C was the diagnosis standard for intraoperative hypothermia.
Data collection methods: Screening medical records, examination reports, imaging data, and other relevant patient information using a combination of paper and electronic records ensured the completeness and accuracy of the patient information that was collected. During collection, attention was paid to protecting patient privacy and complying with legal regulations and medical ethics norms. After surgery, completed survey forms were given to a dedicated person for safekeeping, and 10% of the patient data was randomly selected for verification.
Supplementing missing data: For datasets with missing information, modeling and fitting was done to fill in missing values to ensure the completeness and accuracy of the dataset. By learning the correlation between the data before and after the model learning, the missing data values were predicted and added.
Building machine learning models: The included data were randomly sampled in a 7:3 ratio, with 70% entering the training set and 30% entering the validation set. Patients in the training set were further divided into a hypothermia group and a non-hypothermia depending on whether the they had developed hypothermia during surgery. The training set queue obtained the optimal hyperparameters through 100 iterations of fivefold cross-validation. The optimal training mode was obtained by combining all training sets, which was then brought into the corresponding validation group for verification to evaluate the model's fitting and generalization ability. The validation set selected a logistic regression classifier to construct a prediction model and used the area under the curve (AUC) to evaluate the model's discrimination. A large AUC indicated good discrimination ability of the prediction model. The model performance was evaluated by the AUC, and sensitivity, specificity, accuracy, and recall rates. The prediction model was visualized by a nomogram, and the scores of the predictive variables in the model were added together to find the corresponding point on the total score scale and a line was drawn vertically downward. The value on the corresponding probability scale was the probability of an individual experiencing an outcome event. The model's calibration was evaluated using calibration curves and the Hosmer–Lemeshow χ2 test, which reflects the consistency between the predicted risk of intraoperative hypothermia and the actual risk in different risk-stratification patients.
The collected data were analyzed using R software (4.3.1). Normally distributed metric data were reported as means ± SD and compared by t-tests. Counting data were reported as numbers and percentage (%) and compared by χ2 tests. P values < 0.05 indicated a significant difference. The predictive model was constructed using logistic regression, and the Hosmer–Lemeshow test was used to verify the model's goodness of fit, with a large P value indicating a good fit. The model's predictive ability was indicated by the AUC of the receiver operating characteristic (ROC) curve analysis. Sensitivity, specificity, and accuracy were used to verify the model's actual application efficiency.
We found no statistically significant differences of the clinical data in the training and validation sets (P > 0.05) as shown in Table 1).
| Parameter | Training (n = 154) | Validation (n = 66) | Statistical value | P value |
| Sex | χ2 = 0.383 | 0.536 | ||
| Male | 84 (54.55) | 33 (50.00) | ||
| Female | 70 (45.45) | 33 (50.00) | ||
| Age in years | 54.34 ± 11.58 | 55.87 ± 10.79 | t = 0.916 | 0.361 |
| Basal body temperature in °C | 34.05 ± 0.22 | 34.01 ± 0.29 | t = 1.119 | 0.264 |
| BMI in kg/m2 | 23.27 ± 2.82 | 23.65 ± 2.75 | t = 0.923 | 0.357 |
| Diabetes | χ2 = 2.013 | 0.156 | ||
| Yes | 19 (12.34) | 13 (19.70) | ||
| No | 135 (87.66) | 53 (80.30) | ||
| Hypertension | χ2 = 2.685 | 0.101 | ||
| Yes | 31 (20.13) | 20 (30.30) | ||
| No | 123 (79.87) | 46 (69.70) | ||
| Smoking | χ2 = 2.013 | 0.156 | ||
| Yes | 19 (12.34) | 13 (19.70) | ||
| No | 135 (87.66) | 53 (80.30) | ||
| Drinking | χ2 = 2.127 | 0.145 | ||
| Yes | 15 (9.74) | 11 (16.67) | ||
| No | 139 (90.26) | 55 (83.33) | ||
| Operating room temperature in °C | 21.65 ± 4.16 | 21.52 ± 4.12 | t = 0.213 | 0.832 |
| ASA | χ2 = 5.034 | 0.169 | ||
| I | 16 (10.39) | 10 (15.15) | ||
| II | 116 (75.32) | 41 (62.12) | ||
| III | 21 (13.64) | 13 (19.70) | ||
| IV | 1 (0.65) | 2 (3.03) | ||
| Mode of anesthesia | χ2 = 0.542 | 0.462 | ||
| General anesthesia | 71 (46.10) | 34 (51.52) | ||
| General anesthesia joint board anesthesia | 83 (53.90) | 32 (48.48) | ||
| Duration of anesthesia in min | 150.48 ± 76.21 | 139.39 ± 75.97 | t = 0.990 | 0.323 |
| Operation duration in min | 127.61 ± 78.16 | 124.65 ± 75.77 | t = 0.260 | 0.795 |
| Intraoperative fluid infusion in mL | 1067.84 ± 616.12 | 1073.92 ± 610.11 | t = 0.067 | 0.946 |
| Infusion of crystal solution in mL | 1050.13 ± 567.08 | 1041.37 ± 600.42 | t = 0.103 | 0.918 |
| Infusion of colloidal solution in mL | 92.68 ± 15.22 | 91.15 ± 15.14 | t = 0.684 | 0.495 |
| Pneumoperitoneum flow in L/min | 261.22 ± 16.13 | 258.09 ± 15.96 | t = 1.323 | 0.187 |
| Intraoperative blood loss in mL | 98.27 ± 35.82 | 94.18 ± 35.61 | t = 0.777 | 0.438 |
| Hemoglobin in g/L | 115.65 ± 18.15 | 113.74 ± 17.05 | t = 0.728 | 0.467 |
| Platelet as × 109/L | 180.88 ± 56.32 | 176.87 ± 56.76 | t = 0.483 | 0.630 |
| Glu in mmol/L | 4.15 ± 0.87 | 4.25 ± 0.86 | t = 0.784 | 0.434 |
| ALT in U/L | 25.58 ± 10.19 | 27.02 ± 10.12 | t = 0.962 | 0.337 |
| AST in U/L | 20.27 ± 8.82 | 19.18 ± 8.75 | t = 0.842 | 0.401 |
Statistically significant differences (P < 0.05) of sex, age, baseline temperature, intraoperative temperature, anesthesia duration, operation duration, intraoperative infusion of fluids, crystalloid infusion, colloid infusion, and pneumoperitoneum flow rate were observed between the hypothermia group and the non-hypothermia group. Differences were observed in other characteristics were not significant (P > 0.05), as shown in Table 2.
| Item | Hypothermia, n = 53 | Non-hypothermia, n = 101 | Statistical value | P value |
| Sex | χ2 = 9.210 | 0.002 | ||
| Male | 20 (37.74) | 64 (63.37) | ||
| Female | 33 (62.26) | 37 (36.63) | ||
| Age in years | 61.11 ± 12.15 | 57.23 ± 8.71 | t = 2.282 | 0.024 |
| Basal body temperature in °C | 36.15 ± 0.28 | 36.74 ± 0.28 | t = 12.423 | < 0.001 |
| BMI in kg/m2 | 23.73 ± 2.71 | 23.75 ± 3.25 | t = 0.038 | 0.970 |
| Diabetes | χ2 = 1.715 | 0.190 | ||
| Yes | 4 (7.55) | 15 (14.85) | ||
| No | 49 (92.45) | 86 (85.15) | ||
| Hypertension | χ2 = 0.498 | 0.480 | ||
| Yes | 9 (16.98) | 22 (21.78) | ||
| No | 44 (83.02) | 79 (78.22) | ||
| Smoking | χ2 = 0.077 | 0.781 | ||
| Yes | 6 (11.32) | 13 (12.87) | ||
| No | 47 (88.68) | 88 (87.13) | ||
| Drinking | χ2 = 0.442 | 0.506 | ||
| Yes | 4 (7.55) | 11 (10.89) | ||
| No | 49 (92.45) | 90 (89.11) | ||
| Operating room temperature in °C | 21.12 ± 4.05 | 22.84 ± 5.02 | t = 2.153 | 0.033 |
| ASA | χ2 = 4.325 | 0.228 | ||
| I | 3 (5.66) | 13 (12.87) | ||
| II | 43 (81.13) | 73 (72.28) | ||
| III | 6 (11.32) | 15 (14.85) | ||
| IV | 1 (1.89) | 0 | ||
| Mode of anesthesia | χ2 = 0.238 | 0.625 | ||
| General anesthesia | 23 (43.40) | 48 (47.52) | ||
| General anesthesia joint board anesthesia | 30 (56.60) | 53 (52.48) | ||
| Duration of anesthesia in min | 162.52 ± 75.16 | 118.63 ± 58.35 | t = 4.006 | < 0.001 |
| Operation duration in min | 141.27 ± 75.11 | 99.21 ± 56.22 | t = 3.916 | < 0.001 |
| Intraoperative fluid infusion in mL | 1157.12 ± 615.51 | 793.15 ± 422.17 | t = 4.319 | < 0.001 |
| Infusion of crystal solution in mL | 1046.01 ± 560.12 | 769.34 ± 389.25 | t = 3.585 | 0.001 |
| Infusion of colloidal solution in mL | 103.41 ± 15.06 | 27.79 ± 7.15 | t = 42.274 | < 0.001 |
| Pneumoperitoneum flow in L/min | 270.51 ± 17.21 | 187.29 ± 11.19 | t = 36.200 | < 0.001 |
| Intraoperative blood loss in mL | 118.33 ± 33.95 | 112.79 ± 35.31 | t = 0.937 | 0.350 |
| Hemoglobin in g/L | 134.98 ± 20.85 | 136.58 ± 19.69 | t = 0.469 | 0.639 |
| Platelet as × 109/ | 208.76 ± 62.16 | 211.41 ± 59.52 | t = 0.259 | 0.796 |
| Glu in mmol/L | 5.14 ± 0.95 | 5.21 ± 1.06 | t = 0.403 | 0.687 |
| ALT in U/L | 26.89 ± 10.41 | 25.79 ± 10.68 | t = 0.612 | 0.541 |
| AST in U/L | 21.27 ± 9.12 | 20.39 ± 8.97 | t = 0.575 | 0.566 |
Assignment of the independent variables and the dependent variable is shown in Table 3. The results of logistic regression analysis indicated that age, baseline temperature, intraoperative temperature, anesthesia duration, and operation duration were independent influencing factors for intraoperative hypothermia during laparoscopic surgery (P < 0.05), as shown in Table 4.
| Variable | Factor | Assignment |
| Y | Hypothermia | 1 = hypothermia; 0 = non-hypothermia |
| X1 | Age | Continuous variable, original value input |
| X2 | Basal body temperature | Continuous variable, original value input |
| X3 | Operating room temperature | Continuous variable, original value input |
| X4 | Duration of anesthesia | Continuous variable, original value input |
| X5 | Operation duration | Continuous variable, original value input |
| X6 | Intraoperative fluid infusion | Continuous variable, original value input |
| X7 | Infusion of crystal solution | Continuous variable, original value input |
| X8 | Infusion of colloidal solution | Continuous variable, original value input |
| X9 | Pneumoperitoneum flow | Continuous variable, original value input |
| X10 | Sex | 0 = Male; 1 = Female |
| Risk factor | β | SE | Wald χ2 | P value | OR | 95%CI |
| Age | 0.056 | 0.017 | 11.434 | 0.001 | 1.058 | 1.024-1.093 |
| Basal body temperature | −1.211 | 0.365 | 11.157 | 0.001 | 0.286 | 0.231-0.976 |
| Operating room temperature | −0.066 | 0.041 | 2.688 | 0.101 | 0.936 | 0.864-1.013 |
| Duration of anesthesia | −0.008 | 0.003 | 8.458 | 0.004 | 0.992 | 0.986-0.997 |
| Operation duration | 0.011 | 0.003 | 12.787 | < 0.001 | 1.011 | 1.005-1.018 |
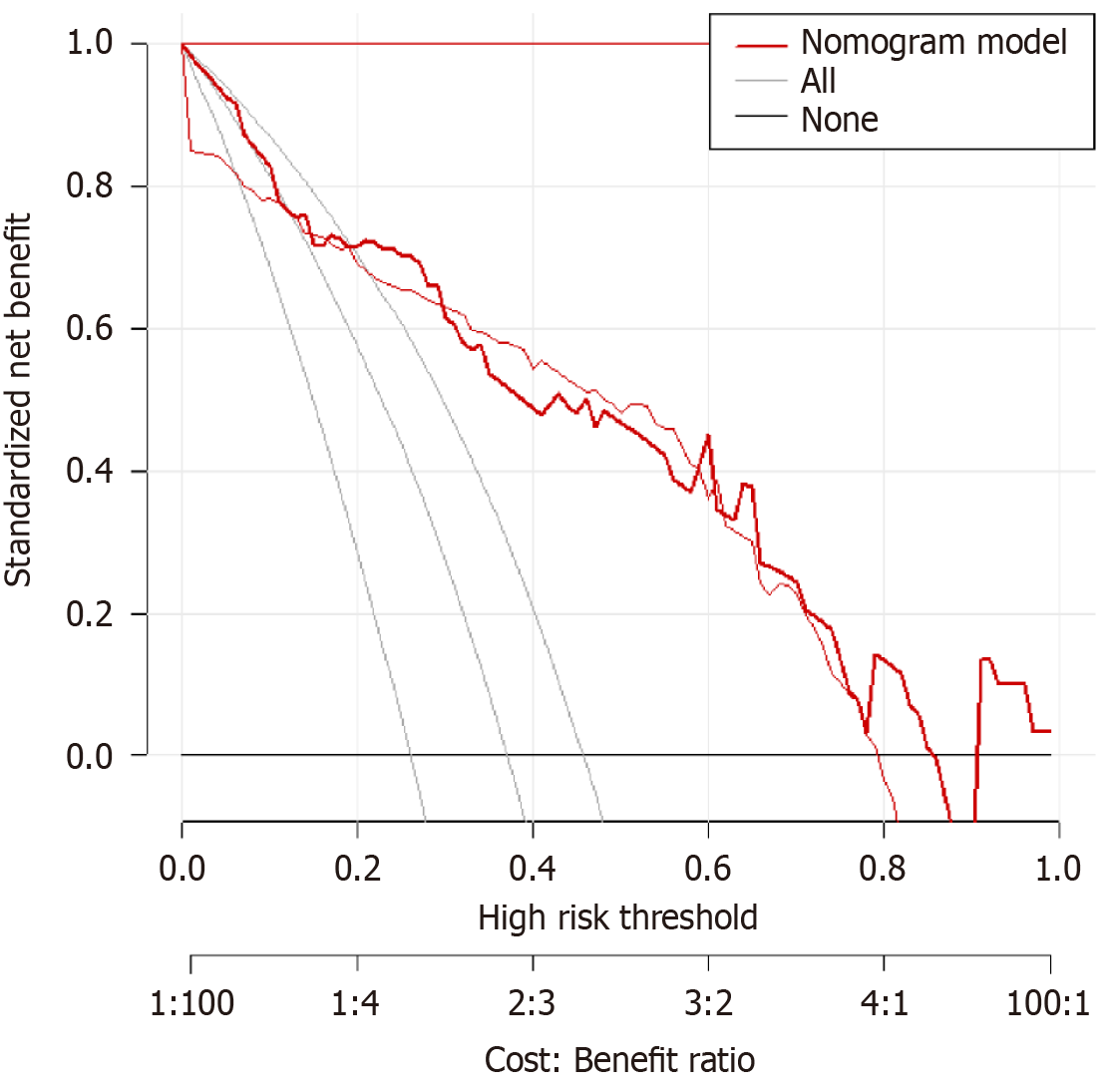
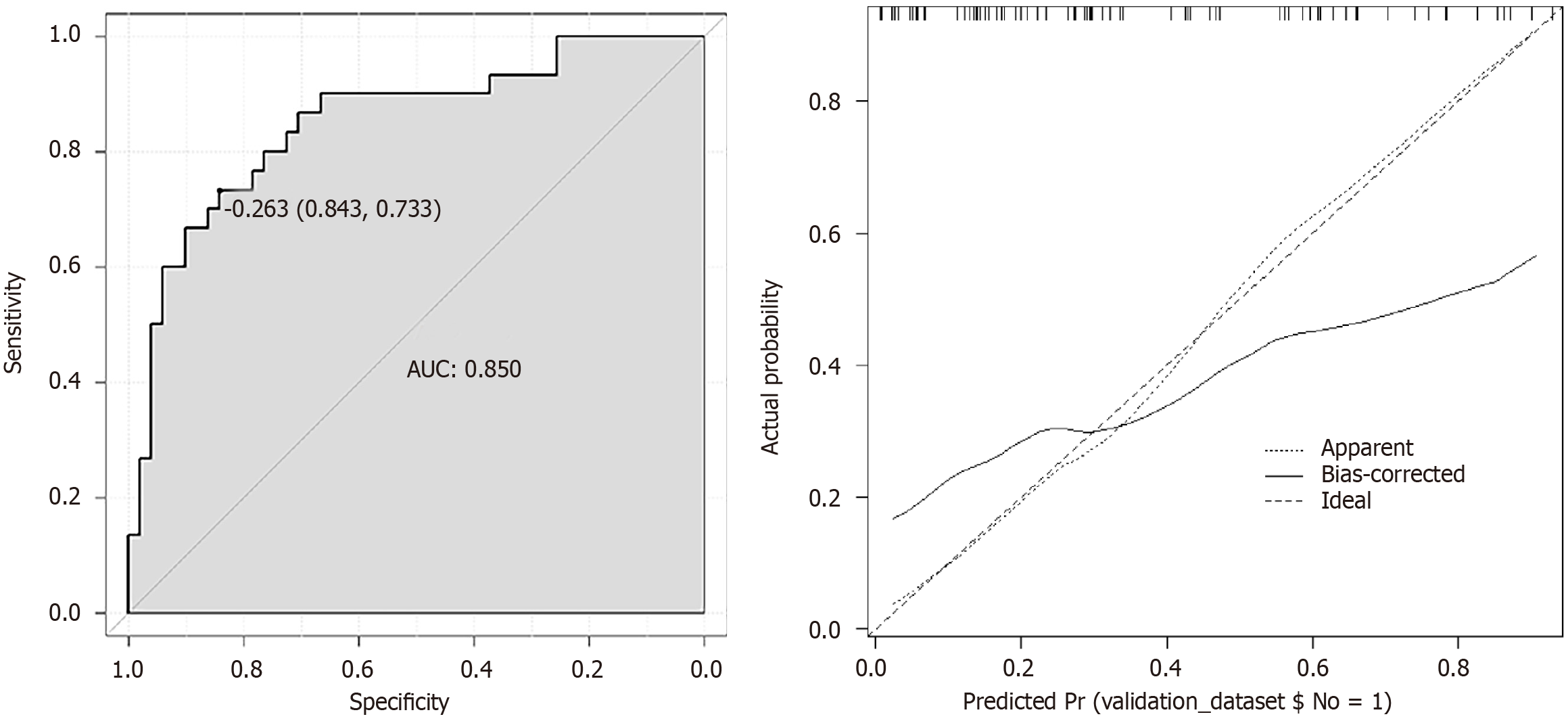
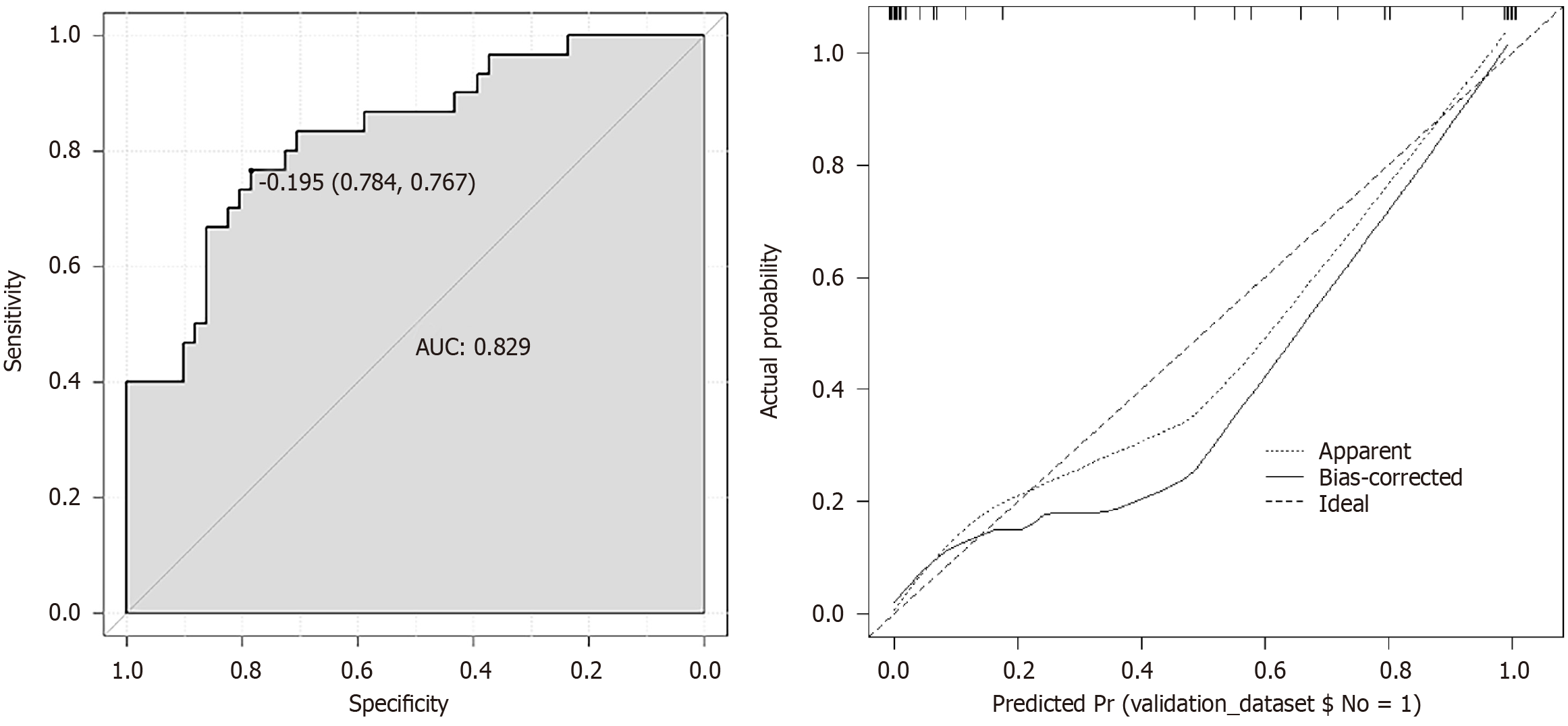
A predictive model was established using the logistic regression analysis results. Calibration curve analysis demonstrated good consistency between the predicted and actual occurrence of intraoperative hypothermia (P > 0.05), as shown in Figure 1. The ROC curve analysis results indicated that the model had AUC values of 0.850 and 0.829 for predicting the occurrence of hypothermia in the training and validation set patients, respectively, as shown in Table 5 and Figures 2
| Index | AUC | SEN, % | SPE, % | 95%CI | P value |
| Training | 0.850 | 94.69 | 91.47 | 0.909-0.962 | < 0.001 |
| Validation | 0.829 | 95.38 | 90.53 | 0.850-0.988 | < 0.001 |
Currently, the hazards of intraoperative hypothermia are recognized by most medical staff, and the benefits of perioperative thermal insulation are recognized and recommended by many international guidelines. Relevant research in China has also begun to focus on its importance[7]. Laparoscopic techniques have become the preferred approach for most surgical procedures, yet there is a relative lack of research and data on intraoperative hypothermia in laparoscopic surgery patients. This study found that age, baseline temperature, intraoperative temperature, anesthesia duration, and operation duration were independent influencing factors for intraoperative hypothermia during laparoscopic surgery (P < 0.05).
Age is an important factor influencing the risk of intraoperative hypothermia in patients with multiple trauma undergoing laparoscopic surgery. With increasing age, the body's tolerance decreases, and its self-regulation functions are impaired, leading to an increased risk of intraoperative hypothermia in patients with multiple trauma. As age increases, the basal metabolic rate decreases, particularly due to an increase in fat tissue mass and a decrease in muscle tissue mass, which reduces the ability to produce heat and maintain body temperature[8]. Increasing age can also lead to a decline in temperature regulation, narrowing of the range of temperature regulation, delay of the response to change in external temperature and increase of the risk of intraoperative hypothermia[9]. Furthermore, as age increases, sympathetic nervous regulation decreases and the ability to withstand cold is reduced, resulting in weakened vascular constriction, reduced heat loss during surgery, and increased risk of intraoperative hypothermia[10].
During laparoscopic surgery, the use of surgical trauma and anesthesia drugs may further reduce heat generation. Therefore, patients with multiple trauma and low baseline temperatures are highly likely to experience insufficient heat and increased heat loss during laparoscopic surgery, thereby increasing the risk of intraoperative hypothermia[11]. Furthermore, baseline temperature may reflect the body's temperature regulation function. Body temperature is regulated by multiple physiological systems, including the central nervous system, sympathetic nervous system, and skin vascular system[12]. Patients with multiple trauma and low baseline temperatures may have abnormal or impaired temperature regulation because of weakened sympathetic nervous system activity and reduced skin blood flow that can lead to reduced heat loss during surgery and result in an increased risk of intraoperative hypothermia[13].
Regarding intraoperative temperature, the body is constantly exchanging heat with the external environment. Body temperature is closely related to environmental temperature, and heat is lost to the surrounding environment by radiation, convection, conduction, and evaporation. If more heat is lost than is generated, body temperature decreases. A low temperature can cause a decrease in body temperature, vascular constriction, and the metabolic rate of various organs. Factors affecting the metabolic rate of organs include an increase in cell membrane osmotic pressure (due to temperature reduction), which limits the exchange of substances inside and outside the cell, and reduced adenosine triphosphate (ATP) synthesis. As the metabolism is a thermodynamic process, so the energy of synthesizing ATP by the body at low temperatures decreases[14]; and enzyme catalytic activity is inhibited, which reduces the rate of chemical reactions. Thermoregulation is mainly controlled by the nervous and endocrine systems, including mechanisms such as vasodilation and constriction, sweating, and skin-surface heat loss[15]. At low temperatures, responses like shivering, capillary constriction, decreased blood flow, and increased metabolic rate occur, but cannot be maintained for a long time. The result is a decrease in body temperature[16]. One of the common side effects of anesthesia drugs is inhibition of the thermoregulation center and loop, resulting in a decrease in body temperature. At the same time, anesthesia drugs can increase muscle relaxation and slow respiration, thereby reducing the flow of deoxygenated blood and lowering the body's metabolic rate[17].
Regarding anesthesia duration, a long duration can damage the temperature regulation center, thereby affecting the ability to control temperature. Anesthesia drugs can inhibit the activity of the temperature regulation center and reduce the metabolic rate, leading to a decrease in body temperature. During long surgical procedures, the body-heat conduction is restricted[18]. During surgery, the patient is exposed on the surgical table, which is a relatively cold surface that causes increased heat loss from the body surface[19]. In addition, the surgical procedure may expose internal organs, which can lead to increased heat loss.
Regarding operation duration, patients with multiple trauma undergoing long operations are in a state of dormancy due to the need for general anesthesia, and their metabolic rate slows, leading to a decrease in body temperature. Anesthesia drugs can inhibit the metabolic activity of the central nervous system, thereby reducing energy consumption, slowing the body's heat production and ability to preserve heat, and thus lower body temperature[20]. In laparoscopic surgery, carbon dioxide is used to inflate the abdomen to expand the surgical space. Given the ability of carbon dioxide to absorb heat, it absorbs surrounding heat into the abdomen and affects the gas exchange of the alveoli, leading to an increase in the concentration of carbon dioxide in the body[21]. These factors can cause a decrease in body temperature. Local tissue damage and bleeding during surgery can also increase energy consumption, leading to a decrease in body temperature. Research has shown[22] that the incidence of intraoperative hypothermia increases significantly when the operation time exceeds 2 h, which is consistent with the results of this study.
BMI is an important factor influencing intraoperative hypothermia during laparoscopic surgery[23]. Previous studies demonstrated[24] that patients with high BMIs have reduced surface heat loss owing to the protective effect of fat, which results in a small difference between core temperature and surface temperature and a low incidence of hypothermia. However, this study did not find BMI was a factor that influenced the occurrence of hypothermia during laparoscopic surgery.
Based on the logistic regression analysis results, age, baseline temperature, intraoperative temperature, anesthesia duration, and operation duration were included in the predictive model. Calibration curve analysis demonstrated good consistency between the predicted and actual occurrence of intraoperative hypothermia (P > 0.05). ROC curve analysis results showed that the model had AUC values of 0.850 and 0.829, respectively, for predicting the occurrence of hypothermia in the training set and validation set patients. The above results indicate that the model had good predictive ability and accuracy.
A predictive model of the risk of intraoperative hypothermia in laparoscopic surgery patients was developed using data from multiple dimensions, including demographic data, surgery, anesthesia, and the environment, using a random forest algorithm. The random forest algorithm has significant advantages in predicting the risk of intraoperative hypothermia in laparoscopic surgery patients. It can identify important influencing factors of intraoperative hypothermia from a complex group of multiple factors by comprehensive evaluation. The result is of great significance for clinical and medical staff to identify high-risk patients in a timely manner and adopt effective intervention measures. However, the study had limitations. It included only laparoscopic surgery patients from our hospital, which may have introduced information bias and patient selection bias in the data collection process. Moreover, the study sample was relatively small. In future studies, we will collaborate with multiple centers, increase the sample size, include additional variables, and continuously optimize this predictive model of the risk of intraoperative hypothermia in laparoscopic surgery patients.
| 1. | Polick CS, Polick SR, Stoddard SA. Relationships between childhood trauma and multiple sclerosis: A systematic review. J Psychosom Res. 2022;160:110981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Sumann G, Moens D, Brink B, Brodmann Maeder M, Greene M, Jacob M, Koirala P, Zafren K, Ayala M, Musi M, Oshiro K, Sheets A, Strapazzon G, Macias D, Paal P. Multiple trauma management in mountain environments - a scoping review : Evidence based guidelines of the International Commission for Mountain Emergency Medicine (ICAR MedCom). Intended for physicians and other advanced life support personnel. Scand J Trauma Resusc Emerg Med. 2020;28:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Jiang Y, Han W, Jiang L, Mao C, Wang M. Establishment and obstacle analysis of evidence-based nursing indexes for unplanned hypothermia management in patients undergoing laparoscopic operation. Gland Surg. 2022;11:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Birch DW, Dang JT, Switzer NJ, Manouchehri N, Shi X, Hadi G, Karmali S. Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev. 2016;10:CD007821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Binda MM. Humidification during laparoscopic surgery: overview of the clinical benefits of using humidified gas during laparoscopic surgery. Arch Gynecol Obstet. 2015;292:955-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Wittenborn J, Clausen A, Zeppernick F, Stickeler E, Meinhold-Heerlein I. Prevention of Intraoperative Hypothermia in Laparoscopy by the Use of Body-Temperature and Humidified CO (2) : a Pilot Study. Geburtshilfe Frauenheilkd. 2019;79:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Manja V, Kirpalani H, Lakshminrusimha S. Factors influencing decision-making: Delayed hypothermia in a late preterm infants with hypoxic-ischemic encephalopathy. Early Hum Dev. 2019;128:102-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Suri TS, Sardesai A, Volpin A, Muniz-Terrera G, Khanduja V. Incidence of hypothermia and factors affecting variation in core body temperature in patients undergoing arthroscopic surgery of the hip. Acta Orthop Belg. 2019;85:535-539. [PubMed] |
| 9. | Lai LL, See MH, Rampal S, Ng KS, Chan L. Significant factors influencing inadvertent hypothermia in pediatric anesthesia. J Clin Monit Comput. 2019;33:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Simegn GD, Bayable SD, Fetene MB. Prevention and management of perioperative hypothermia in adult elective surgical patients: A systematic review. Ann Med Surg (Lond). 2021;72:103059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Dresden SM, O'Connor LM, Pearce CG, Courtney DM, Powell ES. National trends in the use of postcardiac arrest therapeutic hypothermia and hospital factors influencing its use. Ther Hypothermia Temp Manag. 2015;5:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Tander B, Baris S, Karakaya D, Ariturk E, Rizalar R, Bernay F. Risk factors influencing inadvertent hypothermia in infants and neonates during anesthesia. Paediatr Anaesth. 2005;15:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Pabello NG, Tracy SJ, Snyder-Keller A, Keller RW Jr. Regional expression of constitutive and inducible transcription factors following transient focal ischemia in the neonatal rat: influence of hypothermia. Brain Res. 2005;1038:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Sahin Akboga O, Dikmen Aydin Y. Barriers and Solutions in Implementing Evidence-Based Recommendations to Prevent Intraoperative Inadvertent Hypothermia: A Qualitative Study. Ther Hypothermia Temp Manag. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Shou BL, Zhou AL, Ong CS, Alejo DE, DiNatale JM, Larson EL, Lawton JS, Schena S. Impact of intraoperative blood products, fluid administration, and persistent hypothermia on bleeding leading to reexploration after cardiac surgery. J Thorac Cardiovasc Surg. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Ma Z, Ma P, Huang N, Li C, Cao Y, Chen J. Incidence of Unintentional Intraoperative Hypothermia and Its Risk Factors in Oral and Maxillofacial Surgery: A Prospective Study. J Perianesth Nurs. 2023;38:876-880. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Dibiasi C, Agibetov A, Kapral L, Zeiner S, Kimberger O. Predicting Intraoperative Hypothermia Burden during Non-Cardiac Surgery: A Retrospective Study Comparing Regression to Six Machine Learning Algorithms. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Morishige S, Ohyama T, Fujita N, Kameda M, Yonekura Y, Endo F, Abe S. Risk Factors for Intraoperative Hypothermia during Holmium Laser Enucleation of the Prostate. Urol Int. 2023;107:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Prabhu SS, Driscoll CR, Davidson AL, Peoples AE, Katz AJ. The effects of prolonged intraoperative hypothermia on patient outcomes in immediate implant-based breast reconstruction. J Plast Reconstr Aesthet Surg. 2023;77:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Chataule SM, Hazarika A, Jain K, Chauhan R, Luthra A, Meena S, Aggarwal S, Sethi S. Preoperative Forced-Air Warming Strategy: Is It Effective in Averting Intraoperative Hypothermia in Elderly Trauma Surgical Patients? Cureus. 2022;14:e29305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Lee C, Park S, Kim B, Yim H, Lee M, Lee J, Kim H. Effects of Female Reproductive Hormone Levels on Inadvertent Intraoperative Hypothermia during Laparoscopic Gynecologic Surgery: A Retrospective Study. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Sultana R, Allen JC, Siow YN, Bong CL, Lee SY. Development of local guidelines to prevent perioperative hypothermia in children: a prospective observational cohort study. Can J Anaesth. 2022;69:1360-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 23. | Park S, Yoon SH, Youn AM, Song SH, Hwang JG. Heated wire humidification circuit attenuates the decrease of core temperature during general anesthesia in patients undergoing arthroscopic hip surgery. Korean J Anesthesiol. 2017;70:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Frisch NB, Pepper AM, Rooney E, Silverton C. Intraoperative Hypothermia in Total Hip and Knee Arthroplasty. Orthopedics. 2017;40:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |