Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.12028
Peer-review started: August 7, 2022
First decision: August 22, 2022
Revised: August 29, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 16, 2022
Primary testicular neuroendocrine tumors (TNETs) are sporadic, accounting for only 0.23% of all testicular tumors. Few cases have been reported in the literature, and no uniform treatment protocol exists. We report a case of a primary TNET with liver lymph node metastasis diagnosed at the age of 24 years and discuss its clinicopathological features, diagnosis, differential diagnosis, treatment, and prognosis.
We report the case of a 24-year-old patient with a primary TNET with liver lymph node metastasis. The patient was found to have a right testicular swelling of about 3 cm × 4 cm in size with unclear borders and no testicular pressure pain seven years ago without any examination or treatment. One month ago, an ultrasound examination was performed for persistent enlargement of the right testis, which showed an occupying lesion of the right testis approximately 110 mm × 102 mm × 82 mm in size. Magnetic resonance imaging scan of the testis (plain scan) showed that the right testis was an occupying lesion with inhomogeneous density and mixed signal, the boundary was still clear, and the possibility of seminoma was considered; chest X-ray and computed tomography did not show any apparent abnormalities. The patient underwent radical orchiectomy, and the pathological examination suggested a right TNET with a typical carcinoid tumor histological type. One month after the surgery, the patient received nine cycles of lanreotide chemotherapy at a dose of 90 mg/mo without adverse effects. No distant lymph node or other organ metastases were detected at follow-up. He is in good physical condition and attends regular follow-up visits.
Neuroendocrine tumors are rare in clinical practice, and the diagnosis mainly relies on the characteristics of microscopic tumor cells and immunohistochemical features. Treatment involves radical orchiectomy. If it is accompanied by distant lymph node metastasis and the metastatic lesion can be resected, it should be surgically removed; if it cannot be resected, growth inhibitor analog octreotide or lanreotide chemotherapy can be administered to obtain good results, with close postoperative follow-up to prevent recurrence and metastasis.
Core Tip: Neuroendocrine tumors are rare in the clinic, and the diagnosis mainly depends on the characteristics of tumor cells and immunohistochemistry. Radical orchiectomy is the main treatment. If it is accompanied by distant lymph node metastasis and the metastatic lesion can be resected, it should be removed. If it cannot be resected, it can be treated with somatostatin analog octreotide or lanreotide chemotherapy. Good results can be obtained. Close follow-up can be conducted to prevent recurrence and metastasis.
- Citation: Xiao T, Luo LH, Guo LF, Wang LQ, Feng L. Primary testicular neuroendocrine tumor with liver lymph node metastasis: A case report and review of the literature. World J Clin Cases 2022; 10(32): 12028-12035
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/12028.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.12028
Neuroendocrine tumors are composed of a series of malignant tumors arising from neuroendocrine cells throughout the body, which are often referred to as carcinoid tumors and are characterized by the ability to produce peptides that lead to typical hormonal syndromes[1]. Neuroendocrine tumors occur mainly in the gastrointestinal tract (85%), ileum, and appendix, as well as in other sites, including the lung, pancreas, biliary tract, thymus, ovaries, and, rarely, the testis[2]. Testicular neuroendocrine tumors (TNETs) account for less than 1% of all testicular tumors[3]. We report a case of a primary TNET and discuss the clinicopathological features, diagnosis, differential diagnosis, treatment, and prognosis of this rare tumor.
A 24-year-old male presented with a 7-year history of painless progressive enlargement of the right testicle.
This 24-year-old male patient found a right testicular mass, hard and indistinctly demarcated from the testicle, about 3 cm × 4 cm in size, without pain, without further examination and treatment seven years ago during physical examination. Due to the gradual increase of the swelling, he was seen at Jiangxi Provincial People's Hospital on September 15, 2021, and an ultrasound examination suggested that the right testicle was an occupying lesion approximately 110 mm × 102 mm × 82 mm in size, which was considered to be seminoma.
The right testicle was enlarged and more challenging in texture, with indistinct testicular epididymis demarcation. No enlarged lymph nodes were palpated in the groin.
Levels of the following parameters were observed: Alpha-fetoprotein: 3.09 ng/mL; human chorionic gonadotropin: < 0.20 mIU/mL; lactate dehydrogenase: 176.9 U/L; hemoglobin: 125 g/L; red blood cells: 4.24 × 1012; mean platelet volume: 11.10 fl; luteinizing hormone: 13.10 mIU/mL; follicle stimulating hormone: 15.40 mIU/mL; estradiol: 45.90 pg/mL; pituitary prolactin: 30.50 ng/mL; testosterone: 279.00 ng/dL; albumin: 38.2 g/L (Table 1).
| Laboratory tests | Examination value | Reference value |
| RBC | 4.24 × 1012 | 4.30-5.80 × 1012 |
| HB | 125 g/L | 130-175 g/L |
| MPV | 11.10 fL | 125-350 × 109/L |
| LDH | 176.90 U/L | 120-250 U/L |
| HCG | < 0.20 mIU/mL | — |
| AFP | 3.09 ng/ml | 0-7.0 ng/mL |
| LH | 13.10 mIU/mL | ≤ 8.6 mIU/mL |
| FSH | 15.40 mIU/mL | ≤ 12.4 mIU/mL |
| E | 45.90 pg/mL | 0.0-36.5 pg/mL |
| PRL | 30.50 ng/mL | 2.1-17.7 ng/mL |
| T | 279.00 ng/dL | 241-827 ng/dL |
| ALB | 38.2 g/L | 40-55 g/L |
Pre-admission: September 16, 2021. Jiangxi Provincial People's Hospital ultrasound showed an occupying lesion in the right testicle, approximately 110 mm × 102 mm × 82 mm in size, and varicose veins in the right spermatic cord.
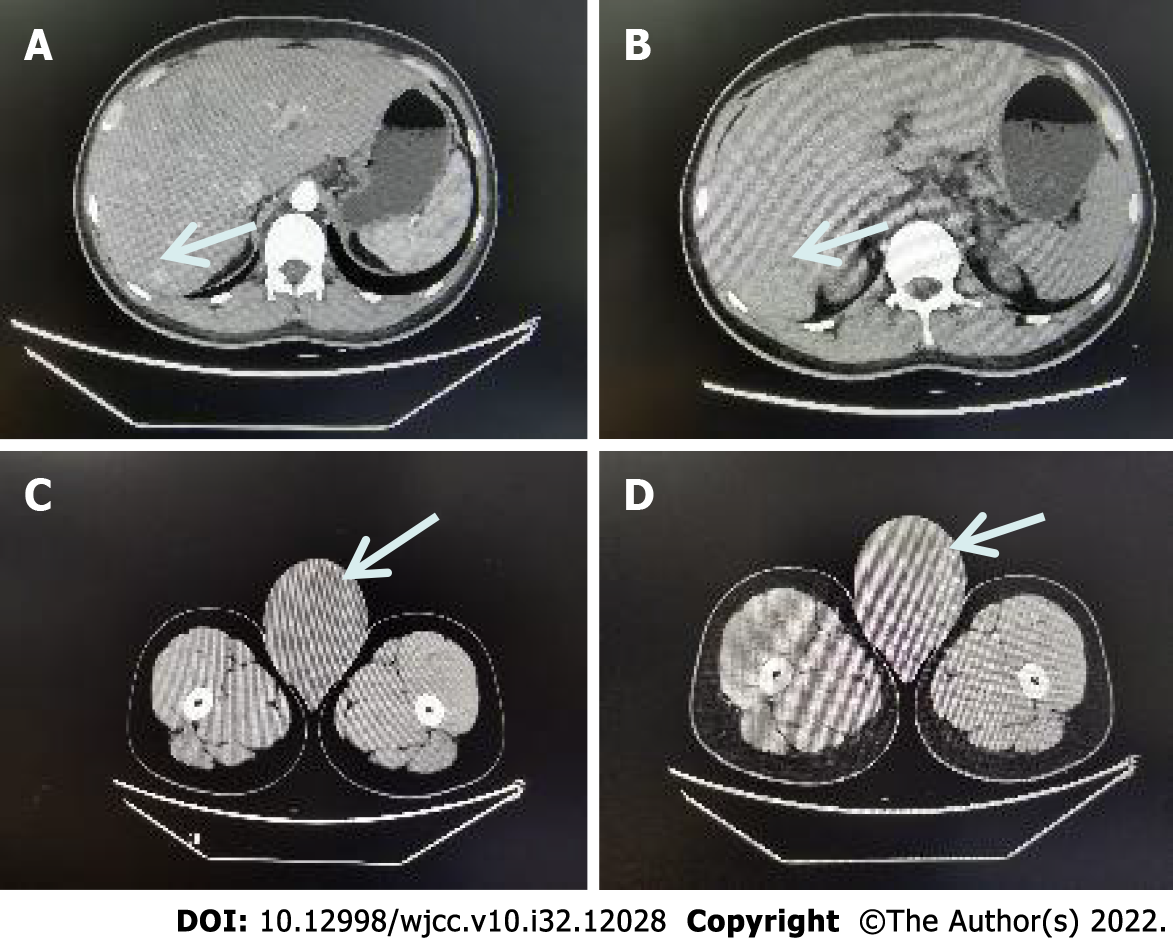
After-admission: September 16, 2021. Computed tomography (CT) showed that the right testis was significantly enlarged, measuring about 8.1 cm × 9.9 cm, with mild to moderate heterogeneous enhancement on the enhanced scan, malignancy was considered, with uneven enhancement of the liver parenchyma, and multiple abnormally enhanced nodules in the right lobe of the liver, with metastasis not excluded (Figure 1).
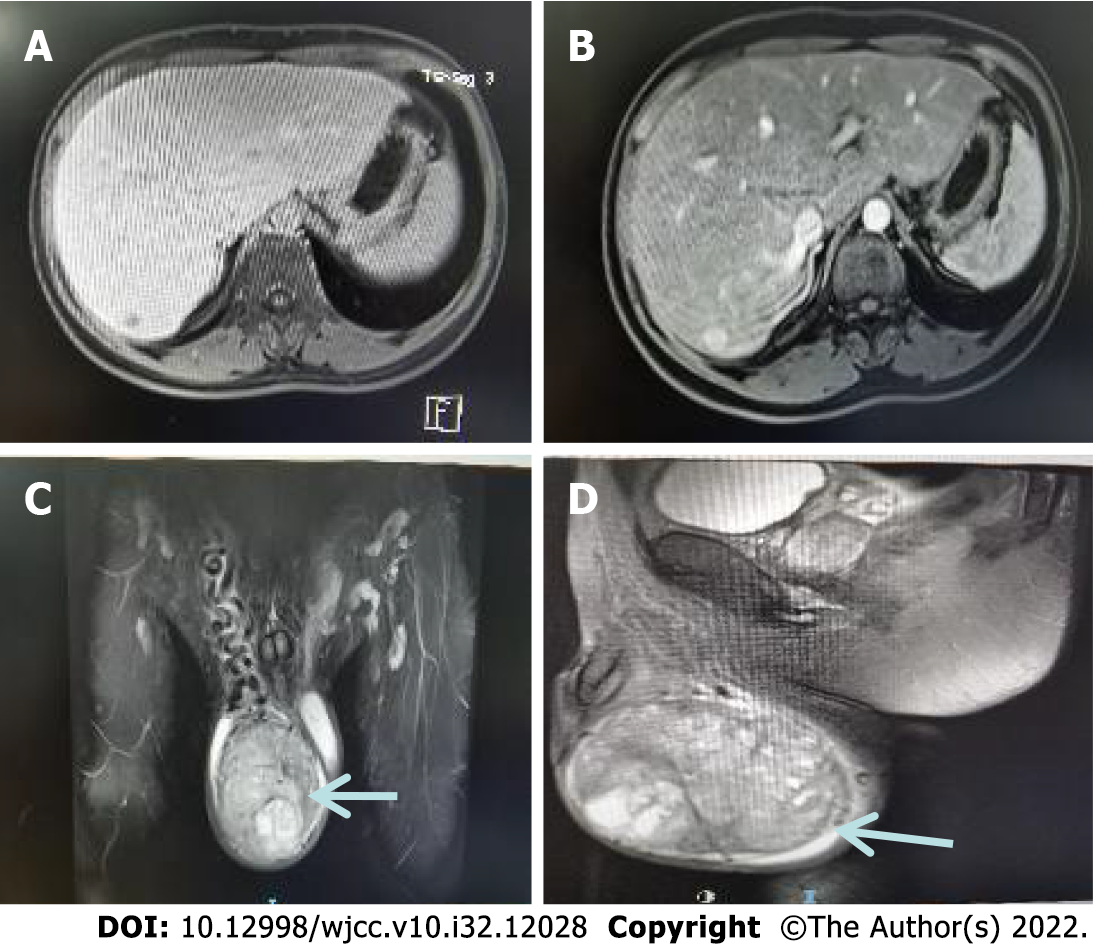
September 16, 2021. Magnetic resonance scan of the testis (plain scan) showed an occupying lesion in the right testis with an inhomogeneous density and mixed-signal with clear borders. A seminomatous cell tumor was considered a high probability (Figure 2).
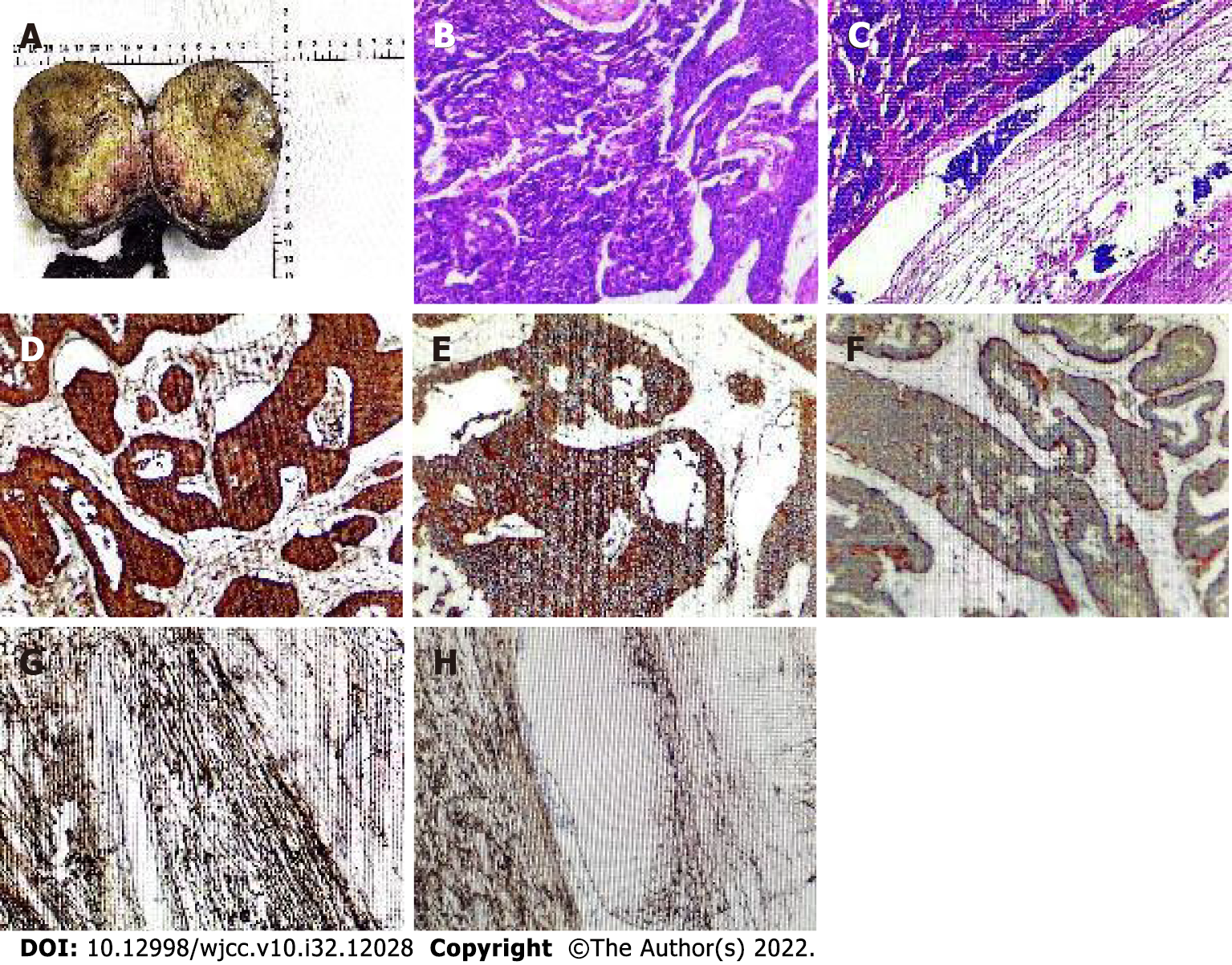
Postoperative pathology showed that the resected specimen consisted of the right testis, tumor, spermatic cord, and epididymis. The incisional surface of the right testis showed a firm, grayish-yellow solid mass measuring 10 cm × 7.5 cm × 9.5 cm with focal calcification and no hemorrhage or necrosis. The right epididymis measured 12.5 cm in length and 1.5 cm in ductal diameter, and its portion was yellowish white, solid, soft, and without tumor. Microscopic findings: The tumor was irregularly adenoid, nest-like, with a sieve-like structure, contracted fissures were seen around the nest, cancer cells were abundant in the envelope, red staining, consistent in size and shape, round and oval nuclei, finely granular chromatin, abundant interstitial vessels, vitreous changes, nuclear fission was not easily seen < 2 per 10 HPF. Immunohistochemistry showed: "A03 "CK (3+); CK8 (3+); CD56 (3+); CgA (3+); Syn (3+); Ki-67 (3%+); EMA (-); Vimentin (-); SALL4 (-); CD117 (-); PLAP (-); CD30 (-); Gly-3 (-); OCT-4 (-); Hep (-); CR (-); a-inhibin (-); CD34 (-); D2-40 (-), and (right testis) neuroendocrine tumor was considered (Figure 3).
Right radical orchiectomy was performed in September 2021; the patient recovered well and was discharged 7 d after surgery. Regular chemotherapy with the growth inhibitor analog lanreotide was started one month after surgery, and nine chemotherapy sessions have been given so far.
The patient was reviewed on July 20, 2022, and the CT scan showed no distant lymph node metastases (follow-up CT image is missing); he was in good health and was attending regular follow-up visits.
Neuroendocrine tumors (NETs) were first described by Langhans in 1887[4]. In 1954, Simon et al[5] reported the first case of primary testicular carcinoid tumor. Primary TNETs are extremely rare, accounting for only 0.23% of all testicular tumors[6]. Patients with TNETs are usually between 20 and 90 years, with a mean presentation age of 46 years[7,8]. According to the latest 2016 World Health Organization (WHO) testicular tumor classification system, TNETs are germ cell tumors unrelated to in situ germ cell neoplasia[9]. TNETs can be divided into three subgroups: Pure primary TNETs, primary TNETs associated with teratomas, and secondary NETs[10]. Amine et al[11] reported the clinical characteristics of 132 cases with TNETs reported from 1930 to February 2015, with patients' ages ranging from 10 to 83 years (mean, 39 years). The distribution of tumor types, sizes, and immunohistochemical findings are shown in Tables 2 and 3.
| Tumor type | Number of cases | Rate | Size (mm) | mean size (mm) |
| Pure primary TNET | 101 | 76.52% | 3-100 | 41.75 |
| TNET associated teratoma | 22 | 16.67% | 10-80 | 36.91 |
| Secondary TNET | 9 | 6.82% | 5-60 | 22.13 |
| Primary TNET associated with metastases | — | — | 5-100 | 52.70 |
| Marker | Number of patients | Positive cases | Rate |
| Cytokeratin | 29 | 27 | 93.10% |
| Chromogranin | 59 | 59 | 100% |
| Synaptophysin | 45 | 45 | 100% |
| PLAP | 20 | 0 | 0 |
| Serotonin | 15 | 13 | 86.67% |
| NSE | 14 | 14 | 100% |
| p53 | 11 | 0 | 0 |
| CD56 | 7 | 7 | 100% |
| Vimentin | 6 | 5 | 83.30% |
| Inhibin | 4 | 0 | 0 |
| TTF 1 | 3 | 1 | 33.33% |
| AFP | 2 | 0 | 0 |
NETs are heterogeneous tumors originating from peptidergic neurons and neuroendocrine cells, distributed in different tissues and organs, and occurring in two main categories: Neuroendocrine organs, such as the pituitary gland, thymus, and adrenal gland. The other category is non-neuroendocrine organs, such as the gastrointestinal tract, pancreas, lung, genitourinary system, etc[12]. Currently, there is no classification method for TNETs, and the clinical classification is mainly based on the 2019 WHO Gastrointestinal Tumor Classification System[13-16]. According to the morphological characteristics and biological behavior of this tumor, NETs are classified into two types: (1) NETs, including NET grades 1, 2, and 3; and (2) neuroendocrine carcinomas (NECs), including small and large cell carcinomas and mixed neuroendocrine-non-neuroendocrine tumors; in addition, based on the nuclear schwannoma count and/or Ki-67 index, NETs are histologically classified into three grades, namely low (G1, with < 2 nuclear schwannomas/10 HPF and Ki-67 < 3%), intermediate (G2, with 2–20 nuclear schwannomas/10 HPF and Ki-67 3%–20%) and high (G3, with > 20 nuclear schwannomas/10 HPF and Ki-67 > 20%) grades. Secondary NEC is usually metastasized from lung or gastrointestinal NEC to the testis[17].
Studies have shown that most testicular carcinoid tumors are NETs originating from Kulchitsky cells in the embryonic primitive intestinal mucosa[18]. The main clinical manifestation of these tumors is painless testicular swelling or masses, and some patients may experience testicular pressure pain. TNET cells secrete biologically active substances such as 5-hydroxytryptamine, histamine, and prostaglandins. These substances are inactivated in the liver and lungs through blood circulation; some cannot be inactivated. As a result, about 10% of patients show symptoms of flushed skin, diarrhea, asthma, and heart damage (carcinoid syndrome)[19]. Primary TNETs rarely cause carcinoid syndrome or metastasis. In the present case, the primary TNET was accompanied by liver lymph node metastasis with < 2 nuclear schwannomas/10 HPF. The rest of the body tissues, organs, and lymph nodes showed no lesions or metastases and no neuroendocrine syndrome. Therefore, the diagnosis of primary TNET (G1) with liver lymph node metastasis was supported by clinical, imaging, and histopathological findings.
Primary NETs (G1) are diagnosed based on clinical, ultrasound, histopathological, and immunohistochemical findings[12]. TNET should be differentiated from testicular teratomas combined with carcinoid tumors, metastatic carcinoid tumors, supportive cell tumors, and seminomas. (1) Teratoma combined with carcinoid tumor: The mass has typical sarcoid features of teratomas, and the microscopic composition of tumor tissue is complex, with three germinal teratoma components and carcinoid components; (2) Metastatic carcinoid tumor: It often involves the bilateral testes, with a multifocal pattern, lymphatic vessels, and vascular infiltration, and there is a primary tumor outside the testes; (3) Supporting cell tumor: Tumor cells are arranged in striated, solid nests, solid tubular or sieve-like shapes, surrounded by an encapsulated basement membrane, with dense fibrous mesenchyme often accompanied by hyaline degeneration. Immunophenotype: Tumor cells express α-inhibin and vimentin but are negative for cytokeratin, synaptophysin, chromogranin A, and neuron-specific enolase; and (4) Seminoblastoma: This tumor consists of diffusely uniform, large, well-defined round tumor cells separated by slender fibers into sheets and cords, with interstitial infiltration of lymphocytes. Differential diagnosis was not difficult when supplemented with CD117 and placental alkaline phosphatase immunostaining[20-23].
Most TNET cases are reported to be moderate-to-low malignant tumors; therefore, radical surgical resection is recommended with long-term postoperative follow-up[24]. Some studies have indicated that the choice of surgical approach is based on the tumor size, lymph node presence, and distant metastases. Radical orchiectomy is recommended for low-intermediate grade primary TNET patients with good results, and close postoperative follow-up is required to prevent recurrence and metastasis[25]. Surgical resection combined with postoperative radiotherapy and chemotherapy is currently the treatment for TNETs. The extent and mode of resection depend on the nature, size, and location of the tumor, the depth of tumor infiltration, whether there are lymph node metastases, etc[26,27]. Chemotherapy (cisplatin and etoposide, ifosfamide, epirubicin, and octreotide) or radiotherapy improves survival in patients with primary TNETs with lymph node and lung metastases[28]. It has been shown that growth inhibitor analogs, such as octreotide and lanreotide, have antiproliferative effects on both primary and metastatic tumors in the case of NETs[29]. Octreotide is a growth inhibitor analog that inhibits the release of hormones and neurotransmitters, causing symptomatic improvement in approximately 80% of patients[30]. In contrast, patients with carcinoid syndrome have a poor prognosis[31]. If the metastatic lesion is resectable, surgery should be performed; if it is non-resectable, a trial of octreotide therapy can be performed due to its antiproliferative and anti-hormonal properties. Our patient underwent radical orchiectomy and radiofrequency ablation of the liver lymph nodes 1 mo after surgery and received nine cycles of chemotherapy with lanreotide at a dose of 90 mg/mo after radiofrequency ablation treatment. No distant lymph nodes or other organ metastases have been detected at follow-up. He is in a good physical condition and attends regular follow-up visits.
In conclusion, TNETs are clinically rare, the clinical manifestations are not specific, and the diagnosis mainly relies on the characteristics of microscopic tumor cells and immunohistochemical features. The diagnosis primarily depends on microscopic tumor cell characteristics and immunohistochemical features. Once the diagnosis of a neuroendocrine tumor is made, it is necessary to exclude metastatic cancer or the presence of metastatic foci as approximately 10% of testicular tumors may be metastatic, so it is essential to exclude metastatic TNETs. Radical orchiectomy is advocated for patients with low-intermediate grade primary TNETs. In addition, distant lymph node metastases, and metastatic lesions can be resected. If they cannot be resected, growth inhibitor analogs octreotide or lanreotide chemotherapy can be administered to obtain good results, with close postoperative follow-up to prevent recurrence and metastasis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Limaiem F, Tunisia; Stabellini N, Brazil S-Editor: Liu GL L-Editor: Webster JR P-Editor: Liu GL
| 1. | Munshi SA, Saada H, Mujtaba S, Elkoushy MA. Primary testicular neuroendocrine tumor with azoospermia: Extending indications for testicle-sparing surgery. Urol Case Rep. 2019;23: 78-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4:526-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Mai KT, Park PC, Yazdi HM, Carlier M. Leydig cell origin of testicular carcinoid tumour: immunohistochemical and electron microscopic evidence. Histopathology. 2006;49:548-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Langhans T. Ueber einen Drüsenpolyp im Ileum. Virchows Arch. 1887;38:559-600. [Cited in This Article: ] |
| 5. | Simon HB, Mcdonald JR, Culp OS. Argentaffin tumor (carcinoid) occurring in a benign cystic teratoma of the testicle. J Urol. 1954;72:892-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Berdjis CC, Mostofi FK. Carcinoid tumors of the testis. J Urol. 1977;118:777-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kato N, Motoyama T, Kameda N, Hiruta N, Emura I, Hasegawa G, Murata T, Kimura M, Tsuda H, Ishihara T. Primary carcinoid tumor of the testis: Immunohistochemical, ultrastructural and FISH analysis with review of the literature. Pathol Int. 2003;53:680-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Reyes A, Moran CA, Suster S, Michal M, Dominguez H. Neuroendocrine carcinomas (carcinoid tumor) of the testis. A clinicopathologic and immunohistochemical study of ten cases. Am J Clin Pathol. 2003;120:182-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 9. | Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1478] [Cited by in F6Publishing: 1814] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 10. | Stroosma OB, Delaere KP. Carcinoid tumours of the testis. BJU Int. 2008;101:1101-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 11. | Amine MM, Mohamed B, Mourad H, Majed H, Slim C, Mehdi B, Hela M, Nouri R, Rim K, Tahya B, Nabil MM. Neuroendocrine Testicular Tumors: A Systematic Review and Meta-Analysis. Curr Urol. 2017;10:15-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Grozinsky-Glasberg S, Lines KE, Avniel-Polak S, Bountra C, Thakker RV. Preclinical drug studies in MEN1-related neuroendocrine neoplasms (MEN1-NENs). Endocr Relat Cancer. 2020;27:R345-R355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bordi C, Yu JY, Baggi MT, Davoli C, Pilato FP, Baruzzi G, Gardini G, Zamboni G, Franzin G, Papotti M. Gastric carcinoids and their precursor lesions. A histologic and immunohistochemical study of 23 cases. Cancer. 1991;67:663-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 14. | Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Chinese Expert Group on Pathology of Gastroenteropancreatic Neuroendocrine Tumors. Chinese Consensus on Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors. honghua Binglixue Zazhi. 2011;40:6. [DOI] [Cited in This Article: ] |
| 16. | Chen H, Chen Y. Consensus and controversy of endoscopic diagnosis and treatment of gastroenteropancreatic neuroendocrine tumors. Zhonghua Weichang Waike Zazhi. 2017;20:982-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 17. | Rathert M, Ubrig B, Atkins DJ, Roth S. [Carcinoid tumor of the testis]. Urologe A. 2011;50:340-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Wang WP, Guo C, Berney DM, Ulbright TM, Hansel DE, Shen R, Ali T, Epstein JI. Primary carcinoid tumors of the testis: a clinicopathologic study of 29 cases. Am J Surg Pathol. 2010;34:519-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Son HY, Ra SW, Jeong JO, Koh EH, Lee HI, Koh JM, Kim WB, Park JY, Shong YK, Lee KU, Kim GS, Kim MS. Primary carcinoid tumor of the bilateral testis associated with carcinoid syndrome. Int J Urol. 2004;11:1041-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Li H, Zheng JF, Liu Y. A case of primary neuroendocrine tumor of the testis and review of the literature. Linchuang Yu Shiyan Binglixue Zazhi. 2013;29:1255-1257. [Cited in This Article: ] |
| 21. | Feng LC, Chen GH. A case of testicular metastasis from nasopharyngeal carcinoma. INE ISTIC PKU CSCD. 1992;27:c7. [DOI] [Cited in This Article: ] |
| 22. | Rong S, Mao QZ, Ji ZG, Zhang YS, Shi BB. Report of three cases of testicular supporting cell tumor and review of the literature. Beijing Yixue. 2013;37:941-943. [DOI] [Cited in This Article: ] |
| 23. | Liu FF, Zheng JF, Zhou LT, Wang CC, Wang JJ, Shen Q, Yu B, Ma HH, Wang JD, Shi QL. Clinicopathological study of primary testicular neuroendocrine tumors (report of 7 cases). Zhonghua Nankexue Zazhi. 2014;1:63-67. [Cited in This Article: ] |
| 24. | Epperson JR, Pope NM, Abuzeid MJ. Rare testicular tumor discovered by assault: an unusual presentation of a primary testicular neuroendocrine tumor grade 2. Case Rep Pathol. 2013;2013:709352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Wu HF, Li GF, Liu XL, Wang J, Wu CH, Li GY. Clinical and pathological analysis of primary testicular neuroendocrine tumors. Ningxia Yike Daxue Xuebao. 2014;4. [Cited in This Article: ] |
| 26. | Pectasides D, Glotsos J, Bountouroglou NG, Dadioti PA, Athanassiou AE. Primary carcinoid of the testis with metastases. Case report and review of the literature. J BUON. 2002;7:153-156. [PubMed] [Cited in This Article: ] |
| 27. | Neely D, Gray S. Primary carcinoid tumour of the testis. Ulster Med J. 2011;80:79-81. [PubMed] [Cited in This Article: ] |
| 28. | Sasaki M, Emura M, Kim U, Shinbo M, Shima T, Suzuki N, Tomioka S, Tanaka M, Nakatsu H, Murakami S, Suzuki Y. Primary carcinoid tumor of the testis metastatic to the para-aortic lymph nodes in six years after the first operation: a case report. Hinyokika Kiyo. 2009;55:233-236. [PubMed] [Cited in This Article: ] |
| 29. | Penke M. Primary neuroendocrine tumor of the testis and osseous, cardiac, and lymph node metastases in a young patient. Case Rep Oncol. 2014;7:815-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Zacharias DG, Jensen MH, Farley DR. Long-term survival with metastatic carcinoid tumors: a case report and review of the literature. J Surg Educ. 2010;67:99-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Lubana SS, Singh N, Chan HC, Heimann D. Primary neuroendocrine tumor (carcinoid tumor) of the testis: a case report with review of literature. Am J Case Rep. 2015;16:328-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |