Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6218
Peer-review started: November 23, 2021
First decision: January 11, 2022
Revised: January 19, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Vancomycin is the most commonly used drug for methicillin-resistant Staphylococcus aureus. The empirical clinical doses of vancomycin based on non-obese patients may not be optimal for obese ones.
This study reports a case of vancomycin dosing adjustment in an obese patient (body mass index 78.4 kg/m2) with necrotizing fasciitis of the scrotum and left lower extremity accompanied with acute renal failure. Dosing adjustment was performed based on literature review and factors that influence pharmacokinetic parameters are analyzed. The results of the blood drug concentration monitoring confirmed the successful application of our dosing adjustment strategy in this obese patient. Total body weight is an important consideration for vancomycin administration in obese patients, which affects the volume of distribution and clearance of vancomycin. The alterations of pharmacokinetic parameters dictate that vancomycin should be dose-adjusted when applied to obese patients. At the same time, the pathophysiological status of patients, such as renal function, which also affects the dose adjustment of the patient, should be considered.
Monitoring vancomycin blood levels in obese patients is critical to help adjust the dosing regimen to ensure that vancomycin concentrations are within the effective therapeutic range and to reduce the incidence of renal injury.
Core Tip: We report the medical records of dose adjustment of vancomycin in an obese patient (body weight 240 kg), including the dose adjustment protocol in acute renal injury. This article also reviews the current literature on the application of vancomycin in the obese population and provides recommendations on how to make dose adjustments based on available evidence.
- Citation: Xu KY, Li D, Hu ZJ, Zhao CC, Bai J, Du WL. Vancomycin dosing in an obese patient with acute renal failure: A case report and review of literature. World J Clin Cases 2022; 10(18): 6218-6226
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6218.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6218
Since 1980, the prevalence of obesity has more than doubled worldwide. It is estimated that by 2030, 60% of the world's adult population will be classified as obesity[1]. In the United States from 2013 to 2014, the prevalence of obesity was 35.0% for male and 40.4% for female adults, and there was a significant linear increasing trend among women in the prevalence of obesity from 2005 through 2014[2]. Obesity has also become a major public health burden in China. Over the past 40 years, the prevalence of obesity has increased significantly. The nationally representative survey showed that more than half of the Chinese adults are obese according to the Chinese standards[3]. The increased prevalence of obesity poses a challenge for clinicians to deliver optimized doses of antimicrobial drugs in the intensive care unit. Obesity is a key risk factor for community and hospital-acquired infections[4], and increases risks of incidence and mortality compared to non-obese individuals[5]. It may affect the pharmacokinetics of antimicrobial agents, particularly in patients requiring high-dose antimicrobial therapy[6], and can also influence the immune response and increase susceptibility to infections[7], resulting in a high risk of infection in obese patients[8]. As a consequence, clinicians are increasingly facing severely obese patients requiring antibiotic treatment. However, few studies have summarised the published data and provided clinical guidance for effective dosing in these patients.
Since the early 1980s, as the number of methicillin-resistant Staphylococcus aureus (MRSA) infections began to increase, vancomycin has become the drug of first choice for this microbial infection[9]. Vancomycin belongs to glycopeptide antibiotic which acts by inhibiting bacterial cell wall synthesis[10]. It is the most widely used antibiotic worldwide for the treatment of severe Gram-positive bacterial infections[11]. The binding of vancomycin to protein is approximately 50% to 55%[10]. The volume of distribution is 0.4-1 L/kg[9]. Vancomycin is primarily cleared via renal excretion[12]. The actual body weight of obese subjects increases the chance of vancomycin exposure and the incidence of vancomycin-associated nephrotoxicity[13]. Therefore, dose adjustment is required when vancomycin is used in obese patients, because of the effect of obesity on vancomycin pharmacokinetic parameters. One study shows that therapeutic drug monitoring (TDM) significantly improves the clinical curative effect and reduces the incidence of nephrotoxicity in patients treated with vancomycin[14].
Although the pharmacokinetics of vancomycin in the general population is well-described, to the best of our knowledge, only a few studies have investigated the effect of vancomycin dose in the obese population. This study reports the medical records of dose adjustment of vancomycin in an obese patient weighing up to 240 kg, including the dose adjustment protocol in the acute renal injury. This article also reviews the current literature on the application of vancomycin in the obese population and provides recommendations on how to make dose adjustments based on the available evidence.
A 40-year-old man was referred to our intensive care unit (ICU), with the complaints of chest tightness and shortness of breath with no obvious cause for 3 mo.
In November 2020, the patient reported chest tightness and shortness of breath with no obvious cause. Three days later, the patient’s symptoms aggravated with abdominal distension and edema of both lower limbs. He was admitted to the ICU of a local hospital for acute respiratory failure. After 2 wk of treatment, the patient still had persistent fever and was transferred to the ICU of our hospital on November 18, 2020.
The patient had suffered from hypertension for 3 years and erysipelas of the right lower extremity for 2 years.
The patient had no specific personal or family history.
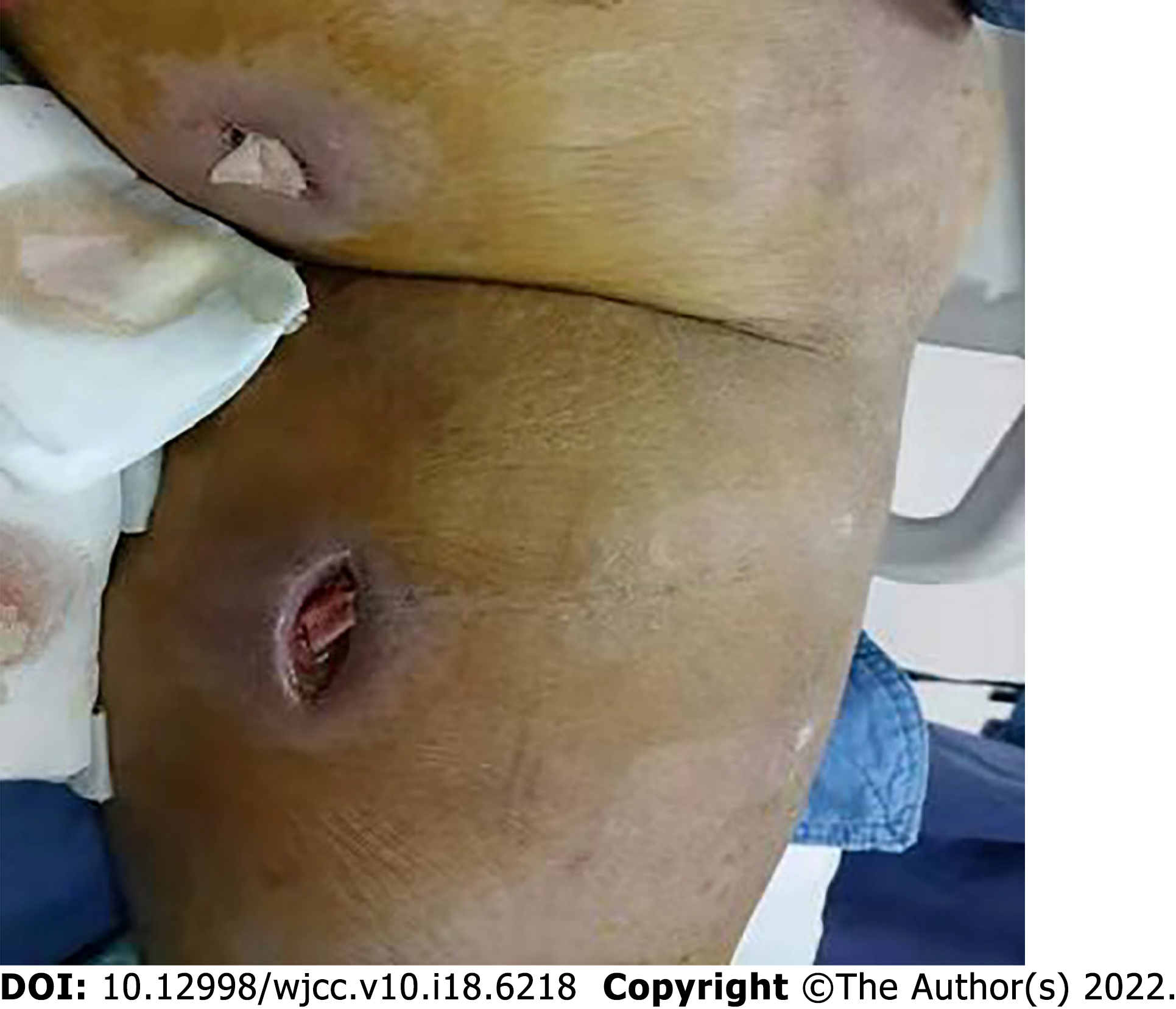
The patient’s height and body weight were 175 cm and 240 kg, respectively. The patient had necrotizing fasciitis of the scrotum and left lower extremity, and large brown skin pigmentation of the left calf, and two approximately 2-cm surgical incisions with built-in gauze drainage and cloudiness drainage fluid were visible in the left thigh and the middle of the left calf (Figure 1).
The culture of secretion revealed Staphylococcus hemolyticus at a local hospital.
There were no abnormal imaging data findings.
The final diagnoses were: (1) Sepsis; (2) Acute respiratory distress syndrome; (3) Pneumonia; (4) Heart failure; (5) Necrotizing fasciitis of the scrotum and left lower extremity; and (6) Severe obesity.
The patient had pulmonary infection and Staphylococcus hemolyticus was detected in his secretion at the local hospital. His initial serum creatinine was 63.3 μmol/L and creatinine clearance (CrCl) was greater than 90 mL/min. Based on the patient's history and drug sensitivity testing results, intravenous levofloxacin 0.75 g/d and tigecycline 0.2 g/d were started empirically for anti-infection treatment. Then, linezolid 0.6 g intravenous injection every 12 h was prescribed to replace levofloxacin, and the patient's temperature decreased to normal after 3 d of treatment. On November 27, the patient developed a high fever (temperature up to 40.2 °C), and his high-sensitivity C-reactive protein (hs-CRP) rose to 183.51 mg/L (Table 1). Considering the infection from the lower extremity and the scrotum, the patient received enhanced drainage and dressing change. Meanwhile, the culture of sputum and scrotal revealed Acinetobacter baumannii. The linezolid was subsequently discontinued and intravenous infusion of vancomycin was started. Because the patient was severely obese, after reviewing the literature, we determined the dosing regimen of a loading dose (vancomycin administered as continuous infusion of 2 g over 2 h) and a maintenance dose (vancomycin 1 g infused over 60 min every 8 h). The vancomycin blood trough concentration was 11.7 μg/mL after the patient had received three doses of vancomycin. The patient developed acute renal failure due to the aggravation of infection, the serum creatinine levels showed a gradual increase, and the vancomycin trough concentration was greater than 20 μg/mL (up to 34.3 μg/mL). We then adjusted the vancomycin administration dose according to the blood drug concentration monitoring. On December 16, continuous renal replacement therapy (CRRT) was used because of anuria of the patient. Given using continuous veno-venous hemodiafiltration mode, we adjusted the vancomycin administration dose to 1 g every 12 h, during which vancomycin blood drug concentration fluctuated between 10 and 20 μg/mL.
| Check item/date | November 19 | November 28 | December 1 | December 11 | December 16 | December 20 | December 25 |
| White blood cell count (× 109/L) | 5.50 | 9.59 | 8.16 | 14.02 | 35.29 | 20.19 | 7.71 |
| Neutrophil percentage (%) | 62.6 | 75.80 | 75.00 | 85.60 | 85.90 | 82.20 | 68.30 |
| Procalcitonin(ng/mL) | 0.130 | 0.190 | 0.190 | 9.170 | 53.760 | 4.930 | 1.160 |
| High-sensitivity C-reactive protein(mg/L) | 183.51 | 194.91 | 140.00 | 68.80 | 99.00 | 22.30 | 8.91 |
| Serum creatinine (μmol/L) | 67.8 | 61.8 | 48.9 | 266.8 | 453.1 (CRRT) | 120.9 (CRRT) | 264.1 (CRRT) |
The treatment produced significant improvement in the patient’s respiratory status and the infection. Vancomycin and CRRT treatment were subsequently discontinued on December 24. Two days later, the patient was transferred out of the ICU to continue treatment. He was well with no further complaints at the routine 1-mo follow-up.
In recent years, body mass index (BMI) is a world-accepted grading method to assess the degree of obesity. According to the criteria of the guideline, obesity is defined as a BMI of 30.0 kg/m2 or higher[15]. Based on the body weight and height of this patient, his BMI was calculated to be 78.4 kg/m2, which met the threshold for obesity. Numerous physiopathological changes occur in obese individuals, including changes in distribution (Vd) and renal excretion[16].
Vancomycin is a time-dependent antibiotic and a number of factors influence its clinical activity, including variable tissue distribution, dose size, and clearance rate[17]. One study showed that total body weight (TBW) influenced the Vd and clearance (CL) of vancomycin (Table 2)[18]. As expected, obesity is a known factor affecting drug pharmacokinetics[19]. Vancomycin, as a hydrophilic drug, is able to penetrate and distribute, to a certain extent, in adipose tissues, thereby increasing the Vd[20]. A large retrospective study by Ducharme et al[21] showed that the Vd was greater in obese subjects than in normal subjects by examining pharmacokinetics of vancomycin in 704 patients. Blouin and his colleagues[22] also demonstrated statistically significant differences in weight-indexed Vd between two groups of subjects. A recent study suggests that Vd changes in obese patients can be ascribed to the physicochemical properties of the drugs in most cases[23]. In addition, the degree of the Vd depends on the lipophilicity, hydrophilicity, protein binding, and molecular weight of the antibiotic[24]. In the obese population, higher cardiac output and blood volume may increase blood flow, and lead to larger Vd[25]. Edema combined with fluid resuscitation can increase the Vd of different antibacterial agents in obese, critically ill patients[26].
| Title (year) | Design | Results | Conclusions | Ref. |
| Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed staphylococcus aureus infections (2015) | Prospective pharmacokinetic study | When the minimum inhibitory concentration (MIC) was 1 μg/ml, the probability of the concentration-time curve (AUC)/MIC rate of 400 for vancomycin at 4000 to 5000 mg/d was 93% | Vd and clearance of vancomycin were affected by total body weight, respectively | Adane et al[18] |
| To assess vancomycin pharmacokinetic parameters in obese patients | ||||
| n = 31 | ||||
| Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight (1994) | Retrospective review | Vd is 0.69 L/kg IBW in normal females compared with 0.58 in men | Vancomycin dosing can be improved by adapting the initial estimates of Vd in obese people | Ducharme et al[21] |
| Comparative pharmacokinetics of vancomycin using steady-state peak and trough serum concentrations | ||||
| The Vd for obese women and men was 1.17 and 0.90 L/kg IBW respectively | ||||
| n = 704 | ||||
| Vancomycin pharmacokinetics in normal and morbidly obese subjects (1982) | An uncontrolled study | Significant differences in mean terminal half-life and volume of distribution values between normal and morbidly obese individuals | TBW should be used for dosing of vancomycin in obese individuals | Blouin et al[22] |
| Vancomycin pharmacokinetics was determined in normal and morbidly obese populations | ||||
| n = 10 | ||||
| Strong correlations between TBW and Vd and total body clearance | ||||
| Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens (2011) | A retrospective data collection | Patients with a creatinine clearance of 100 ml/min/1.73 m2 should receive a continuous infusion at least 35 mg/kg/d to maintain target concentrations | TBW should be considered for the initial dose | Roberts et al[39] |
| To perform a pharmacokinetic analysis of vancomycin in subjects | ||||
| The maintenance dose can be directed by creatinine clearance | ||||
| n = 206 | ||||
| Dosing vancomycin in the super obese: less is more (2018) | Retrospective study | Maintenance dose > 4500 mg/d is not required in obese patients to reach the pharmacodynamic AUC target | Using AUC-targeted TDM can optimize the treatment of obese adults | Crass et al[40] |
| Determining an experiential vancomycin dosing strategy for obese individuals | ||||
| n = 346 | ||||
| The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock (2016) | A prospective, non-comparative study | The two-compartmental first-order elimination model | In the early stages of septic shock, the total clearance of vancomycin increased, while the volumes of distribution of the central and peripheral compartments did not increase | Katip et al[52] |
| To investigate the pharmacokinetics of vancomycin in patients with early septic shock | The mean ± SD of the total vancomycin clearance (3.70 ± 1.25 L/h) was higher than in patients with non-septic shock | |||
| n = 12 | There was no increase in the volume of the central compartment (8.34 ± 4.36 L) or the volume of peripheral compartment (30.99 ± 7.84 L) compared to patients with non-septic shock | |||
| Multicenter evaluation of vancomycin dosing: emphasis on obesity (2008) | A random sampling | Adequate initial doses were achieved in 93.9% of overweight patients and 27.7% of obese patients | The patient receives a weight-based dose | Hall et al[53] |
| Patients receiving vancomycin were categorised by body mass index and randomly chosen from the computer-generated query | ||||
| n = 421 | ||||
| Performance of a vancomycin dosage regimen developed for obese patients (2012) | Retrospective review | Revised strategy resulted in a higher frequency of target troughs | Compared with the original strategy, the revised strategy improved the attainment of target trough concentrations with minimal nephrotoxicity | Reynolds et al[54] |
| Comparison of original and revised dosing regimens for achieving target serum trough concentrations and occurrence of nephrotoxicity in obese subjects | ||||
| n = 138 |
Previous studies indicated that CL of vancomycin was much higher in the obese population, especially in young obese patients, and they required high doses to obtain adequate trough concentrations[9]. Han et al[27] demonstrated that obese adults exhibited higher drug clearance rates than non-obese ones. Unlike Vd, the physicochemical properties of drugs have little effect on CL, which is largely controlled by physiological processes[23]. The change in clearance was mainly attributed to an increase in kidney mass and renal blood flow in obese subjects[28]. Greater glomerular filtration rate and renal perfusion in obese individuals increase the CL of vancomycin[29]. At the same time, greater renal volume, hypertrophy of the renal unit, and hydrostatic pressure of the glomerulus were also associated with greater CL of vancomycin in the obese group[30]. Vancomycin is a hydrophilic drug with predominant renal excretion. Furthermore, augmented renal clearance (ARC), defined as a creatinine clearance more than or equal to 130 mL/min/1.73 m2, refers to enhanced elimination of hydrophilic solutes by the kidneys[31]. The results indicate that ARC has been described in the obese, non-critically ill patient[32], and is a common finding in critically ill patients with normal plasma creatinine concentrations[33].
The option of vancomycin loading doses is dependent on the estimate of the Vd. Pharmacokinetic research had demonstrated that vancomycin Vd increases with increasing TBW[34]. The physicochemical properties of drugs lead us not to define a universal body-size parameter for the distribution and clearance of drugs. As a consequence, the body weight was used in dose selection for drug administration[35]. One guideline states that a reasonable approach to the initial dose of vancomycin in obese individuals is to increase the loading dose to 20 to 25 mg/kg TBW and to decrease the maintenance dose, then adjust the dose according to TDM[36]. The 2020 Infectious Diseases Society of America (IDSA) consensus recommends the use of a TBW-based loading dose of 20 to 25 mg/kg in obese adults with severe infections, and considers capping doses of 3000 mg as the most practical dosing regimen[37].
Data have shown an excellent correlation between TBW and CL[38]. Thus, the empirical maintenance dose of vancomycin is dependent on the estimated CL[39]. The initial maintenance doses of vancomycin can be calculated by vancomycin CL and target AUC for obese population[18,40]. The 2020 IDSA consensus points out that the mean vancomycin CL in obese patients is approximately 6 L/h, which corresponds to an AUC of approximately 500 mg·h/L at a daily dose of 3000 mg. The empirical vancomycin maintenance dose for obese adults should not exceed 4500 mg/d because vancomycin CL rarely goes beyond 9 L/h[37].
The pharmacodynamic parameter that best predicts the efficacy of vancomycin is the ratio of the area under the curve (AUC) to the minimum inhibitory concentration (MIC)[9]. In adult patients with suspected or definitive serious MRSA infection, the AUC/MIC ratio (assuming a vancomycin MIC of 1 mg/L) with targets between 400 and 600 was recommended in the American Society of Health-System Pharmacists (ASHP) 2020 guideline[37]. Based on the historical difficulty of AUC estimation in clinical practice, previous expert guidelines recommended monitoring trough concentrations as a surrogate marker for the AUC/MIC ratio[41]. The 2020 Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin recommends maintaining vancomycin steady-state trough concentrations at 10–20 mg/L to achieve clinical efficacy and improve patient safety[42].
CRRT is a common treatment for critically ill patients with acute renal injury[43]. With advances in hemodialysis membrane technology, vancomycin is cleared substantially by effective and high-flux dialyzers[44]. Therefore, vancomycin dosing regimens for CRRT need to be changed, but there is no mention of CRRT dosing recommendations in the latest FDA-approved vancomycin package insert[45]. Vd may be increased in CRRT patients compared to healthy individuals with normal kidney function[46]. During CRRT treatment, vancomycin CL remains a near-steady-state condition over the dosing interval, although vancomycin CL may decline over time as a result of hemodialysis filter plugging[46]. Vancomycin CL is closely related to the flow rate of ultrafiltration/dialysis solution[47]. The recommended loading dose for patients receiving CRRT is based on the actual TBW, at the dose of 20 to 25 mg/kg[48]. In order to achieve the generation of steady-state concentrations between 15 and 20 mg/L, a maintenance dose of 400 to 650 mg/12 h of vancomycin at an ultrafiltration flow rate of 30-40 mg/kg/h is recommended for most critically ill patients[49]. Due to the unstable clinical situation, vancomycin concentration must be strictly monitored in critical patients[50].
In summary, we report a case of adjusting the blood concentration of vancomycin with enhanced effectiveness in an obese patient. The initial TBW of the patient with normal renal function was 240 kg. Thus, the patient should receive an initial TBW-based load of 6 to 7.2 g of vancomycin every day. However, the dose of vancomycin is greater than 4 g/d, which increases the risk of nephrotoxicity[51]. Following the recommended dose limit of 3 g, the patient received an initial TBW-based loading dose of 2 g and a maintenance dose of 1 g of vancomycin every 8 h. The initial serum concentration of 11.7 μg/mL was obtained, after the patient had received three doses of vancomycin. The serum concentration demonstrated that the dosing regimen is reasonable. Due to acute renal failure with reduced urine output or even anuria, intravenous injection of vancomycin at 3 g/d led to a blood concentration of vancomycin that was higher than 20 μg/mL. We immediately reduced the dose of vancomycin and monitored the blood concentration of the drug. On the 29th day, the patient was treated with CRRT, the dosage regimen of vancomycin was 1 g every 12 h considering the clearance of vancomycin by CRRT, and the blood concentration was 13.3 μg/mL. The final blood concentration of vancomycin was maintained in the range of 10 to 20 mg/L.
The clinical dose of drugs administered is generally determined based on the results of pharmacokinetic studies and clinical trial studies in non-obese patients, which may not be optimal in obese individuals. Hence, the difference in pharmacokinetics of different drugs between obese and non-obese patients must be considered during drug treatment. Obesity is also associated with physiological changes that can alter the pharmacokinetics of vancomycin, and the selection of the dose of vancomycin administered needs to take into account the effect of the body weight of patients. Furthermore, both the loading dose and the maintenance dose are different from non-obese patients. During treatment, we should make appropriate dose adjustments based on the patient's therapeutic drug monitoring and renal function. At the same time, altered pharmacokinetics of antibacterial drugs may require dose individualization to achieve target concentrations. Adjustment of loading dose and maintenance dose is critical for the antibiotic treatment in obese patients using vancomycin. Unfortunately, limited data are available analyzing vancomycin concentrations in obese patients.
We thank the intensive care unit multidisciplinary team of the Fourth Hospital of Hebei Medical University for their treatment support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katip W, Thailand; Kothan S, Thailand; Muthu S, India S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1843] [Cited by in F6Publishing: 1897] [Article Influence: 118.6] [Reference Citation Analysis (2)] |
| 2. | Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2025] [Cited by in F6Publishing: 2042] [Article Influence: 255.3] [Reference Citation Analysis (0)] |
| 3. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 538] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 4. | Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 369] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 5. | El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120:1989-1997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Genoni G, Prodam F, Marolda A, Giglione E, Demarchi I, Bellone S, Bona G. Obesity and infection: two sides of one coin. Eur J Pediatr. 2014;173:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Hainer V, Zamrazilová H, Kunešová M, Bendlová B, Aldhoon-Hainerová I. Obesity and infection: reciprocal causality. Physiol Res. 2015;64:S105-S119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 567] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 9. | Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1253] [Cited by in F6Publishing: 1285] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 10. | Schilling A, Neuner E, Rehm SJ. Vancomycin: a 50-something-year-old antibiotic we still don't understand. Cleve Clin J Med. 2011;78:465-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Polso AK, Lassiter JL, Nagel JL. Impact of hospital guideline for weight-based antimicrobial dosing in morbidly obese adults and comprehensive literature review. J Clin Pharm Ther. 2014;39:584-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Choi YC, Saw S, Soliman D, Bingham AL, Pontiggia L, Hunter K, Chuang L, Siemianowski LA, Ereshefsky B, Hollands JM. Intravenous Vancomycin Associated With the Development of Nephrotoxicity in Patients With Class III Obesity. Ann Pharmacother. 2017;51:937-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Ye ZK, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One. 2013;8:e77169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1717] [Cited by in F6Publishing: 1754] [Article Influence: 159.5] [Reference Citation Analysis (0)] |
| 16. | Janson B, Thursky K. Dosing of antibiotics in obesity. Curr Opin Infect Dis. 2012;25:634-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42 Suppl 1:S35-S39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 500] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Adane ED, Herald M, Koura F. Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed Staphylococcus aureus infections. Pharmacotherapy. 2015;35:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Hanrahan TP, Lipman J, Roberts JA. Antibiotic dosing in obesity: a BIG challenge. Crit Care. 2016;20:240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Grace E. Altered vancomycin pharmacokinetics in obese and morbidly obese patients: what we have learned over the past 30 years. J Antimicrob Chemother. 2012;67:1305-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit. 1994;16:513-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO Jr. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 172] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 499] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 24. | Hites M, Taccone FS. Optimization of antibiotic therapy in the obese, critically ill patient. Réanimation. 2015;24:278-294. [Cited in This Article: ] |
| 25. | Durand C, Bylo M, Howard B, Belliveau P. Vancomycin Dosing in Obese Patients: Special Considerations and Novel Dosing Strategies. Ann Pharmacother. 2018;52:580-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: A structured review. Int J Antimicrob Agents. 2016;47:259-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Han PY, Duffull SB, Kirkpatrick CM, Green B. Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther. 2007;82:505-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Lemmens HJ, Ingrande J. Pharmacology and obesity. Int Anesthesiol Clin. 2013;51:52-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817-F822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 392] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 30. | Eknoyan G. Obesity, diabetes, and chronic kidney disease. Curr Diab Rep. 2007;7:449-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 32. | Hites M, Taccone FS, Wolff F, Maillart E, Beumier M, Surin R, Cotton F, Jacobs F. Broad-spectrum β-lactams in obese non-critically ill patients. Nutr Diabetes. 2014;4:e119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, Boots RJ, Lipman J. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 2014;42:520-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 34. | Smit C, Wasmann RE, Goulooze SC, Wiezer MJ, van Dongen EPA, Mouton JW, Brüggemann RJM, Knibbe CAJ. Population pharmacokinetics of vancomycin in obesity: Finding the optimal dose for (morbidly) obese individuals. Br J Clin Pharmacol. 2020;86:303-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32:856-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Meng L, Mui E, Holubar MK, Deresinski SC. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacotherapy. 2017;37:1415-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 552] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 38. | Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54:621-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Roberts JA, Taccone FS, Udy AA, Vincent JL, Jacobs F, Lipman J. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother. 2011;55:2704-2709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73:3081-3086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF; Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1692] [Cited by in F6Publishing: 1848] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 42. | He N, Su S, Ye Z, Du G, He B, Li D, Liu Y, Yang K, Zhang X, Zhang Y, Chen X, Chen Y, Chen Z, Dong Y, Gu J, Guo D, Guo R, Hu X, Jiao Z, Li H, Liu G, Li Z, Lv Y, Lu W, Miao L, Qu J, Sun T, Tong R, Wang L, Wang M, Wang R, Wen A, Wu J, Wu X, Xu Y, Yang Y, Yang F, Zhan S, Zhang B, Zhang C, Zhang H, Zhang J, Zhang W, Zhao L, Zhao R, Zhao W, Zhao Z, Zhou W, Zeng XT, Zhai S. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin Infect Dis. 2020;71:S363-S371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 85] [Article Influence: 28.3] [Reference Citation Analysis (1)] |
| 43. | Clark WR, Mueller BA, Alaka KJ, Macias WL. A comparison of metabolic control by continuous and intermittent therapies in acute renal failure. J Am Soc Nephrol. 1994;4:1413-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Pallotta KE, Manley HJ. Vancomycin use in patients requiring hemodialysis: a literature review. Semin Dial. 2008;21:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Mueller BA, Smoyer WE. Challenges in developing evidence-based drug dosing guidelines for adults and children receiving renal replacement therapy. Clin Pharmacol Ther. 2009;86:479-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | van de Vijsel LM, Walker SA, Walker SE, Yamashita S, Simor A, Hladunewich M. Initial vancomycin dosing recommendations for critically ill patients undergoing continuous venovenous hemodialysis. Can J Hosp Pharm. 2010;63:196-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Covajes C, Scolletta S, Penaccini L, Ocampos-Martinez E, Abdelhadii A, Beumier M, Jacobs F, de Backer D, Vincent JL, Taccone FS. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrob Agents. 2013;41:261-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1192] [Cited by in F6Publishing: 1487] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 49. | Li Q, Liang F, Sang L, Li P, Lv B, Tan L, Liu X, Chen W. Pharmacokinetics of and maintenance dose recommendations for vancomycin in severe pneumonia patients undergoing continuous venovenous hemofiltration with the combination of predilution and postdilution. Eur J Clin Pharmacol. 2020;76:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Chaijamorn W, Jitsurong A, Wiwattanawongsa K, Wanakamanee U, Dandecha P. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Int J Antimicrob Agents. 2011;38:152-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 52. | Katip W, Jaruratanasirikul S, Pattharachayakul S, Wongpoowarak W, Jitsurong A, Lucksiri A. The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock. Infect Drug Resist. 2016;9:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Hall RG 2nd, Payne KD, Bain AM, Rahman AP, Nguyen ST, Eaton SA, Busti AJ, Vu SL, Bedimo R. Multicenter evaluation of vancomycin dosing: emphasis on obesity. Am J Med. 2008;121:515-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Reynolds DC, Waite LH, Alexander DP, DeRyke CA. Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm. 2012;69:944-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |