Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9834
Peer-review started: April 25, 2022
First decision: June 8, 2022
Revised: July 1, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: September 26, 2022
Paragangliomas and extra-adrenal pheochromocytomas are uncommon neuroendocrine tumors with ubiquitous distribution. Malignant paraganglioma is a relatively rare entity. We report the treatment and pathological characteristics of a patient with malignant paraganglioma, and summarize the latest advances in the treatment of malignant paraganglioma based on a literature review.
A 45-year-old Chinese woman presented to the hospital due to pain in the waist (right side) and right buttock, and was diagnosed as malignant paraganglioma after the placement of ureteral stent, implantation of ileus catheter, and trans
Clinical management of paraganglioma is challenging for endocrinologists and oncologists. Prospective studies are required to develop standardized therapeutic strategies for malignant paragangliomas.
Core Tip: Paragangliomas are widely distributed and have diverse clinical manifestations. Although most paragangliomas are benign, the malignancy is involved in approximately 10% of paragangliomas. The traditional treatment of malignant paragangliomas is surgery to the primary site. Surgery followed by adjuvant radiation is used less frequently, and chemotherapy is typically reserved for the distant disease. This study indicated the diagnostic features and therapeutic strategies of malignant paraganglioma. Therapeutic strategies for malignant paragangliomas are lacking. After the initial treatment, the patient’s progression-free survival reached 21 mo. Subsequently, the patient progressed and was treated again with chemoradiotherapy, surgery, and targeted therapy.
- Citation: Gan L, Shen XD, Ren Y, Cui HX, Zhuang ZX. Diagnostic features and therapeutic strategies for malignant paraganglioma in a patient: A case report. World J Clin Cases 2022; 10(27): 9834-9844
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9834.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9834
Pheochromocytomas and paragangliomas (PPGLs) are rare catecholamine-secreting endocrine tumors. Paraganglioma, first reported by Felix Frankel in 1886[1], is a neuroendocrine tumor arising from the neural crest cells and may occur anywhere along the paraganglia of the autonomic nervous system[2,3]. PGL is characterized by persistent hypertension and sympathetic activation. The main clinical features include hypertension, headache, hypermetabolism, hyperglycemia, and excessive sweating[4]. Approximately 10% of paragangliomas are malignant[5] and are characterized by metastatic spread. The traditional treatment for malignant paragangliomas is surgical excision of the primary lesion. Surgery followed by adjuvant radiation is used less frequently, and chemotherapy is typically reserved for cases with distant metastasis[6-8]. On immunohistochemical staining, paragangliomas generally exhibit positivity for neuron specific enolase (NSE), synaptophysin (Syn), and chromogranin A (CgA) within the chief cells, and S-100 and GFAP within the sustentacular cells[9-11]. Here we report the treatment of a middle-aged Chinese woman with malignant paraganglioma who was diagnosed based on immunohistochemical analyses, clinical symptoms, hematological examination, and imaging findings.
A 45-year-old Chinese woman presented with pain in right side of waist and right buttock, which has been present since April 2018.
She was experiencing pain extending from the right waist to the right buttock since April 2018. Initial physical examination and vital signs showed no significant abnormalities, and initial laboratory results were within the normal range.
Twenty-three years ago, the patient had a history of removal of a vaginal mass and a cesarean section. The previously removed vaginal mass was considered benign (not available for review). She was told that additional clinical or radiological follow-up was not necessary.
There was no family history of specific genetic or infectious diseases.
Physical examination revealed tenderness over the kidney region on percussion. Her vital parameters were: temperature, 36.5 °C; pulse rate, 75 beats/min; blood pressure, 135/88 mmHg.
The laboratory test findings were unremarkable. Biochemical tests showed normal levels of glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, total bilirubin, direct bilirubin, indirect bilirubin. Her electrolyte profile and renal function tests were normal. Tumor markers such as α-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 199 were negative, and there was no evidence of hepatitis A, B, C or E virus infection. Her blood pressure increased during hospitalization, and auxiliary examination showed increased levels of 24-h urine vanillylmandelic acid (VMA) (22.40 mg/24 h) and urine methoxynorepinephrine (NMN) levels (3880 μg/24 h).
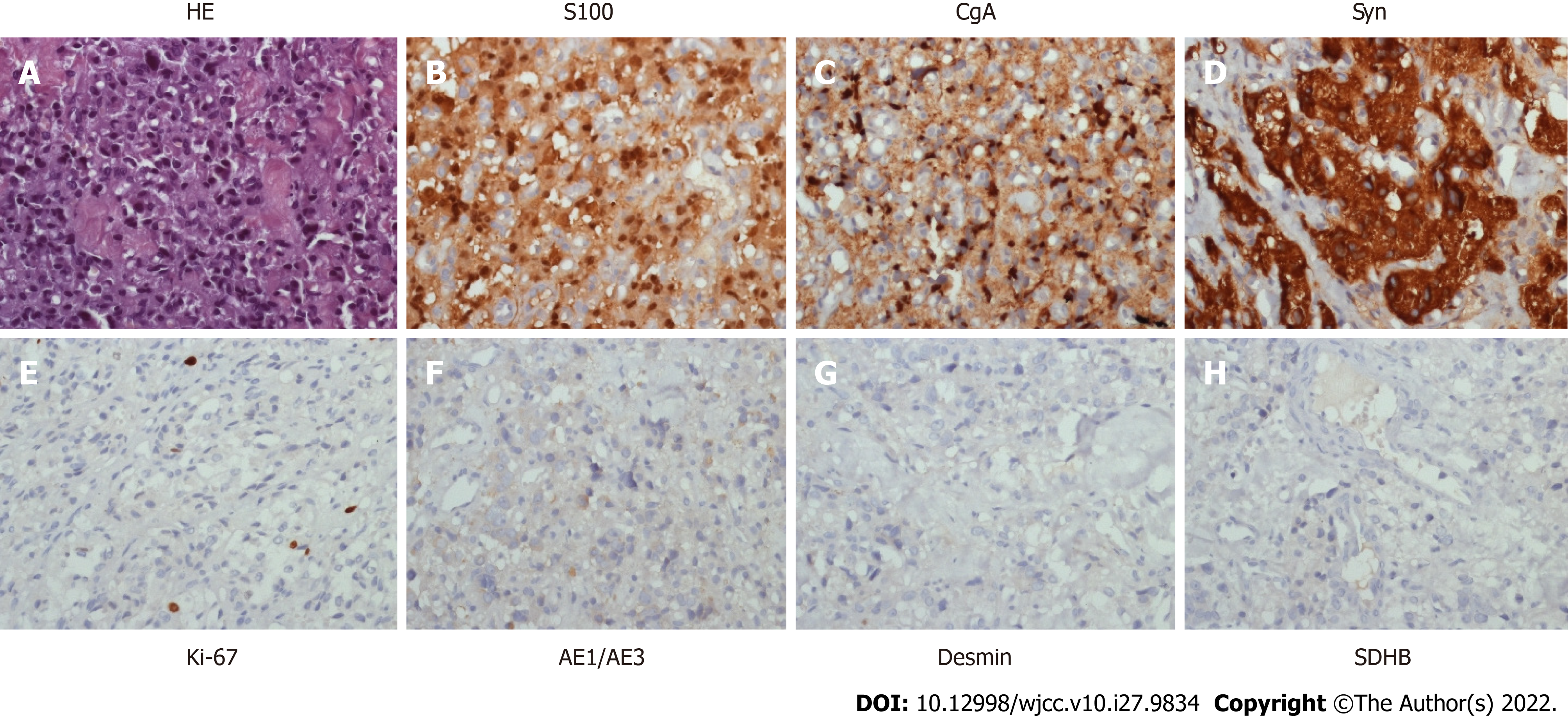
Ureteral stent implantation and laparoscopic pelvic mass biopsy were performed on May 17, 2018. Routine postoperative pathology showed that the cystic wall-like tissues of the pelvic mass were covered with squamous epithelium and other nerve tissues, suggesting the possibility of mature cystic teratoma. The patient recovered well after the operation and was referred to the gynecology department for further treatment. On December 6, 2018, the patient underwent transvaginal resection of the vaginal mass and laparoscopic release of pelvic adhesions. Routine postoperative pathology showed that the vaginal wall nodule was a malignant tumor, pending further diagnosis by the immunopathology. After the surgery, the patient developed abdominal distension and failed to pass flatus. X-ray indicated ileus and an ileus catheter was placed. However, the abdominal distension continued to aggravate. On December 24, 2018, CT-guided peritoneal puncture and drainage were performed; cytological examination revealed no tumor cells in the ascites fluid. The immunopathological examination of the vaginal mass was consistent with malignant paraganglioma. On immunohistochemical analysis, the tumor cells expressed S100 (+), Syn (+), CgA (+), CD56 (+), CD10 (-), Ki67 (+, 5%) and CK (-).
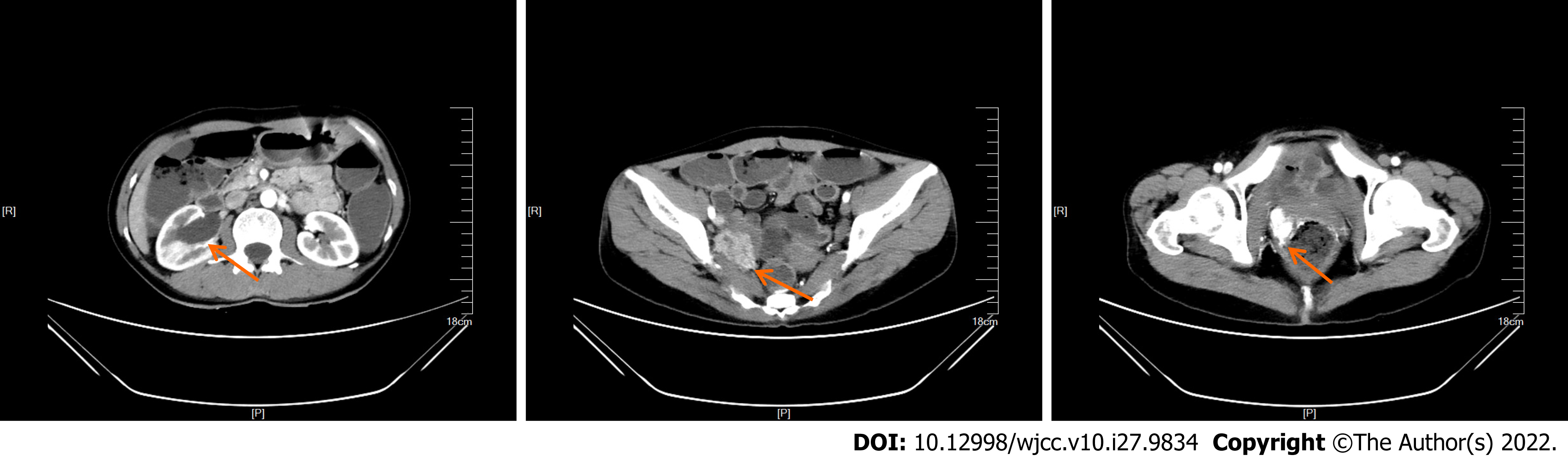
At the onset of symptoms in early April 2018, the patient had undergone B-ultrasonography at a local hospital, which showed right-sided hydronephrosis accompanied by distension of the right upper ureter and hypoechoic pelvic cavity on the right side. On admission at our hospital, enhanced computed tomography (CT) showed right hydronephrosis, right lower ureter mass, and right-sided vaginal mass (Figure 1).
The final pathological diagnosis after biopsy was malignant paraganglioma (Figure 2).
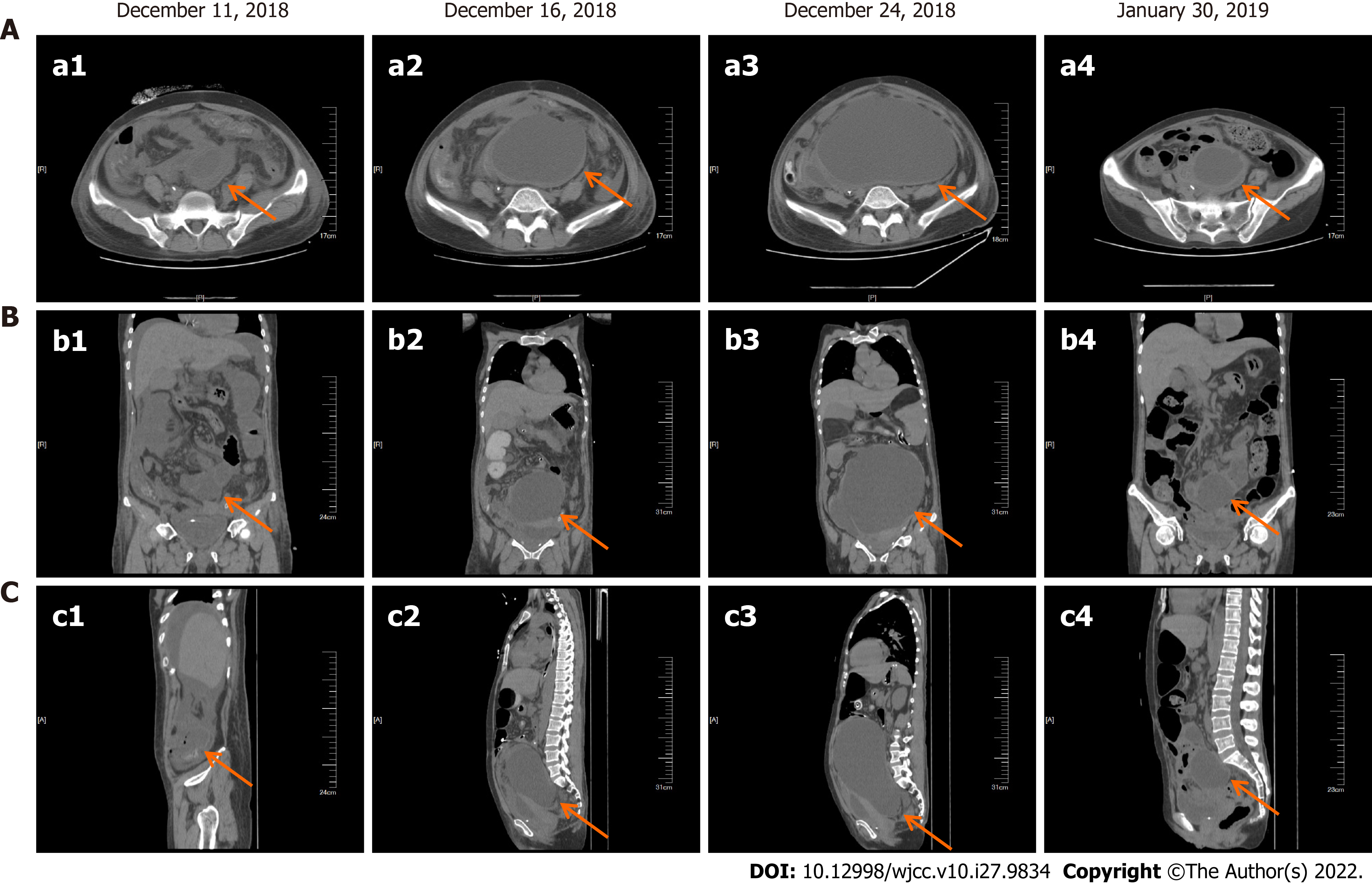
After the patient was relieved of intestinal obstruction, she received 2 cycles of liposome doxorubicin (25 mg/m2) intravenous chemotherapy combined with cisplatin (75 mg/m2) intraperitoneal infusion chemotherapy, which resulted in significant reduction in the pelvic effusion (Figure 3). The patient received a total of 7 cycles of liposome doxorubicin (25 mg/m2) in combination with cisplatin (75 mg/m2) intravenous chemotherapy until June 2019.
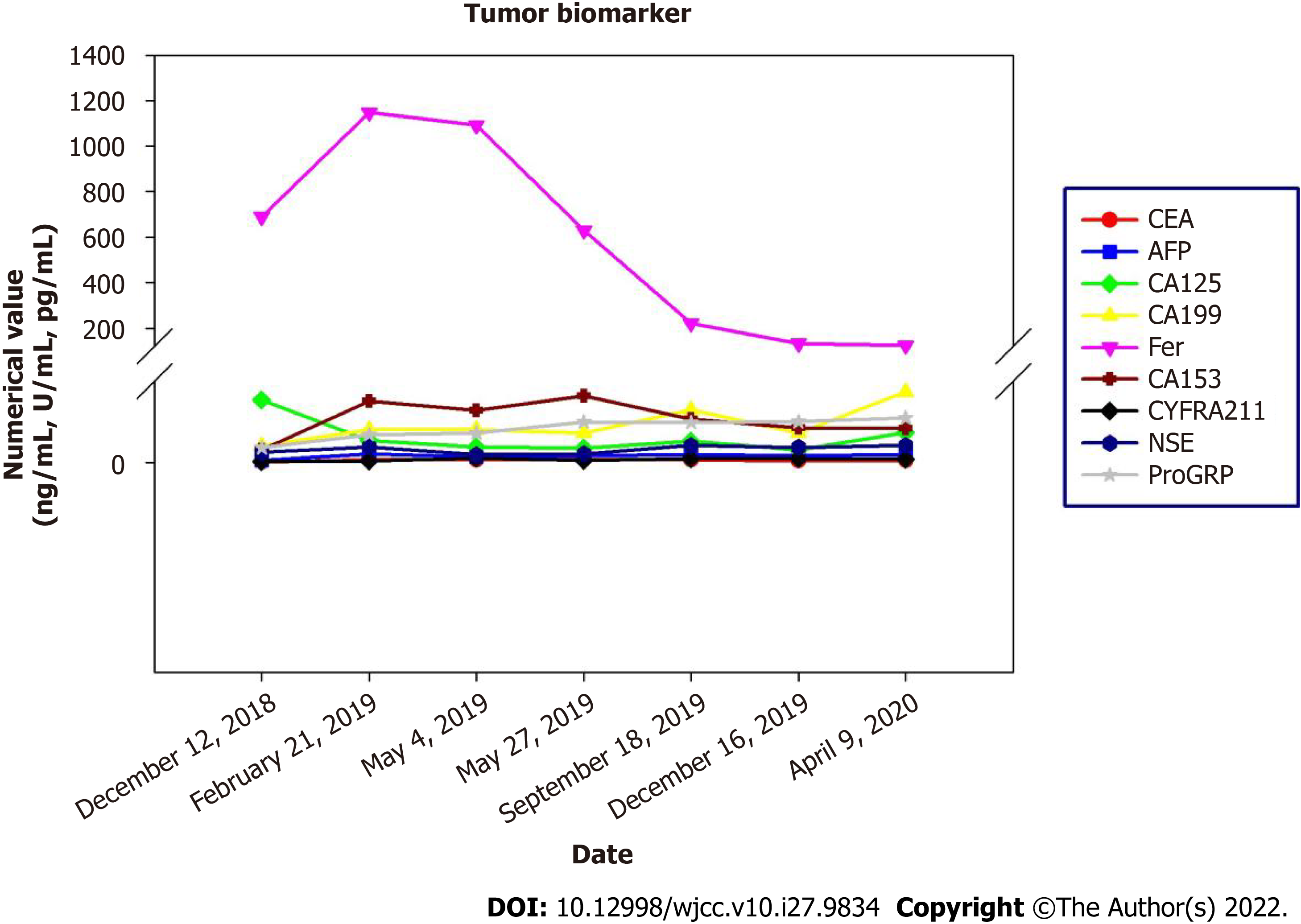
After completion of chemotherapy, the patient was followed up regularly, and the patient's symptoms were stable until April 2020 (Figure 4). During the diagnosis, treatment and follow-up of this patient, we simultaneously analyzed her blood tumor biomarkers. There are no specific tumor biomarkers for malignant paraganglioma, but on follow-up examinations, we found a gradual decrease in the ferritin content of this patient following the effective treatment. This indicated that ferritin content is a potential evaluation index of therapeutic efficacy in these patients (Figure 5).
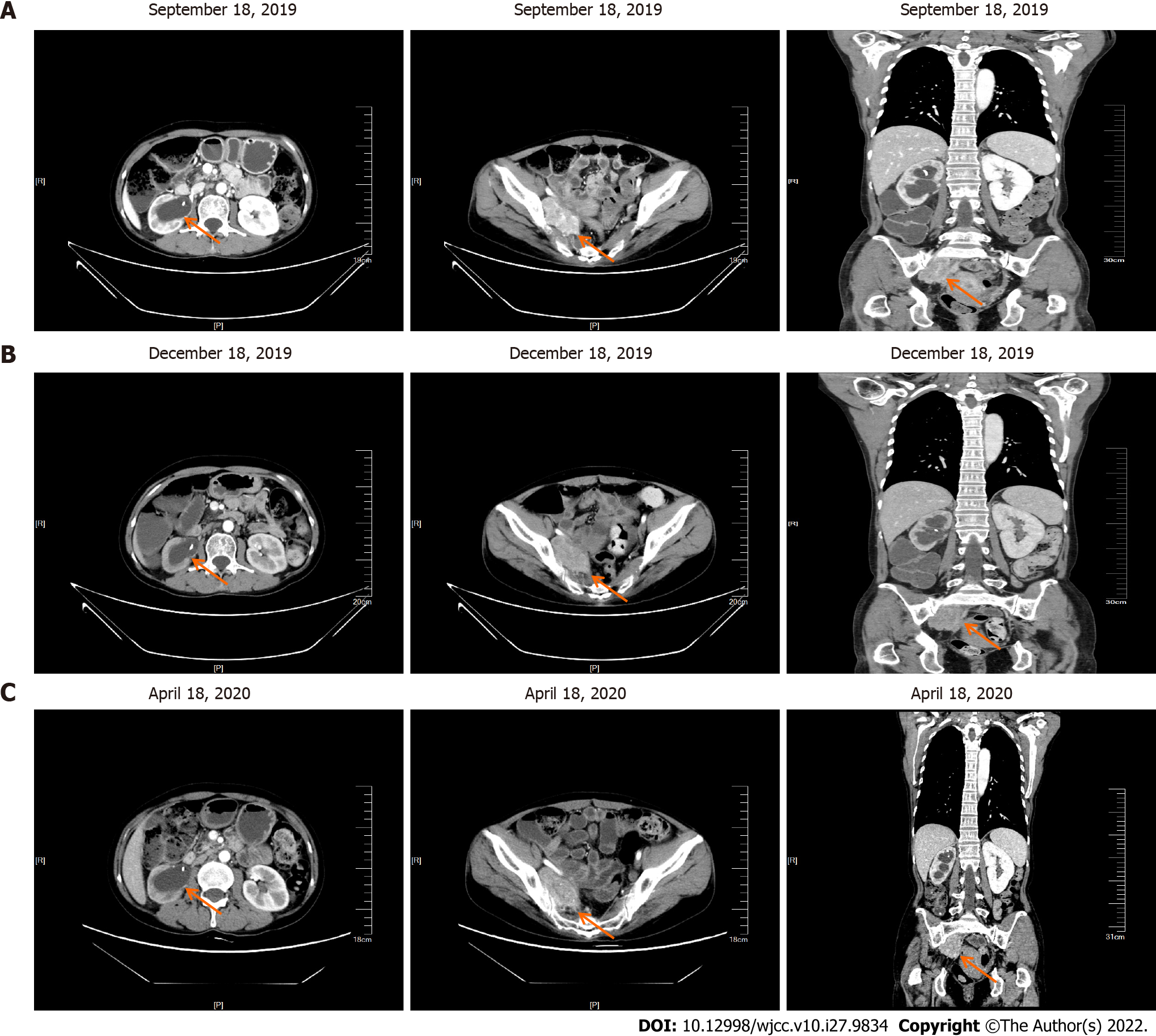
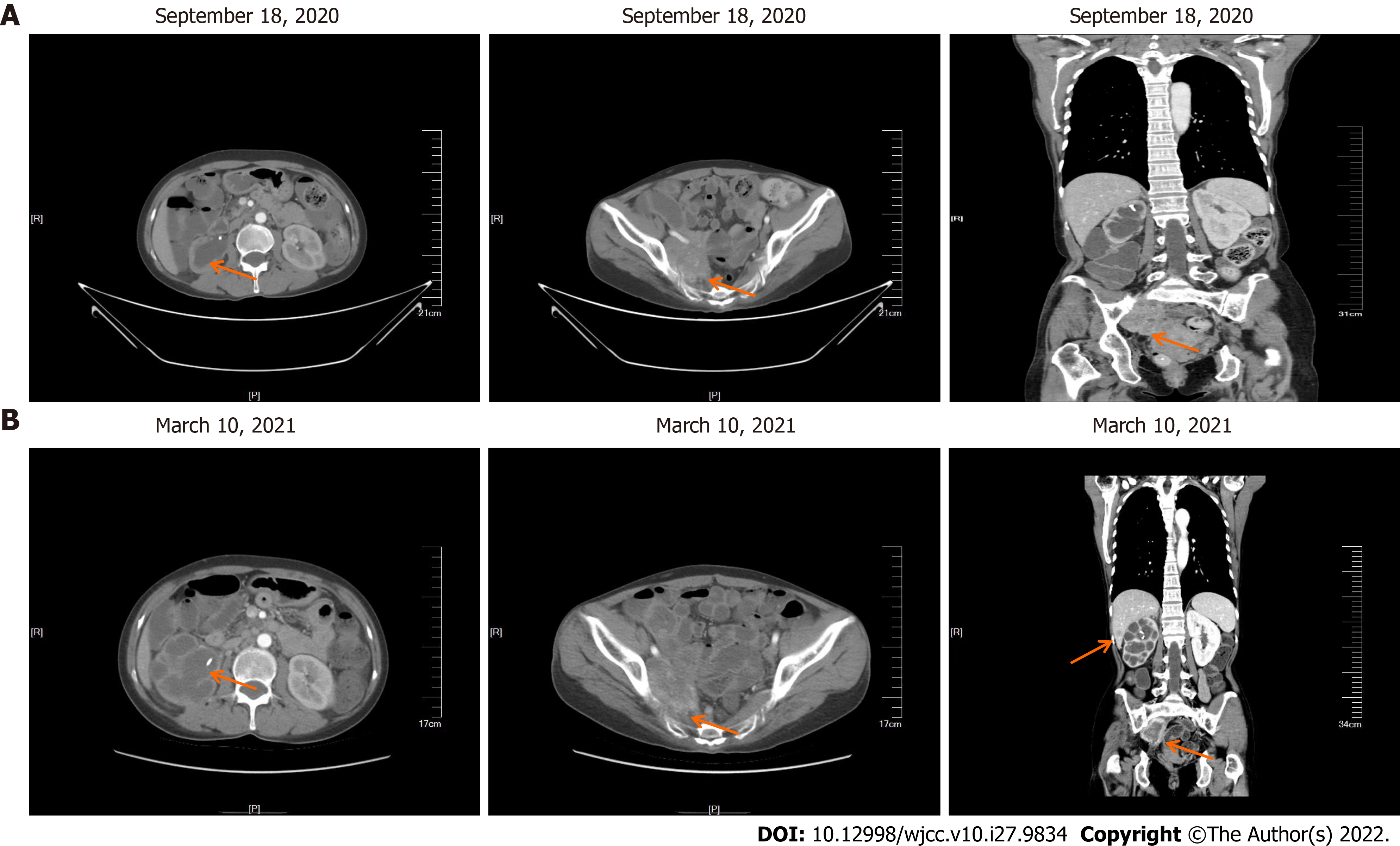
In September 2020, the patient came to our hospital for re-examination. Enhanced CT scan indicated an increase in the size of the right pelvic mass (Figure 6A). After comprehensive evaluation, the patient's disease was classified as progressive. Palliative radiotherapy was performed for the right pelvic mass (total dose 50 Gy administered in 25 sessions; 2 Gy per fraction). During the radiotherapy, two cycles of albumin-bound paclitaxel (260 mg/m2) in combination with nedaplatin (75 mg/m2) were simultaneously administered. After the radiotherapy, two cycles of intravenous chemotherapy with albumin-bound paclitaxel (260 mg/m2) combined with nedaplatin (75 mg/m2) were continued until December 2020. Repeat evaluation of the patient in March 2021, revealed multiple liver metastasis on enhanced CT scan, and the patient’s disease was classified as progressive once more (Figure 6B).
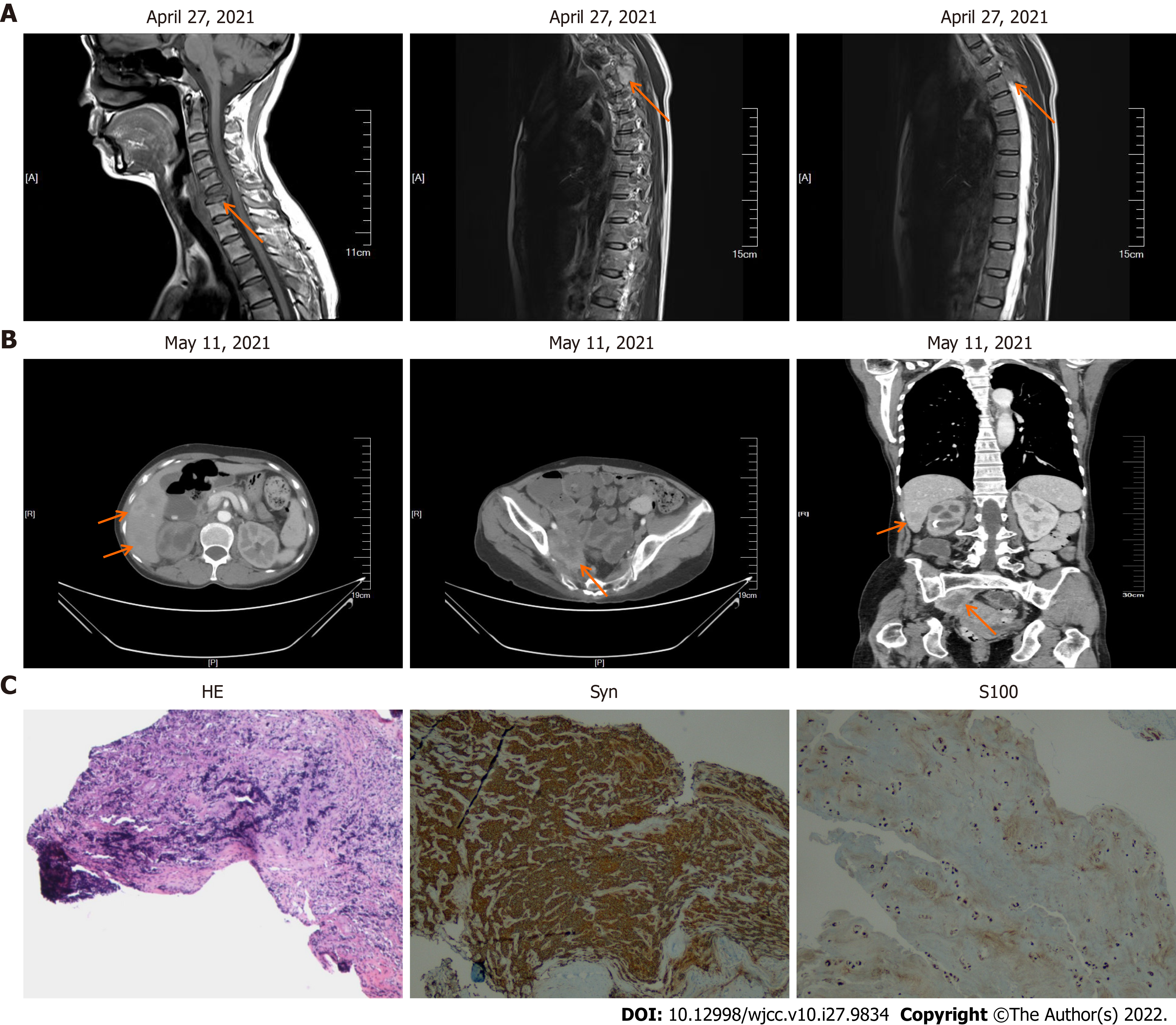
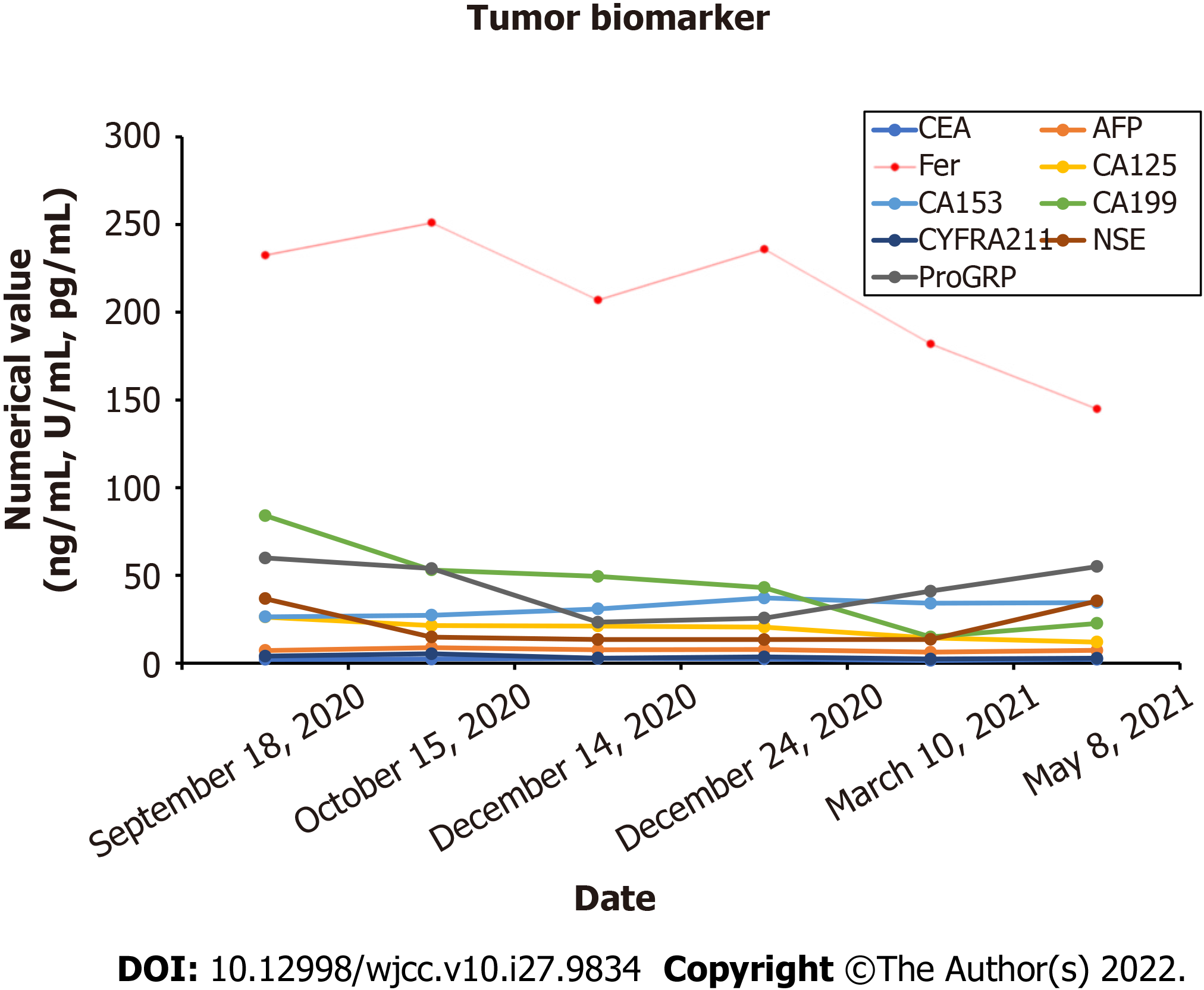
After one month, the patient complained of discomfort in neck and left shoulder, and numbness in left upper limb. Neck and thoracic enhanced magnetic resonance imaging (MRI) showed multiple lesions in the cervical and thoracic vertebral bodies, considered as tumor metastasis. MRI also showed narrowing of the C6 vertebral body (Figure 7A). Enhanced CT showed further increase in the liver metastatic lesions (Figure 7B). In order to relieve the patient's spinal cord compression, anterior jugular tumor resection and iliac bone graft fusion and internal fixation were performed under general anesthesia by the orthopedic surgeon on May 18, 2021. Immunohistochemical analysis of the excised jugular tumor was consistent with bone metastasis of malignant paraganglioma (Figure 7C). In the follow-up process of the patient, the results of the patient's tumor biomarkers were shown in Figure 8. One month after the surgery, the patient received palliative radiotherapy for bone metastases. Finally, the patient was initiated on molecular targeted therapy with apatinib.
Paragangliomas are defined as neuroendocrine tumors which may or may not produce catecholamines. Catecholamines such as dopamine, norepinephrine, and epinephrine are a class of neurotransmitters[12]. The most common symptoms of catecholamine excess are hypertension, tachycardia, headache, pallor, sweating, and anxiety[13, 14]. Pheochromocytomas/paragangliomas originate from chromaffin cells in the neural crest. Chromaffin cells are widely distributed in the adrenal medulla, sympathetic ganglia, and parasympathetic ganglia, and form Luckerkanal body which gradually atrophies in the adulthood in the para-aorta and the root of the inferior mesenteric artery. Paragangliomas were mostly believed to be undegenerated chromaffin tissues. Paragangliomas are widely distributed and have more diverse clinical manifestations than pheochromocytomas. In addition to the symptoms associated with excessive catecholamine secretion, local symptoms caused by tumor invasion may provide clues to the discovery of adrenal mass. Lesions in the retroperitoneal space may cause abdominal pain and/or lower back pain and constipation. Moreover, the mass may be palpable on physical examination. The common manifestations of paraganglioma include a series of sympathetic hyperactivity-related symptoms (such as paroxysmal hypertension and metabolic disorders), which are mainly attributable to the increase in blood catecholamine levels. Plasma and urine metanephrines and 24-h VMA levels may also increase. The first symptom of our patient was right-sided pain in the waist and hip. During hospitalization, her blood pressure increased and auxiliary examination revealed elevated 24-h urine VMA and urine NMN levels.
However, early diagnosis of malignant paraganglioma is difficult; moreover, pathological distinction between benign and malignant is challenging. Currently, the most reliable evidence for the determination of malignant lesions is the occurrence of vascular tumor emboli, local infiltration or lymph node involvement, and hematogenous metastasis (such as bone metastasis, liver metastasis, lung metastasis). The cytologic diagnosis of paraganglioma can be challenging because of its rarity, wide anatomic distribution, and variable cytomorphological features. On immunohistochemical staining, malignant pa
There is a lack of standardized therapeutic strategies for malignant paragangliomas. Targeted radionuclide therapies using 131I-metaiodobenzylguanidine (131I-MIBG) and peptide receptor radionuclide therapy (177Lu or 90Y) are viable therapeutic options in the management of meta
Because our patient had a large amount of abdominal and pelvic effusion, peritoneal puncture and drainage were performed, and peritoneal perfusion chemotherapy with cisplatin and intravenous chemotherapy with doxorubicin liposomes were administered. After two cycles of chemotherapy, there was significant reduction in abdominal and pelvic effusion. Several molecular targeted therapies, a novel radiopharmaceutical medication that targets the catecholamine transporter, and immunotherapy are under evaluation for the treatment of patients with malignant PPGs. Doxorubicin (DOX) is the most effective chemotherapeutic drug developed against a broad range of cancers such as solid tumors, leukemias, and lymphomas. Conventional DOX-induced cardiotoxicity has limited its use. FDA-approved drugs such as non-pegylated liposomal (Myocet®) and pegylated liposomal (Doxil®) formulations have no doubt shown comparatively reduced cardiotoxicity, but have raised new toxicity issues. The entrapment of doxorubicin in biocompatible, biodegradable, and safe nano delivery systems can prevent its degradation in circulation, minimize its toxicity with increased half-life, and enhance its pharmacokinetic profile leading to improved patient compliance. In addition, nano delivery systems can actively and passively target the tumor resulting in increased therapeutic index and decreased side effects of drug. In our case report, the patient accepted chemotherapy combined with the pegylated liposomal doxorubicin and cisplatin 7 cycles until June 2019.
Our patient showed good therapeutic response after the initial treatment with 7 cycles of chemo
After the initial treatment, the patient’s PFS was 21 mo. During follow-up, enhanced CT scan showed enlargement of that right-sided pelvic mass, for which radiotherapy and chemotherapy were administered. Repeat imaging evaluation revealed bone metastases and liver metastases. Due to the development of spinal cord compression, she underwent orthopedic surgery, followed by radiotherapy and molecular targeted therapy with apatinib. The clinical management of paraganglioma is cha
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Youth Committee of Oncology Branch of Suzhou Medical Association.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fazilat-Panah D, Iran; Ghimire R, Nepal S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Classics in oncology. A case of bilateral completely latent adrenal tumor and concurrent nephritis with changes in the circulatory system and retinitis: Felix Fränkel, 1886. CA Cancer J Clin. 1984;34:93-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Granados-Garcia M, Herrera-Gomez A. Carotid body tumors: review of a 20-year experience. Oral Oncol. 2005;41: 56-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Lee JH, Barich F, Karnell LH, Robinson RA, Zhen WK, Gantz BJ, Hoffman HT. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer. 2002;94:730-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Cui Q, Lu J, Zhang C, Tan S. Diagnostic challenges and good treatment outcomes in pediatric paraganglioma of the abdomen: A case report. Medicine (Baltimore). 2018;97:e13268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SKG, Murad MH, Naruse M, Pacak K, Young WF. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1592] [Cited by in F6Publishing: 1492] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 6. | Moskovic DJ, Smolarz JR, Stanley D, Jimenez C, Williams MD, Hanna EY, Kupferman ME. Malignant head and neck paragangliomas: is there an optimal treatment strategy? Head Neck Oncol. 2010;2:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Hinerman RW, Amdur RJ, Morris CG, Kirwan J, Mendenhall WM. Definitive radiotherapy in the management of paragangliomas arising in the head and neck: a 35-year experience. Head Neck. 2008;30:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Bastounis E, Maltezos C, Pikoulis E, Leppäniemi AK, Klonaris C, Papalambros E. Surgical treatment of carotid body tumours. Eur J Surg. 1999;165:198-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Srivastava R, Wadhwa N, Gupta S, Razdan U. Nasal Polyp-An Incidental Paraganglioma. Turk Patologi Derg. 2016;32:196-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Welkoborsky HJ, Gosepath J, Jacob R, Mann WJ, Amedee RG. Biologic characteristics of paragangliomas of the nasal cavity and paranasal sinuses. Am J Rhinol. 2000;14:419-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Wasserman PG, Savargaonkar P. Paragangliomas: classification, pathology, and differential diagnosis. Otolaryngol Clin North Am. 2001;34:845-862, v. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NV, Dahia PL, de Krijger RR, Giordano TJ, Greene LA, Goldstein DS, Lehnert H, Manger WM, Maris JM, Neumann HPH, Pacak K, Shulkin BL, Smith DI, Tischler AS, Young WF. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Zelinka T, Eisenhofer G, Pacak K. Pheochromocytoma as a catecholamine producing tumor: implications for clinical practice. Stress. 2007;10:195-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1154] [Cited by in F6Publishing: 1061] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 15. | Edström Elder E, Hjelm Skog AL, Höög A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Pham TH, Moir C, Thompson GB, Zarroug AE, Hamner CE, Farley D, van Heerden J, Lteif AN, Young WF. Pheochromocytoma and paraganglioma in children: a review of medical and surgical management at a tertiary care center. Pediatrics. 2006;118:1109-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Fishbein L, Bonner L, Torigian DA, Nathanson KL, Cohen DL, Pryma D, Cengel KA. External beam radiation therapy (EBRT) for patients with malignant pheochromocytoma and non-head and -neck paraganglioma: combination with 131I-MIBG. Horm Metab Res. 2012;44:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Baudin E, Habra MA, Deschamps F, Cote G, Dumont F, Cabanillas M, Arfi-Roufe J, Berdelou A, Moon B, Ghuzlan AA, Patel S, Leboulleux S, Jimenez C. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. Eur J Endocrinol. 2014;171:R111-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Garibaldi E, Bresciani S, Panaia R, Delmastro E, Malinverni G, Gabriele P. Hereditary paraganglioma syndrome associated with SDHD gene mutations: a patient with multicentric presentation treated with radiotherapy. Case report. Tumori. 2011;97:214-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 20. | van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EP. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;80:487-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Asai S, Katabami T, Tsuiki M, Tanaka Y, Naruse M. Controlling Tumor Progression with Cyclophosphamide, Vincristine, and Dacarbazine Treatment Improves Survival in Patients with Metastatic and Unresectable Malignant Pheochromocytomas/Paragangliomas. Horm Cancer. 2017;8:108-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Cassol CA, Winer D, Liu W, Guo M, Ezzat S, Asa SL. Tyrosine kinase receptors as molecular targets in pheochromocytomas and paragangliomas. Mod Pathol. 2014;27:1050-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | AyalaRamirez M, Chougnet CN, Habra MA, Palmer JL, Leboulleux S, Cabanillas ME, Caramella C, Anderson P, Ghuzlan AA, Waguespack SG, Deandreis D, Baudin E, Jimenez C. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97:4040-4050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Jimenez C, Cabanillas ME, Santarpia L, Jonasch E, Kyle KL, Lano EA, Matin SF, Nunez RF, Perrier ND, Phan A, Rich TA, Shah B, Williams MD, Waguespack SG. Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. J Clin Endocrinol Metab. 2009;94:386-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Roman-Gonzalez A, Jimenez C. Malignant pheochromocytoma-paraganglioma: pathogenesis, TNM staging, and current clinical trials. Curr Opin Endocrinol Diabetes Obes. 2017;24:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Fishbein L. Pheochromocytoma and Paraganglioma: Genetics, Diagnosis, and Treatment. Hematol Oncol Clin North Am. 2016;30:135-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |