Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11974
Peer-review started: July 18, 2022
First decision: September 5, 2022
Revised: September 8, 2022
Accepted: October 18, 2022
Article in press: October 18, 2022
Published online: November 16, 2022
Although gastric cancer is one of the most prevalent cancers worldwide, cases of gastric cancer metastasis to the male reproductive system are rare. Here, we report a case involving testicular and epididymal gastric cancer metastases.
A 75-year-old Chinese man complained of experiencing a palpable painful mass in the right scrotum for 6 mo. He had undergone distal gastrectomy with chemotherapy for pT3N3aMx poorly differentiated gastric adenocarcinoma 9 mo before. Physical examination revealed a moderate right hydrocele and a painful mass in the right testis and epididymis. Serum tumor biomarkers were all normal except for elevated beta-human chorionic gonadotropin levels. Computed tomography urography and B-ultrasound imaging revealed a moderate right hydrocele and a mixed solid-cystic mass in the right testicular and epididymal area. Thus, the patient underwent right radical orchiectomy. Immunohistochemical analysis revealed that the tumor cells were positive for pancytokeratins and caudal related homeodomain transcription 2. Metastatic, poorly differentiated gastric adenocarcinoma of the testis and epididymis was confirmed by pathology. He continued to undergo chemotherapy at the department of oncology of our hospital. Mesenteric lymph node metastases were found at the postoperative 1-mo follow-up.
Palpable, painful scrotal mass, history of gastric cancer, and imaging features may indicate testicular and epididymal metastatic gastric cancer.
Core Tip: Cases of gastric cancer metastasis to the male reproductive system, especially both the testis and epididymis, are rare. Here, we report a 75-year-old Chinese man with testicular and epididymal gastric cancer metastases. Palpable, painful scrotal mass, history of gastric cancer, and imaging features may indicate testicular and epididymal metastatic gastric cancer.
- Citation: Ji JJ, Guan FJ, Yao Y, Sun LJ, Zhang GM. Testis and epididymis–unusual sites of metastatic gastric cancer: A case report and review of the literature. World J Clin Cases 2022; 10(32): 11974-11979
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11974.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11974
In 2020, gastric cancer was the 5th most commonly diagnosed cancer and the 4th leading cause of cancer deaths worldwide[1]. Because gastric cancer is often advanced and metastatic by the time that symptoms like dyspepsia, abdominal pain, and weight loss have appeared[2], its prognosis is poor. Gastric cancer most commonly metastasizes to the liver, peritoneum, lung, and bone[3]. Gastric cancer metastases to the male reproductive system are very rare and such metastases are often diagnosed as primary tumors. Here, we present an unusual case of pathologically confirmed testicular and epididymal gastric adenocarcinoma metastases after primary tumor treatment using surgery and chemotherapy.
A 75-year-old Chinese man visited the department of urology of The Affiliated Hospital of Qingdao University in March 2022 complaining of experiencing a palpable painful mass in the right scrotum for 6 mo.
The patient had received subtotal gastrectomy with Roux-en-Y gastrojejunostomy for gastric cancer more than 9 mo before. Histopathological analysis revealed mixed, poorly differentiated adenocarcinoma of the gastric corpus invading the serosa and lymph node metastasis of the lesser gastric curvature (5/8) (pT3N3aMx). Immunohistochemical examination showed that the neoplastic cells were positive for EGFR, MLH1, MSH2, MSH6, PMS2, PD-L1 (22C3) (CPS:5), and Ki-67 (60%) and negative for HER2 and alpha-fetoprotein (AFP). Interstitial vascular cancer embolus was revealed by positive CD31 and D2-40 staining. Neural invasion and vascular invasion were also observed by positive S100 and elastic fiber staining. The patient underwent six cycles of oxaliplatin-based chemotherapy.
The patient had a history of type 2 diabetes for over 20 years, and his blood glucose was well controlled. He also had a history of juvenile orchitis and had undergone vasoligature more than 10 years before.
The patient had no remarkable personal or family history.
Physical examination revealed hardness and haphalgesia in the right testis, a painful induration in the right epididymis, and a moderate hydrocele on the right testis.
Laboratory tests revealed elevated serum levels of beta-human chorionic gonadotropin (β-HCG) (12.25 IU/L) and normal serum levels of AFP (2.49 ng/mL), prostate alkaline phosphatase (1.83 ng/mL), total prostatic-specific antigen (TPSA) (0.648 ng/mL), free prostatic-specific antigen (FPSA) (0.35 ng/mL), and FPSA/TPSA (0.54).
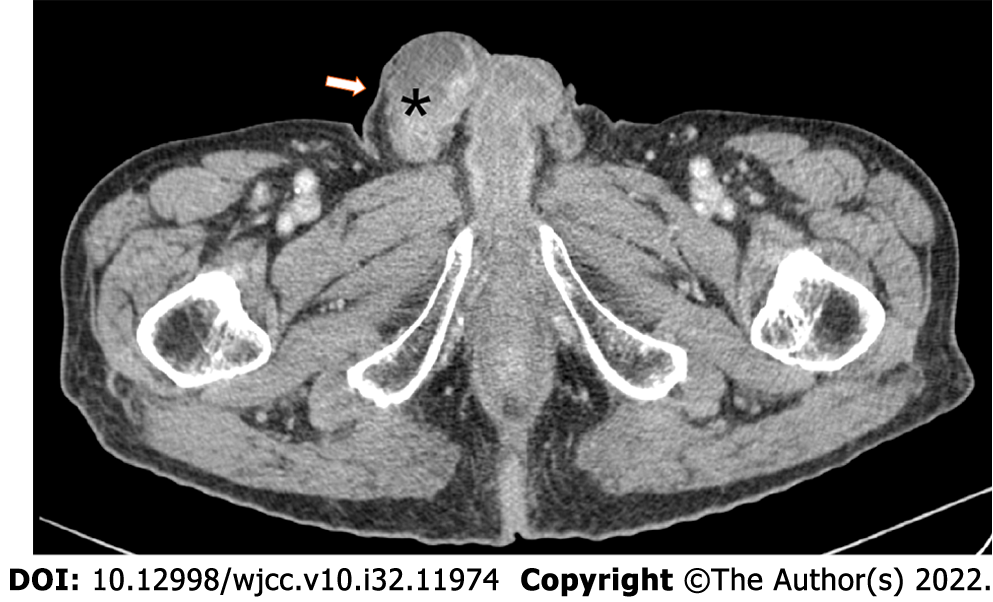
Urinary and scrotal B-ultrasound examination revealed that the right testis was abnormally shaped, with an uneven echo, a 2.3 cm × 1.9 cm hypoechoic mass in the caudal epididymis, and a hydrocele (2.2 cm deep) on the right testis. Computed tomography urography (CTU) revealed a mixed solid-cystic mass in the right testicular and epididymal area (Figure 1).
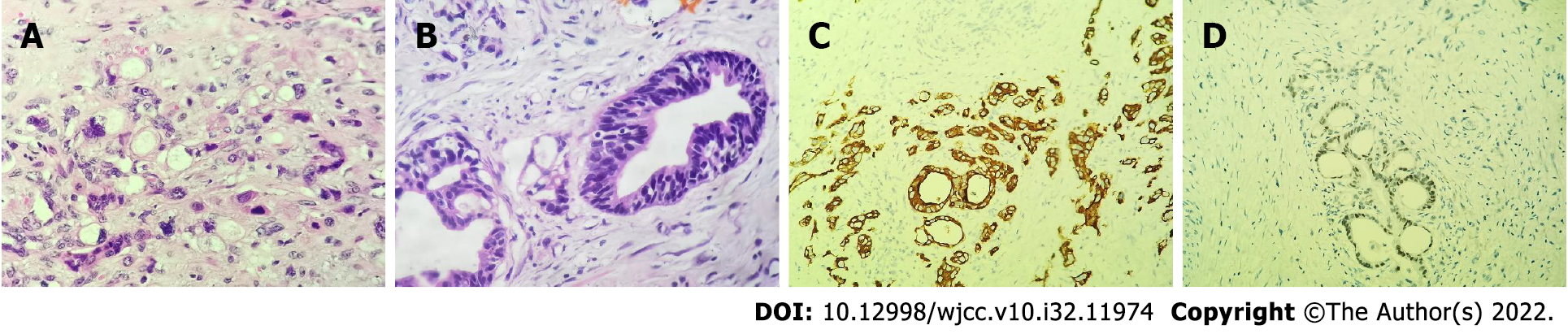
The patient underwent right radical orchiectomy on March 16, 2022. Macroscopically, the incisal surface on the right testis (5 cm × 3 cm × 3 cm) contained an off-white solid tumor with a maximum diameter of 3.6 cm. The incisal surface of the right epididymis (5 cm × 2 cm) also had off-white tumor tissue. Histomorphological analysis of the testicular and epididymal tissue revealed the presence of poorly differentiated adenocarcinoma (Figure 2A and B). Immunohistochemical analysis showed that the tumor cells were positive for Ki-67 (30%), pancytokeratins, and caudal related homeodomain transcription 2 (focal) (Figure 2C and D), but were nonreactive for CD117, PLAP, SALL4, OCT4, CD30, CK7, and CK20.
Based on the patient’s medical history, he was finally diagnosed with testicular and epididymal gastric adenocarcinoma metastases.
Four days after the operation, the patient was discharged from the hospital and transferred to the department of oncology for XELOX adjuvant chemotherapy.
Abdominal computed tomography at the postoperative 1-mo follow-up revealed multiple nodules in the mesenteric region, which were considered to be lymph node metastases. At 2 mo postoperatively, the patient was still alive.
Over 95% of testicular neoplasms are primary tumors derived from germ cells in the testes[4]. Secondary testicular or epididymal tumors originating from other solid neoplasms are rare, making up about 2% of all testicular neoplasms[5]. A previous case series reported that cancers that metastasize to the testes are mainly prostate cancer (45/127) and lung cancer (25/127), and that stomach cancer accounts for only 5% of metastases to the testes[6]. Since testicular gastric cancer metastasis was first reported in 1962[7], only 30 other cases have been published. Similarly, only three patients have been reported to have epididymal gastric cancer metastases since 2000[8-10]. Here, we report a very rare case of gastric adenocarcinoma metastasis to both the testis and epididymis.
The main mechanisms of gastric cancer metastasis are direct invasion, hematogenous metastasis, lymphatic metastasis, and distant seeding via intraperitoneal spread[11]. Because of the blood–testis barrier, solid tumor cells rarely spread to the testes. Moreover, the tunica vaginalis forms an external fibrous protective wall that separates the testes from the peritoneal cavity[12]. However, several mechanisms are suggested to enable gastric cancer cells to overcome these barriers and invade the testis and epididymis. The first hypothesis suggests that metastases may occur via the lymph drainage duct to the testis and epididymis. Gastric metastases have also been reported to originate from testicular cancer, probably via invasion of the stomach by testicular cancer through lymphatic ducts[13-15]. It is reported that testicular and gastric cancers have overlapping lymphatic metastasis pathways[16]. If gastric cancer cells block the thoracic duct, the retrograde lymph flow may carry tumor cells to the scrotum through the receptaculum chyli and urogenital lymph vessel trunks[17]. The multiple nodules that we observed in the mesenteric region upon abdominal computed tomography scanning support this possibility. The second hypothesis suggests that patent processus vaginalis may enable the spread of gastric cancer cells to the testes. Krukenberg tumors, secondary ovarian tumors derived from gastrointestinal signet ring cell cancer, spread via intraperitoneal implantation[18]. However, the incidence of metastatic testicular cancer is much lower than that of metastases to the ovaries, probably because the closed processus vaginalis separates the testes from the peritoneal cavity. During embryonic development, the left testis descends into the scrotum before the right testis. Thus, dysfunctional abdominal inguinal ring and temporary patent processus vaginalis testes are more likely to occur on the right. In adulthood, tumors may spread to the right testis and epididymis through these regressively obliterated structures[19]. Schaefer et al[20] reported that a man who had undergone orchidopexy for left-sided cryptorchidism, which had probably obliterated the left inguinal canal, only had gastric cancer metastases in the non-cryptorchid right testis[20]. This hypothesis is also supported by the case of another gastric cancer patient who had both testicular metastases and extensive peritoneal carcinomatosis that possibly developed via implantation metastasis[12]. Considering that our patient had previously undergone vasoligature, the possible obliteration of bilateral inguinal canals may have prevented tumor cells from spreading through the processus vaginalis. Third, a case involving paraneoplastic leukemoid reactions supported the hypothesis that gastric cancer may hematogenously spread to male genital organs[21]. However, we did not find evidence to prove this. In summary, we hypothesize that the testicular and epididymal metastases in our patient might have spread contrarily via a lymph drainage duct, but the value of this hypothesis is limited due to the lack of tests or examinations.
In this case, the patient initially complained of a palpable painful mass in the right scrotum and also had a hydrocele, which was similar to a case reported by Dai et al[22]. Such cases can easily be misdiagnosed as primary testicular cancers because of their similar presentations as scrotal masses and swelling[23]. If accompanied by a history of juvenile orchitis and pain, they may also easily be misdiagnosed as epididymitis and orchitis. Most forms of primary testicular cancer are seminomas, which appear homogeneous or solid with various proportions of necrosis based on B-ultrasound and CTU imaging, respectively[24]. These features are different from the uneven echo and mixed solid-cystic mass observed in our patient upon B-ultrasound and CTU imaging, respectively. Because nonseminomatous germ cell tumors (NSGCT) can undergo varying degrees of differentiation, they may also exhibit uneven echo and cystic space. Over 70% of NSGCT patients have elevated serum levels of AFP or β-HCG[25]. Although the patient in this case had a history of gastric adenocarcinoma, primary NSGCT could not be completely excluded. Hence, immunohistochemical examination was carried out for pancytokeratins, markers of tumor cells of epithelial origin, and caudal related homeodomain transcription 2, which is ectopically expressed in intestinal metaplasia and is a marker of intestinal-type gastric adenocarcinomas[26]. Several studies have reported that immunohistochemically-confirmed gastric cancer patients have elevated serum levels of β-HCG[27,28]. Although the mechanisms of β-HCG production in gastric cancer cells are unknown, it is known that serum β-HCG can be positive in some patients with gastric cancer. After considering all the possibilities discussed above, the patient was diagnosed with testicular and epididymal gastric adenocarcinoma metastases.
Almost 80% of gastric cancer patients who undergo radical gastrectomy develop recurrent, unresectable metastatic disease[29]. The prognosis of patients with testicular metastases is poor, with an overall survival of 2–52 mo[19]. In this case, the patient underwent testicular metastasectomy, which is consistent with previously reported cases[12,16,19,22,30]. Indeed, mounting evidence has associated metastasectomy with survival benefit in gastric cancer, especially in cases with liver and lymph node metastases[31,32]. However, because these patients usually develop metastases in other sites, such as mesenteric lymph nodes as seen in our patient, they require postoperative therapy, such as systematic chemotherapy, immunotherapy, and targeted therapy. Although well-designed clinical trials on the efficacy of these treatments in patients with testicular gastric cancer metastases are lacking, early and accurate diagnosis is crucial for timely treatment and better survival. To sum up, history of gastric cancer, the presence of palpable painful scrotal masses of unknown cause, and imaging features are important indicators of testicular and epididymal gastric cancer metastases, which are valuable for early diagnosis and precise treatment.
Because gastric adenocarcinoma metastases to the testes and epididymides can easily be misdiagnosed as primary testicular cancer, clinicians should perform careful differential diagnosis. In patients with a history of gastric cancer, the presence of palpable painful scrotal mass of unknown cause and imaging features are important indicators of testicular and epididymal gastric cancer metastases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Huang HL, Japan; Lieto E, Italy; Liu YQ, United States S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 43243] [Article Influence: 14414.3] [Reference Citation Analysis (47)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1784] [Article Influence: 446.0] [Reference Citation Analysis (0)] |
| 3. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307-52316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 4. | Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, Looijenga LHJ. Testicular cancer. Nat Rev Dis Primers. 2018;4:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 5. | Grignon DJ, Shum DT, Hayman WP. Metastatic tumours of the testes. Can J Surg. 1986;29:359-361. [PubMed] [Cited in This Article: ] |
| 6. | Haupt HM, Mann RB, Trump DL, Abeloff MD. Metastatic carcinoma involving the testis. Clinical and pathologic distinction from primary testicular neoplasms. Cancer. 1984;54:709-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Eadie DG. Presentation of carcinoma of the stomach as a left testicular tumor. Br J Surg. 1962;50:156-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Yerko IP, Moloshok AA, Tsiselsky RK. [Rare Case of Stomach Cancer Metastasis]. Urologiia. 2015;81. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Alois M, Valentina P, Andreas G. The "Krukrnberg" tumor in male. Arch Esp Urol. 2005;58:971-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 10. | Ozdal OL, Yakupoglu YK, Ciçek A, Erdem O, Memis L, Memis A. Epididymal metastasis from gastric signet ring cell adenocarcinoma. Can J Urol. 2002;9:1498-1499. [PubMed] [Cited in This Article: ] |
| 11. | Li W, Ng JM, Wong CC, Ng EKW, Yu J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. 2018;37:4903-4920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Alder L, Al-Juhaishi T, Smith SC, Paul AK. Testicular Swelling as an Initial Presentation of a Patient With Metastatic Gastric Cancer. Fed Pract. 2019;36:S26-S28. [PubMed] [Cited in This Article: ] |
| 13. | McLaren A, Baxter MA, Katbeh T, Lynch V, Fullarton G, White J. Metastatic seminoma with isolated gastric metastases: a case report. Scott Med J. 2019;64:133-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Lauro S, Righini R, Onesti CE, Pucci E, Bramini A, Marchetti P. Gastric metastases from testicular cancer: case report and review of literature. J Gastrointest Cancer. 2014;45 Suppl 1:22-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Köksal AS, Kayaçetin E, Torun S, Güneş ZE, Zengin NI. An elusive etiology of upper gastrointestinal bleeding in a young man: testis tumor. Surg Laparosc Endosc Percutan Tech. 2013;23:354-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Park S, Moon SK, Lim JW. Mechanism of metastasis to the spermatic cord and testis from advanced gastric cancer: a case report. BMC Gastroenterol. 2020;20:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Tsao SH, Chuang CK. Krukenberg tumor with concomitant ipsilateral hydronephrosis and spermatic cord metastasis in a man: A case report. World J Clin Cases. 2021;9:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006;30:277-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Li B, Cai H, Kang ZC, Wu H, Hou JG, Ma LY. Testicular metastasis from gastric carcinoma: A case report. World J Gastroenterol. 2015;21:6764-6768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Schaefer IM, Sauer U, Liwocha M, Schorn H, Loertzer H, Füzesi L. Occult gastric signet ring cell carcinoma presenting as spermatic cord and testicular metastases: "Krukenberg tumor" in a male patient. Pathol Res Pract. 2010;206:519-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Güven E, Selvi S, Kilciler M, Baar H. Malign Priapism Secondary to Renal Cell Carcinoma Provoked Paraneoplastic Leukemoid Reaction: Report of The First Case. Acta Oncologica Turcica. 2018;51:259-262. [Cited in This Article: ] |
| 22. | Dai W, Liu D, Zuo J, Tan J, Wang L, Wu H, Cai S, Yuan Y. Metastatic tumor of male genital system from gastric cancer: a case report and review of literature. Int J Clin Exp Pathol. 2017;10:8592-8598. [PubMed] [Cited in This Article: ] |
| 23. | Baird DC, Meyers GJ, Hu JS. Testicular Cancer: Diagnosis and Treatment. Am Fam Physician. 2018;97:261-268. [PubMed] [Cited in This Article: ] |
| 24. | Coursey Moreno C, Small WC, Camacho JC, Master V, Kokabi N, Lewis M, Hartman M, Mittal PK. Testicular tumors: what radiologists need to know--differential diagnosis, staging, and management. Radiographics. 2015;35:400-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Dieckmann KP, Simonsen-Richter H, Kulejewski M, Anheuser P, Zecha H, Isbarn H, Pichlmeier U. Serum Tumour Markers in Testicular Germ Cell Tumours: Frequencies of Elevated Levels and Extents of Marker Elevation Are Significantly Associated with Clinical Parameters and with Response to Treatment. Biomed Res Int. 2019;2019:5030349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | Huang RJ, Choi AY, Truong CD, Yeh MM, Hwang JH. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver. 2019;13:596-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Kafka M, Wöll E, Brunhuber T, Gruber L, Tulchiner G, Staudacher N, Horninger W, Pichler R. A presumed extragonadal germ cell tumor that turned out to be a gastric cancer-a case report. Transl Androl Urol. 2021;10:2528-2533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ota T, Shinohara M, Tanaka M, Date Y, Itakura H, Munakata A, Kinoshita K, Hishima T, Koike M, Kitamura M. Spermatic cord metastases from gastric cancer with elevation of serum hCG-beta: a case report. Jpn J Clin Oncol. 2000;30:239-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 30. | Fu J, Luo J, Ye H, Chen Y, Xie L. Testicular and Spermatic Cord Metastases from Gastric Adenocarcinoma: An Unusual Case. Cancer Manag Res. 2021;13:1897-1900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M, Probst S, Messmann H, Moehler M, Fischbach W, Hartmann JT, Mayer F, Höffkes HG, Koenigsmann M, Arnold D, Kraus TW, Grimm K, Berkhoff S, Post S, Jäger E, Bechstein W, Ronellenfitsch U, Mönig S, Hofheinz RD. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 261] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 32. | Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M, Hanna GB. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg. 2016;263:1092-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |