Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10227
Peer-review started: April 28, 2022
First decision: June 16, 2022
Revised: June 25, 2022
Accepted: August 23, 2022
Article in press: August 23, 2022
Published online: October 6, 2022
Myeloid sarcoma (MS) is relatively rare, occurring mainly in the skin and lymph nodes, and MS invasion of the ulnar nerve is particularly unusual. The main aim of this article is to present a case of MS invading the brachial plexus, causing ulnar nerve entrapment syndrome, and to further clinical understanding of the possibility of MS invasion of peripheral nerves.
We present the case of a 46-year-old man with a 13-year history of well-treated acute nonlymphocytic leukaemia who was admitted to the hospital after presenting with numbness and pain in his left little finger. The initial diagnosis was considered a simple case of nerve entrapment disease, with magnetic resonance imaging showing slightly abnormal left brachial plexus nerve alignment with local thickening, entrapment, and high signal on compression lipid images. Due to the severity of the ulnar nerve compression, we surgically investigated and cleared the entrapment and nerve tissue hyperplasia; however, subsequent pathological biopsy results revealed evidence of MS. The patient had significant relief from his neurological symptoms, with no postoperative complications, and was referred to the haemato-oncology department for further consultation about the primary disease. This is the first report of safe treatment of ulnar nerve entrapment from MS. It is intended to inform hand surgeons that nerve entrapment may be associated with extramedullary MS, as a rare presenting feature of the disease.
MS invasion of the brachial plexus and surrounding tissues of the upper arm, resulting in ulnar nerve entrapment and degeneration with significant neurological pain and numbness in the little finger, is uncommon. Surgical treatment significantly relieved the patient’s nerve entrapment symptoms and prevented further neurological impairment. This case is reported to highlight the rare presenting features of MS.
Core Tip: The main aim of this article is to present a rare case of myeloid sarcoma (MS) invading the ulnar nerve and causing ulnar nerve entrapment syndrome. The patient’s symptoms were relieved by surgical release of the nerve bundle and compressed tissue, and the disease was diagnosed definitively by pathological examination. The case illustrates the rare presenting features of extramedullary MS, enriches the clinical literature, and highlights that it is essential to suspect MS with a history of acute nonlymphocytic leukaemia.
- Citation: Li DP, Liu CZ, Jeremy M, Li X, Wang JC, Nath Varma S, Gai TT, Tian WQ, Zou Q, Wei YM, Wang HY, Long CJ, Zhou Y. Myeloid sarcoma with ulnar nerve entrapment: A case report. World J Clin Cases 2022; 10(28): 10227-10235
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10227.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10227
Myeloid sarcoma (MS), also known as granulocytic sarcoma and chloroma, is a rare haematological tumour consisting of a solid extramedullary mass of immature cells of the myeloid lineage, capable of destroying preexisting tissues and organs. It is more common in males than in females and is often associated with acute nonlymphocytic leukaemia (ANLL), which is frequently the primary mani
The patient was a 46-year-old man who complained of painful numbness in the left little finger for 4 mo with no apparent cause.
The numbness and pain in his left little finger had gradually worsened over the past 4 mo but was not of serious concern.
The patient had a 13-year history of ANLL and a 20-year history of psoriasis; both diseases had been fully treated previously by systemic therapy.
Notably, there was no family history of the same disease.
Physical examination of the whole body revealed no palpable lymph nodes. Specialized examination of the left hand revealed atrophy of the first dorsal interosseous muscle, numbness of the little finger and ulnar aspect of the palm, weakness of the ring and little finger, inability to adduct fingers, positive paper-clip test results, and loss of manual dexterity; all other examination outcomes were normal.
The laboratory results were: White blood cell count (WBC), 14.14 (normal range: 3.50-9.50) × 109/L; neutrophil count (NEUT), 12.12 (1.80-6.30) × 109/L; lymphocyte count (LYMPH), 0.78 (1.20-3.20) × 109/L; monocyte count, 1.22 (0.10-0.60) × 109/L; eosinophil count (EOS), 0.00 (0.02-0.52) × 109/L; %NEUT, 85.8 (40.0-75.0)%; %LYMPH, 5.50 (20-50)%; %EOS, 0.0 (0.4-8.0)%; red blood cell count (RBC), 4.0 (4.3-5.8) × 109/L; haemoglobin count (HGB), 126 (130-175) g/L; haematocrit, 36.2 (40.0-50.0)%; rheumatoid factors, 22.05 (≤ 18.00) IU/mL; hypersensitive C-reactive protein, 4.67 (≤ 4.00) mg/L; uric acid, 463.02 (202.00-416.00) μmol/L; and interferon-γ, 34.21 (≤ 20.36) pg/mL.
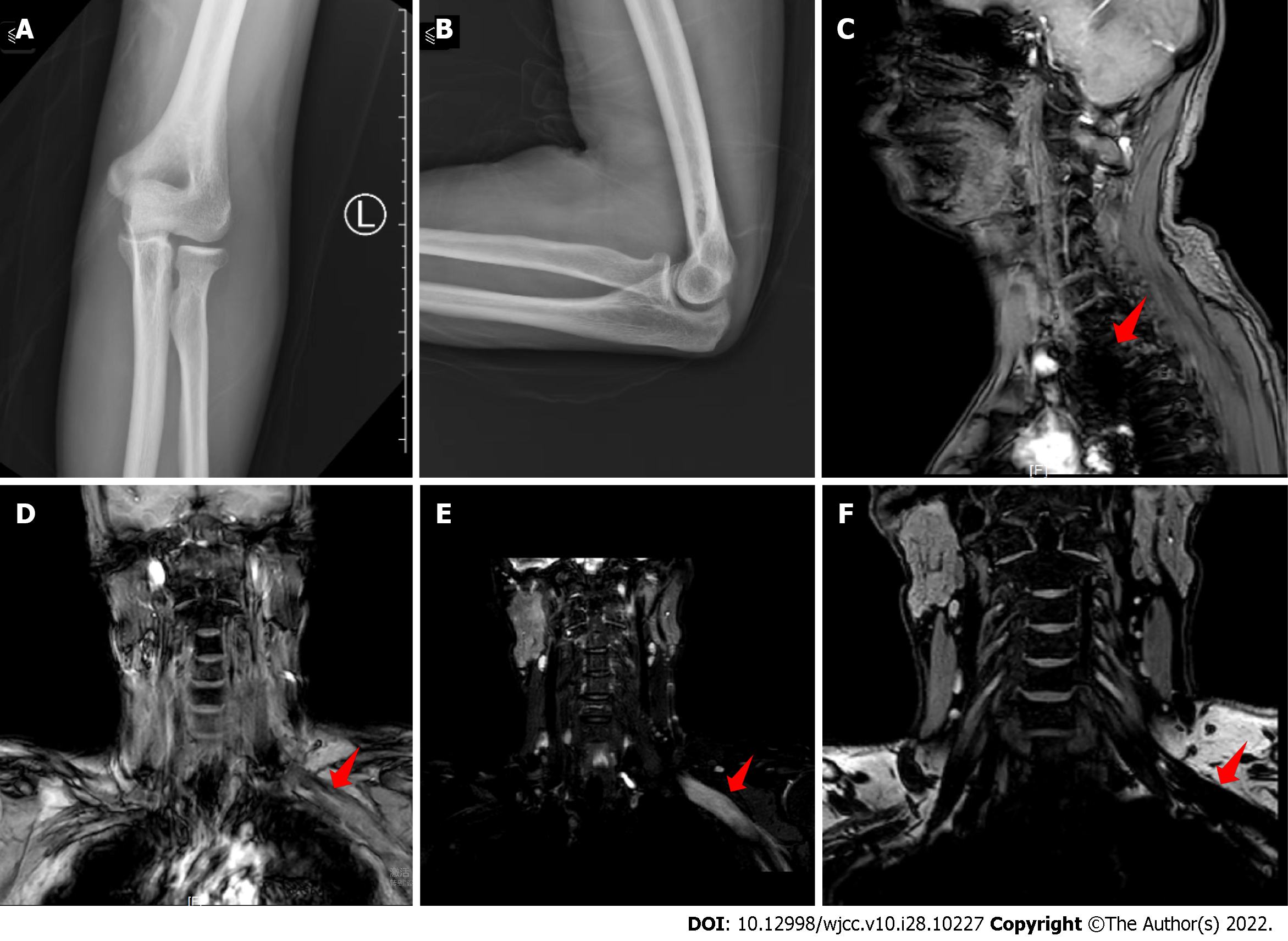
X-rays showed that the bones of the left elbow were regular in shape, with cortical continuity and clear trabecular structures, a normal alignment of the elbow joint with a moderate joint space, and no significant abnormalities in the surrounding soft tissues (Figure 1A and B). Magnetic resonance imaging (MRI) showed a slightly abnormal left brachial plexus nerve alignment with local thickening, entanglement, and a high signal on compression lipid imaging, while the right brachial plexus nerve bundle alignment was normal with no obvious abnormal signal. There were multiple somewhat large lymph nodes in the neck, with the largest being approximately 0.8 cm in short diameter located in the left carotid sheath area (Figure 1C-F).
MS causing ulnar nerve entrapment.
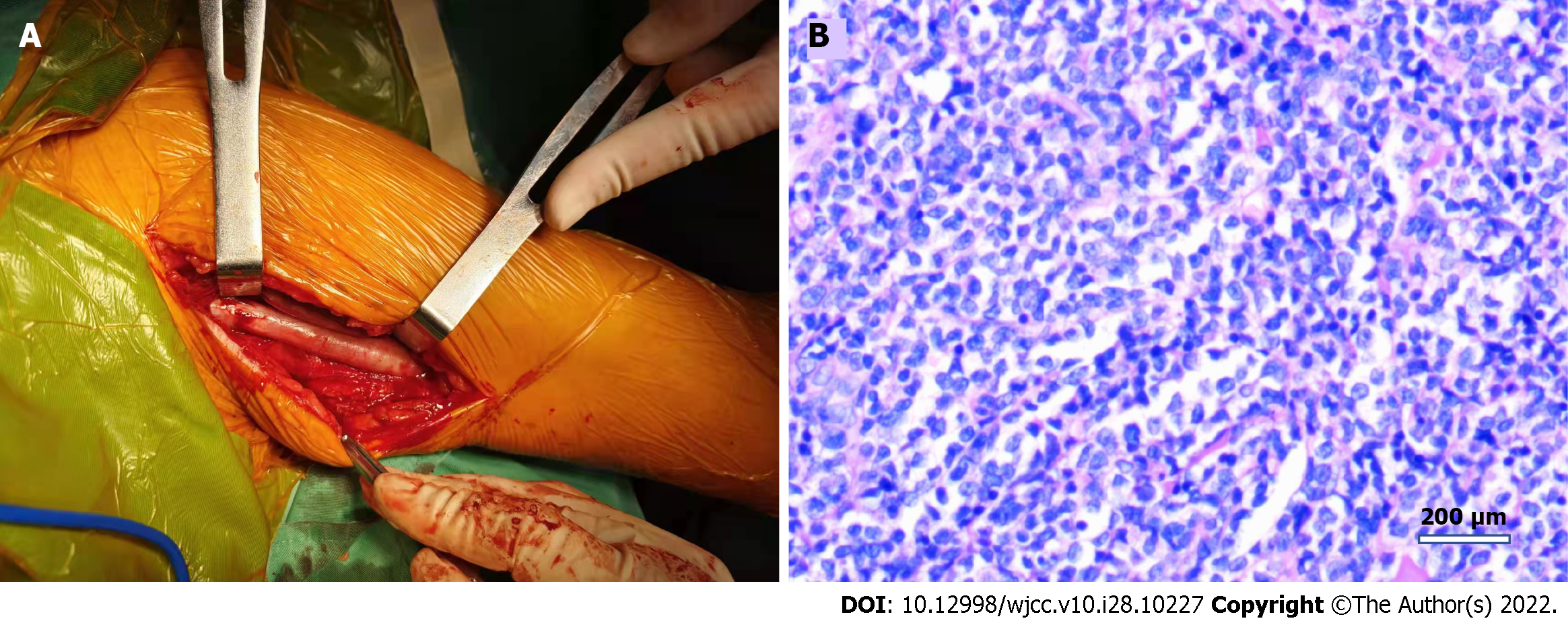
The patient was placed in a supine position, and an upper arm tourniquet was applied. After routine prepping and draping, a longitudinal incision of approximately 20 cm was made on the medial side of the left upper arm. Intraoperatively, the left ulnar nerve was found to be thickened and degenerated, with severe adhesions to the surrounding tissues. Further observation of the ulnar nerve revealed thickening of the epineurium and hyperplasia of the perineurium, trapping the nerve fibres; therefore, interfascicular neurolysis release was performed with careful separation of the hyperplastic tissue between the nerve bundles. In addition, the lymph nodes in the anterior axillary region were cleared and sent for pathological examination. The incision was closed in layers after irrigation and haemostasis.
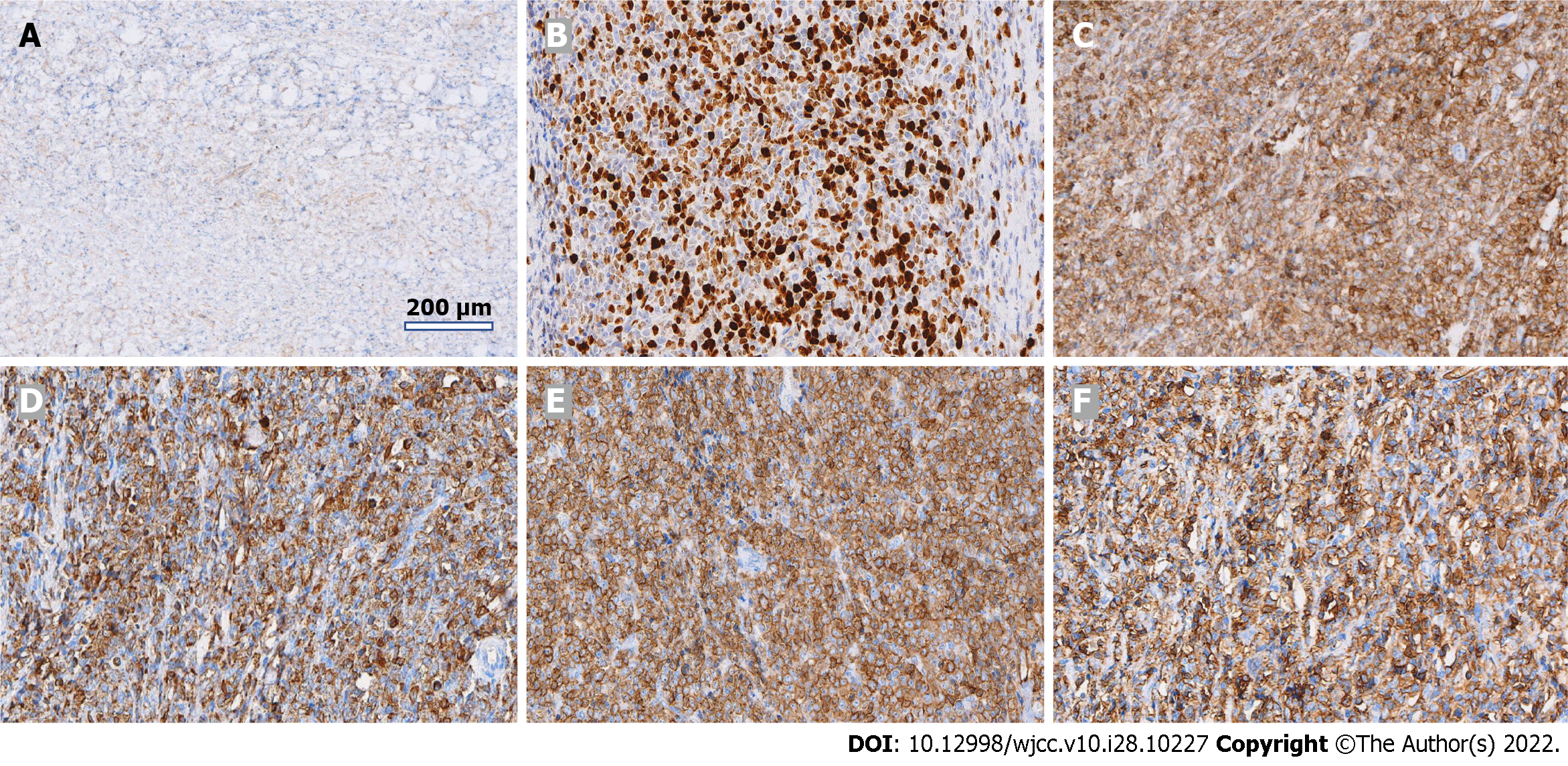
Postoperative pathological findings were as follows: Lymphohaematopoietic tumour (both neuroepithelial and lymph nodes), combined with immunohistochemical staining results consistent with MS [CD3 (-), CD20 (-), CD21:FDC network (+), CD5 (-), Ki-67 (+ 70%), TdT (-), LCA (+), MPO (+), CD117 (+), and CD34 (+)] (Figures 2 and 3).
On the first postoperative day, the patient’s pain and numbness were significantly reduced. After 1 wk of observation, we actively communicated with the patient and his family and transferred him to the external haemato-oncology department for further treatment of the primary disease. Positron emission tomography/computerised tomography (PET/CT) was performed, and the results were consistent with MS as per the pathology report. At the 2-mo follow-up, the patient had recovered well from the hand symptoms, the numbness had disappeared, and the postoperative visual analogue scale (VAS) score of pain had decreased to 1 from 5 preoperatively.
The incidence of MS in acute myeloid leukaemia (AML) patients ranges from 3%-5%[8]. Although the exact pathogenesis of MS is not fully understood, multivariate analysis has demonstrated that the occurrence of MS is significantly associated with intrinsic properties of myeloid tumour cells (e.g., cytogenetics)[9-11]. MS may manifest in many different parts of the body, including peripheral nerves, and its clinical symptoms depend on the specific location and size of the tumour, making it very difficult for doctors to diagnose based on symptoms alone[12-16].
For early MS diagnosis, biopsy and PET/CT images are useful, with the maximum standardized uptake value (SUVmax) on PET/CT images for MS detection often in the range of 2.1-9.3, while MRI can only be used to detect tissue abnormalities and not to provide a definitive diagnosis[7,17]. Ultrasound of areas affected by MS can also be valuable in the assessment of mass size and borders[18]. The aim of tissue biopsy is diagnostic clarification, with cytochemical and immunophenotypic analyses, such as myeloid cell markers, anti-myeloperoxidase, and lysozyme, being particularly helpful in gaining a definitive diagnosis[19-23]. Studies have shown that MS is often misdiagnosed as non-Hodgkin’s lymphoma, and in the absence of immunohistochemical results, the misdiagnosis rate is 50%-75%[24]. It has also been shown that 88% of patients with untreated isolated MS progress to AML within 1 year[25] and that MS is a precursor to relapse of the primary disease[8,26,27]. Due to its rarity, case reports mostly constitute the literature for MS, and studies on treatment options are mainly retrospective analyses of small sample sizes, with a lack of large randomized prospective studies[28-30]. Therefore, a uniform and consistent approach to optimal treatment has not been established. However, given the risk of progression to leukaemia and the poor prognosis, the main aim of treatment is to delay progression and prolong survival as long as possible[19,31-33].
We review cases of peripheral nerve compression due to MS over the last 10 years (Table 1) and found that similar cases were predominantly observed when MS invaded the eye (3 cases) and spine (12 cases), while invasion of the brachial plexus is very rare. Due to the rarity of MS-associated peripheral nerve compression, the resulting misdiagnosis may lead to a delay in optimal MS treatment[31,34].
| Ref. | Gender/age (yr) | Leukaemia-Yes (type)/No | Symptoms | Involved segment | Lesion invasion site | Imaging | Bone marrow biopsy | Laboratory test findings | Treatment regimens | Pathology and immunohistochemistry examination |
| McCarty et al[35] | M/14 | No | Headaches, urinary incontinence | Intracranial, extracranial, sacral spine | Cerebral nerve, sacral nerve root | MRI | Positive | WBC: 51 × 109/L; HGB: 113 g/L; platelets: 130 × 109/L | Radiotherapy, chemotherapy | - |
| Zhu et al[36] | M/36 | No | Abdominal pain, pain in right eye, eye protrusion with inability to close | Pancreas, right eye | Pancreas, right optic nerve | CT, MRI, PET-CT | Negative | Mildly elevated liver and pancreatic enzymes | Radiotherapy, chemotherapy | Ki-67 (+ 80%), P53 (approximately 50%), CD43 (+), MPO (+) |
| Bai et al[24] | M/29 | No | Severe radiating pain in lower limbs, moderate difficulty in urination | Waist | Lumbar spine attachment, spinal cord | CT, MRI, SPECT | Negative | WBC: 6.01 × 109/L; RBC: 5.00 × 1012/L; HGB: 158 g/L; platelets: 236 × 109/L; CRP: 2 mg/L; rrythrocyte sedimentation rate (ESR): 4 mm/1 h; procalcitonin (PCT): 0.27 ng/mL | Lesion excision, chemotherapy | CD33 (+), MPO (+) |
| Valsamis et al[37] | M/56 | No | Pain in right hip radiating down right leg | Right hip | Right sciatic nerve, lumbosacral plexus | Ultrasound, MRI | - | MONO: 0.9 × 109/L; RBC: 3.56 × 1012/L; HGB: 116 g/L; mean cell volume: 102 fL; CRP: 13 mg/L | - | - |
| Pandey et al[38] | (1) F/44; (2) F/16; (3) F/63; (4) M/53 | (1,2,4) No; (3) Yes (AML) | (1) Neck pain, right arm weakness, and muscle wasting; (2) back pain, bilateral lower extremity tingling, and weakness; (3) lower back pain, bilateral lower extremity weakness, and bowel incontinence; and (4) lower back pain, hip pain, lower extremity weakness, and muscle pain | (1) Cervical spine; (2) thoracic spine; (3) thoracic spine; and (4) lumbar spine | Spinal cord compression | - | (1) Negative; (2) positive; (3) negative; and (4) positive | (1) and (3) Negative; (2) and (4) high WBC | Lesion excision | - |
| Snoj et al[39] | F/49 | Yes [MDS (refractory anaemia with excess blasts)] | Weakness and decreased sensation in right arm, right supraclavicular mass | Left upper limb | Left brachial plexus | Ultrasound, MRI | - | - | Radiotherapy, chemotherapy | - |
| Yamamoto et al[40] | F/34 | Yes (Chronic phase chronic myeloid leukaemia) | Pain in left eye, protrusion of eyeball, blurred vision with headache, and vomiting | Left eye | Left optic nerve | MRI | - | - | - | CD13 (+), CD33 (+), CD117 (+) |
| Koh et al[41] | M/68 | Yes (chronic myeloid leukemia) | Low back pain and numbness in both legs with radiating pain | Thoracic and sacral spine | Spinal cord | MRI, CT | - | - | Chemotherapy | - |
| Kim et al[42] | M/62 | Yes (acute myeloid leukaemia, M2) | Abnormal sensation and weakness of ankle flexion in posterior aspect of left calf and foot | Lower left limb | Sciatic nerve | Ultrasound, MRI | - | - | - | MPO (+) |
| Rambeloson et al[43] | M/1 | No | Bilateral ophthalmoplegia with loss of vision | Bilateral eye | Occulomotor nerve | Myelogram, CT | - | - | - | - |
| Slouma et al[31] | M/56 | No | Progressive back pain and bilateral sciatica | Waist | Lumbar 5th nerve root | MRI | - | WBC: 83 × 109/L; absolute neutrophil count: 60 × 109/L | Chemotherapy | MPO (+), CD13 (+), CD33 (+), CD14 (+), CD4 (+), CD46 (+) |
| Anqi et al[44] | M/24 | No | Limp, numbness in right lower limb, mild urinary frequency, urinary and faecal incontinence | Lumbosacral region | Lumbar and sacral spine | MRI | - | Normal | Lesion excision, chemotherapy | MPO, CD34, CD43 (+) |
| Ha et al[34] | F/38 | Yes (AML) | Subacute radiating pain in right upper limb, weakness | Right upper limb | Right brachial plexus | MRI, PET/CT | - | - | Chemotherapy, hormone shock therapy | CD13(+), CD14 (+) ,CD15 (+), CD33 (+), CD54 (+), CD64 (+), CD117 (+) , MPO (+) |
The clearest symptom in the present case was ulnar nerve palsy, most typically caused by cubital tunnel syndrome. As our investigations showed that the nerve signal was abnormal, we first explored and released the nerve. This resulted in a significant reduction in the patient’s pain, and numbness was significantly reduced on the first postoperative day. The subsequent pathology results were unexpected, but we were able to expedite referral to haemato-oncology for primary disease treatment.
MS causing ulnar nerve entrapment and degeneration through invasion of the brachial plexus is a very rare condition. With a history of ANLL with nerve entrapment features, it is essential to suspect MS and send tissue for pathological analysis, as well as to provide prompt surgical decompression for symptom relief and prevent lasting neurological damage.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Chiu CC, Taiwan; Fakhradiyev I, Kazakhstan; Govindarajan KK, India S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Shahin OA, Ravandi F. Myeloid sarcoma. Curr Opin Hematol. 2020;27:88-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Claerhout H, Van Aelst S, Melis C, Tousseyn T, Gheysens O, Vandenberghe P, Dierickx D, Boeckx N. Clinicopathological characteristics of de novo and secondary myeloid sarcoma: A monocentric retrospective study. Eur J Haematol. 2018;100:603-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Meyer HJ, Surov A. [Myeloid sarcoma]. Rofo. 2021;193:7-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kaur V, Swami A, Alapat D, Abdallah AO, Motwani P, Hutchins LF, Jethava Y. Clinical characteristics, molecular profile and outcomes of myeloid sarcoma: a single institution experience over 13 years. Hematology. 2018;23:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Meyer HJ, Beimler M, Borte G, Pönisch W, Surov A. Radiological and clinical patterns of myeloid sarcoma. Radiol Oncol. 2019;53:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Heckl S, Horger M, Faul C, Ebrahimi A, Ioanoviciu SD. [Myeloid sarcoma of nervous plexus - infiltration of the nerve plexus by extramedullary manifestation of acute myeloid leukemia]. Rofo. 2014;186:1059-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Das JP, Riedl CC, Ulaner GA. 18F-FDG PET/CT Helps Differentiate Peripheral Nerve Myeloid Sarcoma From a Presumed Benign Nerve Sheath Tumor. Clin Nucl Med. 2020;45:989-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47:2527-2541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Shi JM, Meng XJ, Luo Y, Tan YM, Zhu XL, Zheng GF, He JS, Zheng WY, Xie WZ, Li L, Ye XJ, Zhang J, Cai Z, Lin MF, Huang H. Clinical characteristics and outcome of isolated extramedullary relapse in acute leukemia after allogeneic stem cell transplantation: a single-center analysis. Leuk Res. 2013;37:372-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Nagamine M, Miyoshi H, Kawamoto K, Takeuchi M, Yamada K, Yanagida E, Kohno K, Ohshima K. Clinicopathological analysis of myeloid sarcoma with megakaryocytic differentiation. Pathology. 2022;54:442-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Abbas HA, Reville PK, Geppner A, Rausch CR, Pemmaraju N, Ohanian M, Sasaki K, Borthakur G, Daver N, DiNardo C, Bueso-Ramos C, Pierce S, Jabbour E, Garcia-Manero G, Konopleva M, Ravandi F, Kantarjian H, Kadia TM. Clinical and molecular characterization of myeloid sarcoma without medullary leukemia. Leuk Lymphoma. 2021;62:3402-3410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Abdelnabi MH, Almaghraby A, Saleh Y, ElSharkawy E. Cardiac chloroma or cardiac myeloid sarcoma: Case Report. Echocardiography. 2019;36:1594-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Arslantaş E, Bayram C, Odaman Al I, Uysalol E, İribaş A, Akı H, Adaletli İ, Ayçicek A, Özdemir N. A Rare Cause of Paraplegia: Myeloid Sarcoma. Turk J Haematol. 2018;35:206-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Inoue M, Hagihara M, Uchida T, Hua J, Nakajima T, Tajima S, Ota Y. A Rare Monocytic Crisis of Chronic Myelogenous Leukemia Presenting with Unusual Extramedullary Manifestations and an Atypical (14;22)(q24;q11.2) Translocation in the Bone Marrow. Intern Med. 2017;56:3341-3346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Mendez-Hernandez A, Andrade XA, Upadhyay S, Parra-Rodriguez LM, Caldeira E, Paz LH, Mann H, Zia M, Sumoza L. Myeloid Sarcomas Causing Unilateral Cranial Nerve Palsies in a Patient with Relapsed Acute Myeloblastic Leukemia. Case Rep Hematol. 2020;2020:3749565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Lim SH, Nam HN, Lim KI, Jeon IS. A case of myeloid sarcoma presenting with an orbital mass, hearing loss, and multiple cranial neuropathies. Turk J Pediatr. 2018;60:322-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Stölzel F, Röllig C, Radke J, Mohr B, Platzbecker U, Bornhäuser M, Paulus T, Ehninger G, Zöphel K, Schaich M. ¹⁸F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica. 2011;96:1552-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Ozsoy A, Akdal Dolek B, Barca N, Aktas H, Araz L, Kulacoglu S. Ultrasound Findings in a Case of Myeloid Sarcoma of the Breast. J Belg Soc Radiol. 2016;100:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk. 2017;17:263-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Hagen PA, Singh C, Hart M, Blaes AH. Differential Diagnosis of Isolated Myeloid Sarcoma: A Case Report and Review of the Literature. Hematol Rep. 2015;7:5709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Coltro G, Mannelli F, Vergoni F, Santi R, Massi D, Siliani LM, Marzullo A, Bonifacio S, Pelo E, Pacilli A, Paoli C, Franci A, Calabresi L, Bosi A, Vannucchi AM, Guglielmelli P. Extramedullary blastic transformation of primary myelofibrosis in the form of disseminated myeloid sarcoma: a case report and review of the literature. Clin Exp Med. 2020;20:313-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Xu G, Zhang H, Nong W, Li C, Meng L, Liu C, Li F. Isolated Intracranial Myeloid Sarcoma Mimicking Malignant Lymphoma: A Diagnostic Challenge and Literature Reviews. Onco Targets Ther. 2020;13:6085-6092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Roy PS, Vohra V, Jain R, Singhal KK, Mahajan S, Chatterjee D, Saxena AK, Rohilla M, Bansal D. Isolated Myeloid Sarcoma and Intracardiac Thrombus Resulting in Superior Mediastinal Syndrome. Indian J Pediatr. 2022;89:591-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Bai CR, Li X, Wang JS, Li JJ, Liu N, Fei Q, Li D, Yang Y. Diagnosis and surgical treatment of primary isolated aggressive lumbar myeloid sarcoma: a rare case report and review of the literatures. BMC Musculoskelet Disord. 2021;22:220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Li X, Fu J, Xue Y, Yang Y, Wang Y, Zhang C, Zhuo S, Irani F. Allogeneic Hematopoietic Stem Cell Transplantation as Treatment for Primary Granulocytic Sarcoma of the Breast. Cell Biochem Biophys. 2015;72:791-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Wang L, Cai DL, Lin N. Myeloid sarcoma of the colon as initial presentation in acute promyelocytic leukemia: A case report and review of the literature. World J Clin Cases. 2021;9:6017-6025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Frietsch JJ, Hunstig F, Wittke C, Junghanss C, Franiel T, Scholl S, Hochhaus A, Hilgendorf I. Extra-medullary recurrence of myeloid leukemia as myeloid sarcoma after allogeneic stem cell transplantation: impact of conditioning intensity. Bone Marrow Transplant. 2021;56:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Shallis RM, Pucar D, Perincheri S, Gore SD, Seropian SE, Podoltsev NA, Zeidan AM. Molecular testing of isolated myeloid sarcoma allows successful FLT3-targeted therapy. Ann Hematol. 2022;101:1145-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Valent P, Sadovnik I, Eisenwort G, Herrmann H, Bauer K, Mueller N, Sperr WR, Wicklein D, Schumacher U. Redistribution, homing and organ-invasion of neoplastic stem cells in myeloid neoplasms. Semin Cancer Biol. 2020;60:191-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Shu X, Wu Q, Guo T, Yin H, Liu J. Acute Promyelocytic Leukemia Presenting With a Myeloid Sarcoma of the Spine: A Case Report and Literature Review. Front Oncol. 2022;12:851406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Slouma M, Rahmouni S, Dhahri R, Khayati Y, Zriba S, Amorri W, Gharsallah I, Metoui L, Louzir B. Epidural myeloid sarcoma as the presenting symptom of chronic myeloid leukemia blast crisis. Clin Rheumatol. 2020;39:2453-2459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Palejwala AH, O'Connor KP, Shi H, Villeneuve L, Scordino T, Glenn CA. Chronic myeloid leukemia manifested as myeloid sarcoma: Review of literature and case report. J Clin Neurosci. 2019;64:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Magdy M, Abdel Karim N, Eldessouki I, Gaber O, Rahouma M, Ghareeb M. Myeloid Sarcoma. Oncol Res Treat. 2019;42:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Ha Y, Sung DH, Park Y, Kim du H. Brachial Plexopathy due to Myeloid Sarcoma in a Patient With Acute Myeloid Leukemia After Allogenic Peripheral Blood Stem Cell Transplantation. Ann Rehabil Med. 2013;37:280-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | McCarty SM, Kuo DJ. Persistent sacral chloroma in refractory acute myelogenous leukaemia. BMJ Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Zhu T, Xi XY, Dong HJ. Isolated myeloid sarcoma in the pancreas and orbit: A case report and review of literature. World J Clin Cases. 2018;6:477-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Valsamis EM, Glover TE. Granulocytic sarcoma: a rare cause of sciatica. BMJ Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Pandey S, Gokden M, Kazemi NJ, Post GR. Hematolymphoid Malignancies Presenting with Spinal Epidural Mass and Spinal Cord Compression: A Case Series with Rare Entities. Ann Clin Lab Sci. 2019;49:818-828. [PubMed] [Cited in This Article: ] |
| 39. | Snoj Ž, Riegler G, Moritz T, Bodner G. Brachial plexus ultrasound in a patient with myelodysplastic syndrome and myelosarcoma. Muscle Nerve. 2017;56:E170-E172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Yamamoto H, Yamamoto M, Omoto E, Kato T, Tajima K. An isolated myeloid blast crisis presenting as optic nerve sarcoma in a patient with chronic myeloid leukaemia treated with imatinib. Br J Haematol. 2015;170:290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Koh HJ, Baek J, Lee MS, Park HJ. Epidural chloroma and spinal cord compression. Chin Med J (Engl). 2019;132:853-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Kim J, Park HJ, Levin J, Won SJ. Neuromuscular Ultrasound for Myeloid Sarcoma Affecting the Sciatic Nerve: A Case Report. Ultraschall Med. 2021;42:654-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Rambeloson R, Ranoasy NF, Randrianjafisamindrakotroka O, Andriamiadanalisoa AO, Raobela L. [Bilateral orbital myeloid sarcoma: the circumstance of the discovery of acute myeloid leukemia (a case report)]. Pan Afr Med J. 2021;39:145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 44. | Anqi X, Siqing H, Zhenlin L, Chao Y. Sacral canal myeloid sarcoma as initial manifestation of granulocytic leukemia: MRI features and differential diagnosis (with a case report). Turk Neurosurg. 2014;24:281-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |