Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12164
Peer-review started: August 7, 2022
First decision: September 25, 2022
Revised: October 4, 2022
Accepted: October 24, 2022
Article in press: October 24, 2022
Published online: November 26, 2022
Accumulating evidences confirm that epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement have coexisted in lung adenocarcinoma (LUAD). However, Its biological mechanism, clinicopathological features, and optimization of targeted drugs have not yet been com
To explore the clinical profile of LUAD patients with co-mutations of EGFR and ALK genes, with hopes of scientifically guiding similar patients towards selected, targeted drugs.
Two hundred and thirty-seven LUAD patients were enrolled. EGFR mutations were detected by the amplification refractory mutation system-peptide nucleic acid technique, while the expression of ALK rearrangement was screened by the 5′/3′ imbalance strategy for reverse transcription followed by quantitative po
There were six cases with co-mutations of EGFR and ALK genes, which were more common in women, non-smoking and stage IV LUAD patients with bone metastasis, hence a positive rate of 2.53% (6/237). EGFR-tyrosine kinase inhibitors (EGFR-TKIs) were their preferred drugs for targeted therapy in these patients, with progression-free survival ranging from two months to six months.
In Gannan region, the positive rate of co-mutations of EGFR and ALK genes in LUAD patients is relatively high, and the co-mutations are more common in women, non-smoking and stage IV patients with bone metastasis. These patients prefer EGFR-TKIs as their preferred targeted drugs, but the therapeutic effect is not good. EGFR/ALK dual-TKIs may be more effective targeted drugs, which needs further study.
Core Tip: This study retrospectively analyzed the clinicopathological features of patients with co-mutations of EGFR and ALK genes in lung adenocarcinoma, and collected follow-up data of these patients, especially focusing on the tyrosine kinase inhibitors (TKIs) selected and its therapeutic effect in the real world from a single institute experience. The results showed that the positive rate of lung adenocarcinoma patients with co-mutations of EGFR and ALK genes, which were more common in women, non-smoking and stage IV patients with bone metastasis, was relatively high in Gannan region, which may be related to regional heterogeneity. EGFR-TKIs were their preferred drugs for targeted therapy in these patients, but the therapeutic effect is not good. EGFR/ALK dual-TKIs may be more effective targeted drugs.
- Citation: Zhong WX, Wei XF. Coexistence of anaplastic lymphoma kinase rearrangement in lung adenocarcinoma harbouring epidermal growth factor receptor mutation: A single-center study. World J Clin Cases 2022; 10(33): 12164-12174
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12164.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12164
With the availability of targeted drugs in non-small cell lung cancer (NSCLC) and the development of molecular detection technology, NSCLC has entered the era of precision medicine. Targeted therapy has brought revolutionary changes to NSCLC patients with epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) rearrangement. EGFR mutation is the most common and important therapeutic target, which is significantly associated with the sensitivity of EGFR-tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib, erlotinib and osimertinib. At present, EGFR-TKIs are widely used in clinical practice and have exhibited favourable anti-tumour effect in the treatment of NSCLC[1,2]. ALK rearrangement is the second target after EGFR mutation, and it is also the first target of fusion gene tyrosine kinase inhibitor found in NSCLC. Targeted drugs for ALK rearrangement have developed rapidly, and the clinical use of ALK-TKIs, including crizotinib[3], alectinib[4], and lorlatinib[5], has significantly improved the survival of advanced NSCLC patients with ALK rearrangement, even better than the efficacy of EGFR-TKIs.
Previous studies[6] have indicated that EGFR mutation and ALK rearrangement are independent molecular events in NSCLC. However, Sporadic cases with Concomitance of EGFR mutation and ALK rearrangement have been increasingly reported[7-10]. The biological mechanism, clinicopathological features and optimal targeted drugs of NSCLC patients with co-mutations of EGFR and ALK genes remain mostly unknown.
In this study, we investigated the clinicopathological features and follow-up data of patients with co-mutations of EGFR and ALK genes in lung adenocarcinoma (LUAD) within Gannan region, aiming to obtain the clinical profile of LUAD patients with co-mutations of EGFR and ALK genes, with intention to scientifically guide the selection of targeted drugs in similar patients. In the experiment, EGFR mutation was detected using amplification refractory mutation system-peptide nucleic acid (ARMS-PNA), and ALK rearrangement was screened by 5′/3′ imbalance strategy for reverse transcription followed by quantitative polymerase chain reaction (RT–qPCR).
Two hundred and thirty-seven patients with primary LUAD confirmed by pathological examination in the First Affiliated Hospital of Gannan Medical University from 2016 to 2020 were enrolled, comprising 132 males (55.70%) and 105 females (44.30%); the average age was (60.3 ± 10.569) years, and the median age was 61 years (range 34-89); 145 cases (61.18%) had no smoking history and 92 cases (38.82%) had smoking history, which was defined as a cumulative minimum of 100 cigarettes. The clinical stage was obtained according to the International Association for the study of lung cancer (LASLC) TNM staging standard, eighth edition. There were 42 cases in stage I (17.72%), 16 cases in stage II (6.75%), 21 cases in stage III (8.86%) and 158 cases in stage IV (66.67%). These patients all came from Gannan region, Jiangxi province, and they were all untreated and had never received chemotherapy, radiotherapy, molecular targeted therapy or immunotherapy.
The data used in this study were all anonymous, which were not involved in the patients’ privacy information, they were obtained after each patient agreed to treatment by written consent. This study have been approved by the Scientific Research Ethics Committee of the First Affiliated Hospital of Gannan Medical University (No. LLSC-2021081601), and conducted in accordance with Declaration of Helsinki (as revised in 2013).
There were 237 tissue samples, including surgical resection tissue, computed tomography (CT) guided percutaneous lung biopsy tissue, and electronic bronchoscopy biopsy tissue. 200 tissue samples were embedded and fixed in paraffin by the Department of Pathology of the hospital, and 15 pathological white films were cut according to a thickness of at least 5 µm and sent out; 37 fresh tissue samples were stored in RNAfixer tissue preservation solution in vitro and sent out after fixation. All tissue samples were sent to Dingjing Medical Laboratory for genetic testing.
DNA Extraction: A QIAamp DNA FFPE Extraction Kit (Qiagen) was used to extract the DNA of samples, while the purity and concentration of DNA were detected by spectrophotometer.
ARMS-PNA: Mutations in exons 18, 19, 20 and 21 of the EGFR gene were detected by ARMS-PNA technology. For the specific operation method, a human EGFR gene mutation detection kit (fluorescence PCR method) was used (Haijili, Registration Certificate No: 3400973).
Total RNA extraction: The total RNA of the samples was extracted by a tissue RNA Extraction Kit (Qiagen), and the concentration (> 30 ng) and purity (OD260/OD280 value of 1.8-2.0) of total RNA in the template were determined by UV spectrophotometer.
Synthesis of complementary DNA by RT: The RT procedure was followed according to the instructions of M-MLV reverse transcription Kit: 25 °C for 5 min, 42 °C for 60 min, 75 °C for 5 min, and 4 °C for 5 min.
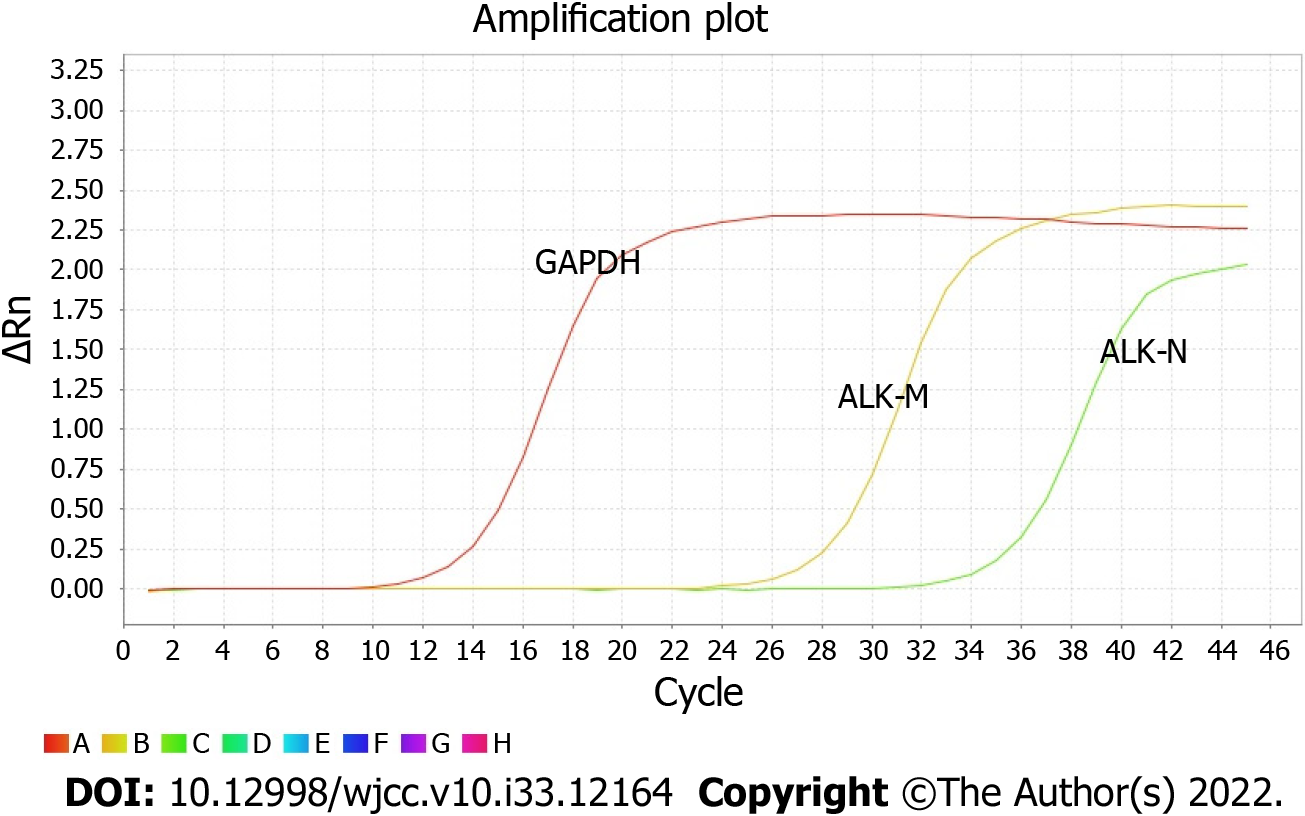
Real-time PCR system: RT products were prepared for PCR by using SYBR Green PCR Master Mix (Applied Biosystems) reagent. Fluorescent substances were added to the reaction system and detected by an ABI7500 PCR instrument (Thermo Fisher Scientific). According to the user manual of the human EML4-ALK fusion gene detection kit (real-time PCR), the PCR amplification was carried out under the following conditions: (1) Ung enzyme reaction: 1 cycle at 50 °C for 2 min; (2) Predenaturation: 1 cycle at 95 °C for 10 min; (3) Denaturation: 95 °C, 15 s; annealing, extension and fluorescence detection (FAM as fluorescence channel) at 60 °C for 32 s; a total of 45 cycles; and (4) Dissolution curve (60 °C-95 °C increases by 1%, and the fluorescence signal is absorbed at this stage): 1 cycle at 95 °C for 15 s; 1 cycle at 60 °C for 1 min; 1 cycle at 95 °C for 30 s; 1 cycle at 60 °C for 15 s. The primer sequences were as follows: EML4-ALK-M (ALK-21/22F1: GCAAGTGGCTGTGAAGAC; ALK-22/23R1: GGTGGTTGAATTTG
The data in this article are count data, expressed as percentage.
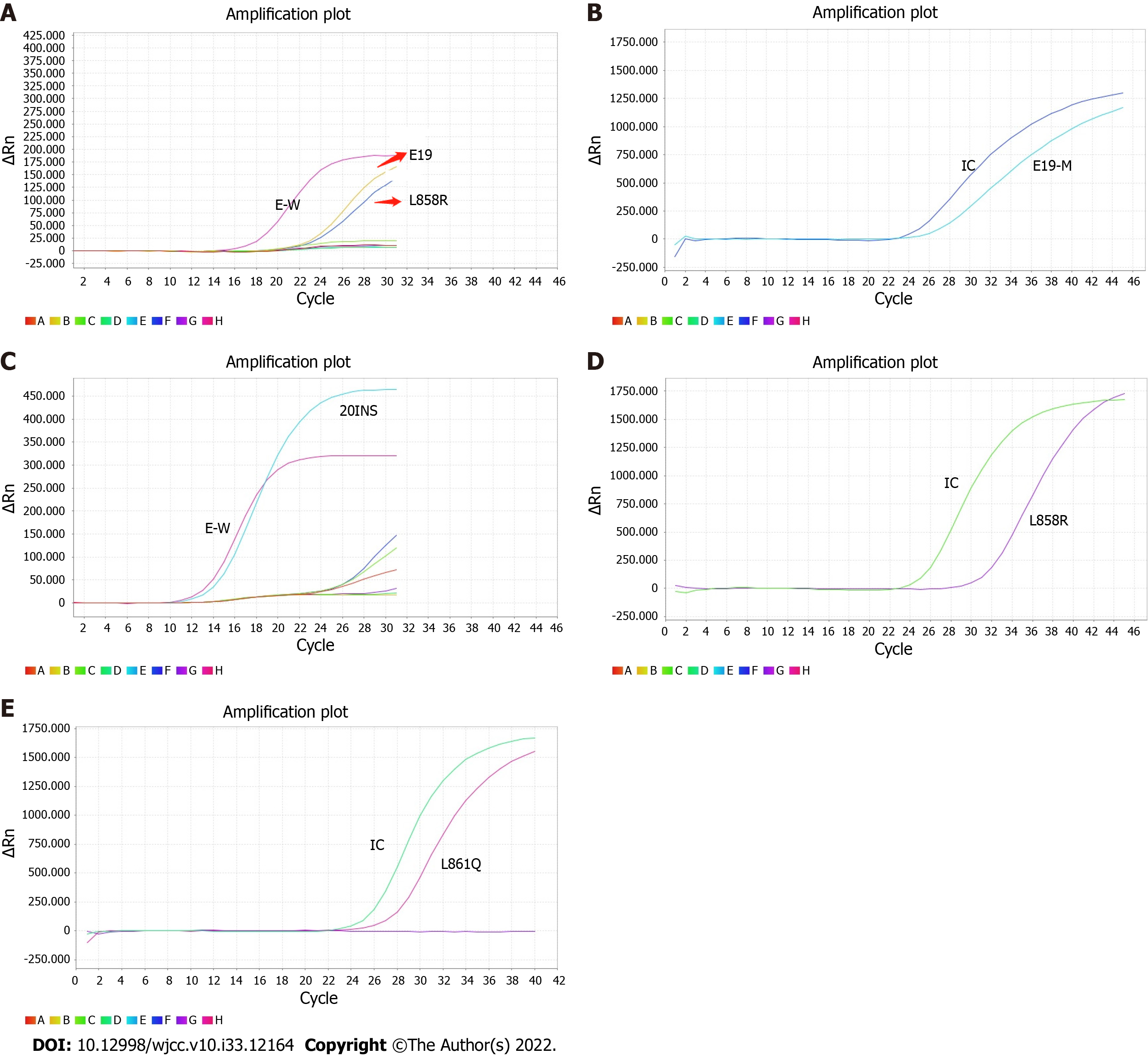
There were six cases of co-mutations of EGFR and ALK genes, and the positive rate was 2.53% (6/237) (Figure 1A-E and Figure 2). One hundred and twenty cases with EGFR mutations were detected, and the mutation rate was 50.63% (120/237); twenty-one cases of ALK rearrangement were expressed, and the expression rate was 8.86% (21/237).
The co-mutations of EGFR and ALK genes in patients with LUAD in Gannan region were more common in women, non-smokers and stage IV patients with bone metastasis. 19del mutation and L858R mutation were the main subtypes of EGFR mutation (Table 1).
| Case | EGFR mutation | Gender | Age (yr) | Smoking history | TNM stage | Common metastatic sites |
| 1# | 19del | Female | 55 | No | IV | Bone |
| 2# | 19del/L858R | Female | 39 | No | IV | Liver, bone, adrenal gland |
| 3# | 20ins | Male | 61 | No | IIIA | None |
| 4# | L858R | Female | 57 | No | IV | Bone |
| 5# | L858R | Female | 73 | No | IV | Bone |
| 6# | L861Q | Female | 53 | No | IV | Bone, brain |
EGFR-TKIs were their preferred drugs for targeted therapy for LUAD patients with co-mutations of EGFR and ALK genes in Gannan region, and the progression-free survival (PFS) ranged from two months to six months (Table 2).
EGFR mutation or ALK rearrangement has become a predictor of therapeutic sensitivity of EGFR-TKIs or ALK-TKIs in NSCLC patients. Accurate detection of EGFR mutation or ALK rearrangement can guide the selection of targeted drugs and ultimately realize individualized therapy. Early study[11] demonstrated that co-mutations of EGFR and ALK genes ware rare in NSCLC, and approximately 94% of ALK rearrangement cases were reported in EGFR wild type NSCLC.
However, a growing number of studies have confirmed that EGFR mutation and ALK rearrangement coexist in NSCLC and are increasingly reported. Wang et al[12] summarized 66 patients with coexistence of EGFR mutation and ALK rearrangement, and displayed that the incidence of co-mutations of EGFR and ALK genes, which were more common in East Asian women, non-smoking and stage IV LUAD, was approximately 1%. In our study, six cases with co-mutations of EGFR and ALK genes were detected, with a higher positive rate of 2.53% (6/237), which may be related to regional heterogeneity. Liu et al[13] found that the frequency of co-mutations of EGFR and ALK genes was as high as 5%, and proposed that more cases with co-mutations of EGFR and ALK genes identified were attributed to the utility of next-generation sequencing (NGS) technology. Our retrospective analysis indicated that co-mutations of EGFR and ALK genes in Gannan region were more common in women, non-smokers and stage IV LUAD with bone metastasis.
Despite the co-mutations of EGFR and ALK genes in NSCLC have been gradually founded, its biological mechanism have not been exhaustively described. There are two hypotheses about the biological mechanism of co-mutations: One is the hypothesis of polyclonal origin, whereby there is heterogeneity between tumours[14], and there may be two tumour cells carrying EGFR mutations or ALK rearrangements in the same tumour tissue; the other is the hypothesis of monoclonal origin[15], wherein tumour cells carry EGFR mutations and ALK rearrangements at the same time.
To the best of our knowledge, targeted therapy is recommended for advanced NSCLC patients with specific oncogenic drivers such as EGFR, ALK. How to select the optimal targeted drugs for NSCLC patients with co-mutations of EGFR and ALK genes? So far, there is no guideline or consensus on the best treatment strategy for patients with coexistence of EGFR mutation and ALK rearrangement. In clinical practice, EGFR-TKIs are the indispensable drugs for first-line treatment of advanced NSCLC patients with concomitant EGFR mutation and ALK rearrangement, and the sequential use of ALK-TKIs is more common after disease progression. Yin et al[16] reviewed the clinical efficacy of EGFR-TKIs and ALK-TKIs in 22 NSCLC patients with EGFR/EML4-ALK co-mutations, and concluded that EGFR-TKIs were the basic treatment for advanced NSCLC patients with concomitant EGFR mutation and ALK rearrangement; Shin et al[17] reported that EGFR-TKIs were more effective than ALK-TKIs in three NSCLC patients with co-mutations of EGFR and ALK genes. Zhao et al[9] found that both EGFR-TKIs and ALK-TKIs were effective in NSCLC patients with co-mutations of EGFR and ALK genes, and sequential use of EGFR-TKIs and crizotinib was a feasible alternative treatment strategy. In contrast, several studies[14,18] shows that NSCLC patients with co-mutations of EGFR and ALK genes have a better response to crizotinib than EGFR-TKIs. A recent case study by Yin et al[16] showed that a female LUAD patient with brain metastases harboured EGFR mutation and ALK rearrangement, and responded to successive osimertinib and alectinib treatment. Surprisingly, alectinib achieved an almost complete response for lung and brain lesions after developing osimertinib resistance, providing a new perspective for the treatment of advanced NSCLC patients with concomitant EGFR mutation and ALK rearrangement. In addition, Mohapatra et al[19] pointed out that, owing to economic stress, clinicians in developing countries preferred to choose EGFR-TKIs as the first-line targeted drugs for NSCLC patients with co-mutations of EGFR and ALK genes. Moreover, Yang et al[20]reports for the first time that EGFR-TKIs-treated patients with EGFR/ALK-L1152R mutations generally had a shorter PFS than patients with other mutation combinations(the mPFS of 4 mo vs 18.2 mo, P < 0.05).
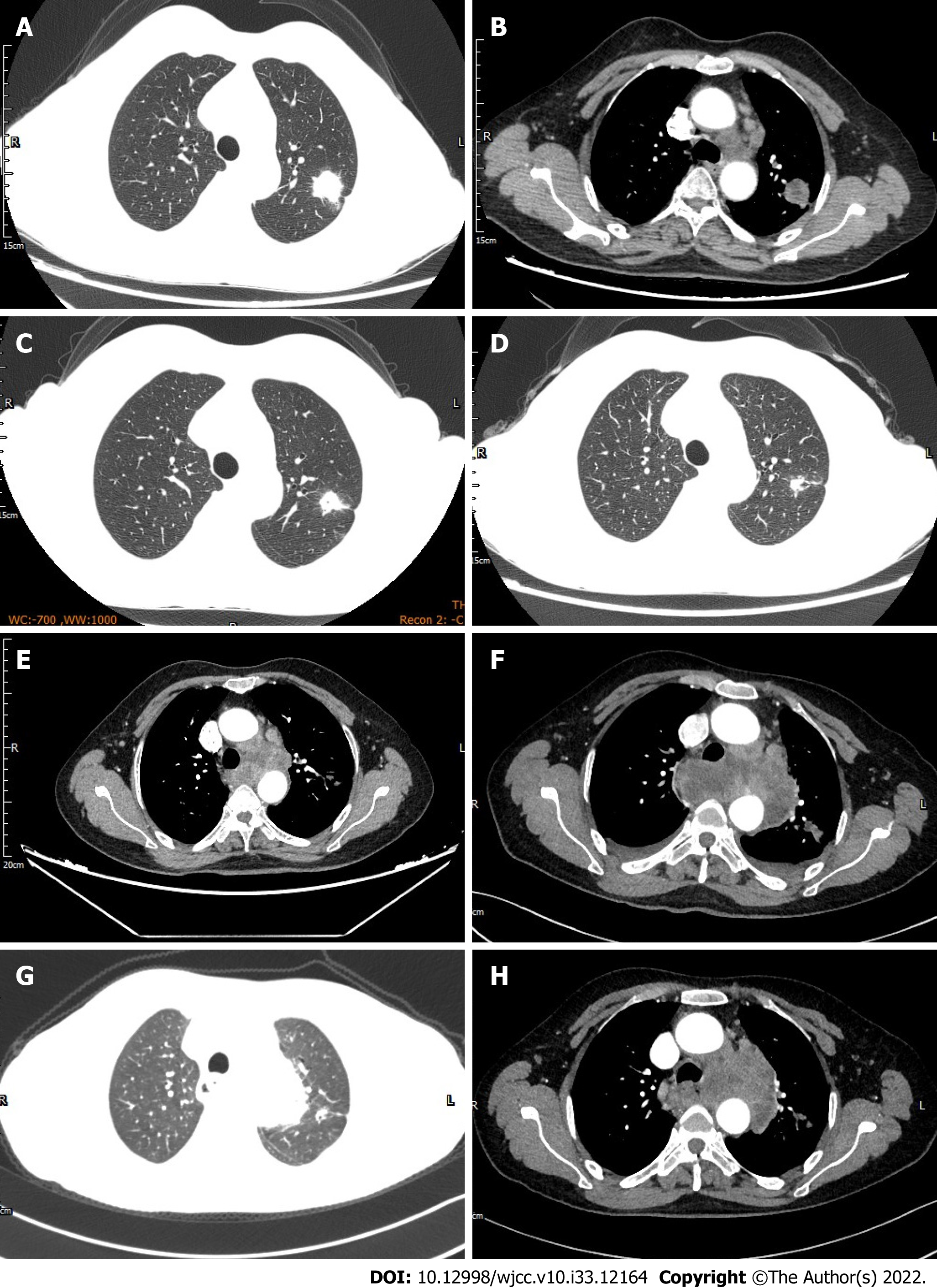
According to the principle of staging treatment of NSCLC, stage I, II and III resectable NSCLC patients preferred surgical treatment; Simultaneous radical radiotherapy and chemotherapy were the main choice for stage III unresectable NSCLC; stage IV NSCLC patients were treated systemically. First-line targeted therapeutic drugs such as gefitinib, erlotinib, and crizotinib are recommended for advanced NSCLC patients with specific oncogene mutations such as EGFR 19del, L858R, and ALK rearrangement. In our study, Follow-up data collected from six LUAD patients with co-mutations of EGFR and ALK genes showed that four cases selected EGFR-TKIs as first-line treatment, one case as second-line treatment and another case as third-line treatment. Here, we focus on the case 6#. The case 6# was a female patient with left upper LUAD (Figure 3A and B) with right hip and left thalamus metastasis. Genetic testing revealed L861Q mutation and ALK rearrangement by real-time PCR. Starting in June 2018, the patient received 150 mg of erlotinib once daily, and further reduction of the left upper lung lesion was thought to be partial response after targeted therapy (Figure 3C and D). After taking erlotinib for 4 mo, her chest CT presented that multiple lymph nodes in left hilum and mediastinum were significantly larger than before, indicating disease progressed (Figure 3E), then she added crizotinib combined with erlotinib for treatment on her own, further enlargement of left hilar and mediastinal lymph nodes (Figure 3F) after 15 d suggesting poor response to targeted therapy. Later, she was treated with bevacizumab combined with pemetrexed combined with carboplatin for 2 courses, subsequent review of chest CT showed that the left upper lung lesion and left hilar and mediastinal lymph nodes shrank (Figure 3G and H), suggesting chemotherapy was effective. Fearing the side effects of chemotherapy, she refused further chemotherapy, and sent the mediastinal lymph nodes biopsy sample for NGS, revealing G719A and L861Q mutations. She then took afatinib orally by herself for targeted therapy, but soon died of massive hemoptysis. In our opinion, although the case 6# received multiline treatment including targeted therapies, chemotherapy, and anti-vascular therapy, and its therapeutic strategy had been adjusted several times in time, the rapid progression of the disease itself and the emergence of new EGFR mutation during targeted therapy ultimately led to poor efficacy of targeted therapy. The retrospective analysis of the follow-up data of six LUAD patients with co-mutations of EGFR and ALK genes showed that EGFR-TKIs were their preferred targeted drugs for first-line targeted therapy, and the PFS ranged between two months and six months, suggesting that the therapeutic effect is not good.
Due to tumour heterogeneity, the optimal treatment of co-mutated NSCLC is still challenging. Tumour heterogeneity, including gene mutation abundance and protein phosphorylation heterogeneity, may be associated with the therapeutic effect of TKIs. Zhou et al[21] pointed out that the baseline abundance values of EGFR mutations may be positively correlated with the efficacy of EGFR-TKIs. Yang et al[22] found that EGFR or ALK protein phosphorylation was associated with the efficacy of EGFR-TKIs or ALK-TKIs. Therefore, patients who are relatively sensitive to EGFR-TKIs have higher levels of EGFR protein phosphorylation and lower levels of ALK protein phosphorylation, and vice versa. Lou et al[23] retrospectively analyzed 11 Asian NSCLC patients with co-mutations of EGFR and EML4-ALK genes, and believed that EGFR-TKIs were a feasible option. The subsequent treatment strategy should be further determined according to ALK rearrangement status and EGFR or ALK protein phosphorylation.
It is well known that EGFR gene and ALK gene have a common signaling pathway. ALK rearrangement may lead to EGFR-TKIs resistance in NSCLC patients with EGFR mutation[23], which is another reason for EGFR-TKIs resistance after KRAS[25]. On the contrary, EGFR mutation and its signal activation are important molecular mechanisms of ALK-TKIs resistance[26,27]. Thus, when tumour cells carry EGFR mutation and ALK rearrangement simultaneously, EGFR-TKIs and ALK-TKIs will eventually acquire drug resistance. Theoretically, EGFR/ALK dual-TKIs may be more effective targeted drugs for NSCLC patients with co-mutations of EGFR and ALK genes, which needs more real cases to confirm.
There are several limitations in this study. First, the cases from a single-Institution enrolled are not large and the collected follow-up data are incomplete, more and more complete cases are needed to further validate our results in multicenter studies in the future. Second, as Shin et al[17] pointed out, since no patient in this study used ALK-TKIs as the first-line therapy, it was not a direct comparison of which targeted drugs are more effective between EGFR-TKIs and ALK-TKIs in NSCLC patients with coexistence of EGFR mutation and ALK rearrangement. Additionally, the specific subtype of ALK rearrangement in this study is unknown, and its effect on TKIs targeted therapy needs to be further evaluated.
In Gannan region, the positive rate of LUAD patients with co-mutations of EGFR and ALK genes is relatively high, and the co-mutations are more common in women, non-smoking and stage IV patients with bone metastasis. These patients prefer EGFR-TKIs as their preferred targeted drugs, but the therapeutic effect is not good. EGFR/ALK dual-TKIs may be more effective targeted drugs, which needs further study.
Epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement are the most two important therapeutic target, which are significantly associated with the sensitivity of EGFR-tyrosine kinase inhibitors (EGFR-TKIs) or ALK-TKIs. At present, EGFR-TKIs and ALK-TKIs are widely used in clinical practice and have exhibited favourable anti-tumour effect in the treatment of non-small cell lung cancer (NSCLC). Accumulating evidences confirm that EGFR mutation and ALK rearrangement have coexisted in lung adenocarcinoma (LUAD). However, its biological mechanism, clinicopathological features, and optimization of targeted drugs have not yet been completely elucidated.
We retrospectively investigated the clinicopathological features and follow-up data of patients with co-mutations of EGFR and ALK genes in LUAD from a single center, aiming to obtain the clinical profile of LUAD patients with co-mutations of EGFR and ALK genes, with intention to scientifically guide the selection of targeted drugs in similar patients, and ultimately achieve individualized precise treatment.
This study aimed to obtain the clinical profile of LUAD patients with co-mutations of EGFR and ALK genes, with hopes of scientifically guiding similar patients towards selected, targeted drugs in similar patients, and ultimately achieve individualized precise treatment.
Two hundred and thirty-seven LUAD patients were enrolled. In the experiment, EGFR mutation was detected using amplification refractory mutation system-peptide nucleic acid, and ALK rearrangement was screened by 5′/3′ imbalance strategy for reverse transcription followed by quantitative polymerase chain reaction. The clinicopathological features of these patients were analysed retrospectively, and the follow-up data were collected.
There were six cases with co-mutations of EGFR and ALK genes, hence a positive rate of 2.53% (6/237), and the co-mutations were more common in women, non-smoking and stage IV LUAD patients with bone metastasis. EGFR-TKIs were their preferred drugs for targeted therapy in these patients, with progression-free survival ranging from two months to six months.
In Gannan region, the positive rate of co-mutations of EGFR and ALK genes in LUAD patients is relatively high, which may be related to regional heterogeneity, and the co-mutations are more common in women, non-smoking and stage IV patients with bone metastasis. These patients prefer EGFR-TKIs as their preferred targeted drugs, but the therapeutic effect is not good. EGFR/ALK dual-TKIs may be more effective targeted drugs.
In this study, the patients with coexistence of EGFR mutation and ALK rearrangement prefer EGFR-TKIs as their preferred targeted drugs, but the therapeutic effect is not good. Theoretically, EGFR/ALK dual-TKIs may be more effective targeted drugs for NSCLC patients with co-mutations of EGFR and ALK genes, which needs more real cases to confirm. Besides, the cases in this study from a single-Institution enrolled are not large and the collected follow-up data are incomplete, more and more complete cases are needed to further validate our results in multicenter studies in the future. Additionally, the specific subtype of ALK rearrangement in this study is unknown, and its effect on TKIs targeted therapy needs to be further evaluated.
The authors thank participants and their families who actively participated in this study; We thank our colleagues from the First Affiliated Hospital of Gannan Medical University for their contribution to the study; The authors also acknowledge Dingjing Medical Laboratory for its strong support and help in the experimental part of this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan; Sezer HF, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC; FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382:41-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1120] [Cited by in F6Publishing: 1409] [Article Influence: 352.3] [Reference Citation Analysis (0)] |
| 2. | Wu L, Zhong W, Li A, Qiu Z, Xie R, Shi H, Lu S. Successful treatment of EGFR T790M-mutant non-small cell lung cancer with almonertinib after osimertinib-induced interstitial lung disease: a case report and literature review. Ann Transl Med. 2021;9:950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, Ho JCM, Ong CK, Tsai CM, Chung CH, Wilner KD, Tang Y, Masters ET, Selaru P, Mok TS. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:1539-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, Yang JJ, Cheng Y, Lee SH, Bu L, Xu T, Yang L, Wang C, Liu T, Morcos PN, Lu Y, Zhang L. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 5. | Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, Thurm H, Calella AM, Peltz G, Solomon BJ; CROWN Trial Investigators. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med. 2020;383:2018-2029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 6. | Gainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ, Iafrate AJ, Arcila ME, Ladanyi M, Engelman JA, Dias-Santagata D, Shaw AT. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273-4281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 448] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 7. | Berradi H, Kaanane H, Kadmiri NE, Nadifi S. Concomitance of EGFR mutations and ALK rearrangement in patients with Lung Cancer. Gene Rep. 2018;11:196-204. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Fan J, Dai X, Wang Z, Huang B, Shi H, Luo D, Zhang J, Cai W, Nie X, Hirsch FR. Concomitant EGFR Mutation and EML4-ALK Rearrangement in Lung Adenocarcinoma Is More Frequent in Multifocal Lesions. Clin Lung Cancer. 2019;20:e517-e530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Zhao Y, Wang S, Zhang B, Qiao R, Xu J, Zhang L, Zhang Y, Han B. Clinical Management of Non-Small Cell Lung Cancer with Concomitant EGFR Mutations and ALK Rearrangements: Efficacy of EGFR Tyrosine Kinase Inhibitors and Crizotinib. Target Oncol. 2019;14:169-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Zhuang X, Zhao C, Li J, Su C, Chen X, Ren S, Li X, Zhou C. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019;8:2858-2866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Tufman A, Kahnert K, Duell T, Kauffmann-Guerrero D, Milger K, Schneider C, Stump J, Syunyaeva Z, Huber RM, Reu S. Frequency and clinical relevance of EGFR mutations and EML4-ALK translocations in octogenarians with non-small cell lung cancer. Onco Targets Ther. 2017;10:5179-5186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wang X, Zhong DS. Research progress of EGFR and ALK gene double mutation in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2018;21:686-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 13. | Liu J, Mu Z, Liu L, Li K, Jiang R, Chen P, Zhou Q, Jin M, Ma Y, Xie Y, Xiang J, Li B, Mao X, Zhang L, Zhang T, Wu D. Frequency, clinical features and differential response to therapy of concurrent ALK/EGFR alterations in Chinese lung cancer patients. Drug Des Devel Ther. 2019;13:1809-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Won JK, Keam B, Koh J, Cho HJ, Jeon YK, Kim TM, Lee SH, Lee DS, Kim DW, Chung DH. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015;26:348-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Max Ma X, Bendell JC, Hurwitz HI, Ju C, Lee JJ, Lovejoy A, Mancao C, Nicholas A, Price R, Sommer N, Tikoo N, Yao L, Yaung SJ, Palma JF. Disease Monitoring Using Post-induction Circulating Tumor DNA Analysis Following First-Line Therapy in Patients with Metastatic Colorectal Cancer. Clin Cancer Res. 2020;26:4010-4017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yin Q, Guo T, Zhou Y, Sun L, Meng M, Ma L, Wang X. Effectiveness of alectinib and osimertinib in a brain metastasized lung adenocarcinoma patient with concurrent EGFR mutations and DCTN1-ALK fusion. Thorac Cancer. 2022;13:637-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Shin HJ, Kho BG, Kim MS, Park HY, Kim TO, Kim YI, Lim SC, Park CK, Kim YC, Choi YD, Oh IJ. Co-alteration of EGFR mutation and ALK rearrangement in non-small cell lung cancer: Case series. Medicine (Baltimore). 2019;98:e14699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Lo Russo G, Imbimbo M, Corrao G, Proto C, Signorelli D, Vitali M, Ganzinelli M, Botta L, Zilembo N, de Braud F, Garassino MC. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget. 2017;8:59889-59900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Mohapatra PR, Sahoo S, Bhuniya S, Panigrahi MK, Majumdar SKD, Mishra P, Patra S. Concomitant echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase rearrangement and epidermal growth factor receptor mutation in non-small cell lung cancer patients from eastern India. J Cancer Res Ther. 2020;16:850-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Yang X, Zhong J, Yu Z, Zhuo M, Zhang M, Chen R, Xia X, Zhao J. Genetic and treatment profiles of patients with concurrent Epidermal Growth Factor Receptor (EGFR) and Anaplastic Lymphoma Kinase (ALK) mutations. BMC Cancer. 2021;21:1107. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 21. | Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang JJ, Xu CR, Yan HH, Chen HJ, Su J, Zhong WZ, Yang XN, An SJ, Wang BC, Huang YS, Wang Z, Wu YL. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:3316-3321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Yang JJ, Zhang XC, Su J, Xu CR, Zhou Q, Tian HX, Xie Z, Chen HJ, Huang YS, Jiang BY, Wang Z, Wang BC, Yang XN, Zhong WZ, Nie Q, Liao RQ, Mok TS, Wu YL. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20:1383-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Lou NN, Zhang XC, Chen HJ, Zhou Q, Yan LX, Xie Z, Su J, Chen ZH, Tu HY, Yan HH, Wang Z, Xu CR, Jiang BY, Wang BC, Bai XY, Zhong WZ, Wu YL, Yang JJ. Clinical outcomes of advanced non-small-cell lung cancer patients with EGFR mutation, ALK rearrangement and EGFR/ALK co-alterations. Oncotarget. 2016;7:65185-65195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17:637-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 577] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 25. | Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27 Suppl 3:iii42-iii50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, Lathan C, Marcoux JP, Du J, Okuda K, Capelletti M, Shimamura T, Ercan D, Stumpfova M, Xiao Y, Weremowicz S, Butaney M, Heon S, Wilner K, Christensen JG, Eck MJ, Wong KK, Lindeman N, Gray NS, Rodig SJ, Jänne PA. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051-6060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 486] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 27. | Kim S, Kim TM, Kim DW, Go H, Keam B, Lee SH, Ku JL, Chung DH, Heo DS. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol. 2013;8:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |