Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6082
Peer-review started: December 22, 2021
First decision: February 8, 2022
Revised: March 21, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 26, 2022
Enhanced recovery after surgery advocates that consuming carbohydrates two hours before anesthesia is beneficial to the patient's recovery. Patients with diabetes are prone to delayed gastric emptying. Different guidelines for preoperative carbohydrate consumption in patients with diabetes remain controversial due to concerns about the risk of regurgitation, aspiration and hyperglycemia. Ultrasonic gastric volume (GV) assessment and blood glucose monitoring can comprehensively evaluate the safety and feasibility of preoperative carbohydrate intake in type 2 diabetes (T2D) patients.
To evaluate the impact of preoperative carbohydrate loading on GV before anesthesia induction in T2D patients.
Patients with T2D receiving surgery under general anesthesia from December 2019 to December 2020 were included. A total of 78 patients were randomly allocated to 4 groups receiving 0, 100, 200, or 300 mL of carbohydrate loading 2 h before anesthesia induction. Gastric volume per unit weight (GV/W), Perlas grade, changes in blood glucose level, and risk of reflux and aspiration were evaluated before anesthesia induction.
No significant difference was found in GV/W among the groups before anes
Preoperative carbohydrate loading < 300 mL 2 h before induction of anesthesia in patients with T2D did not affect GV or increase the risk of reflux and aspiration. Blood glucose levels did not change significantly with preoperative carbohydrate loading of < 200 mL. However, 300 mL carbohydrate loading may increase blood glucose levels in patients with T2D before induction of anesthesia.
Core Tip: Enhanced recovery after surgery advocates that consuming carbohydrates two hours before anesthesia is beneficial to the patient's recovery. Patients with diabetes are prone to delayed gastric emptying. Different guidelines for preoperative carbohydrate consumption in patients with diabetes remain controversial due to concerns about the risk of regurgitation, aspiration and hyperglycemia. In this study, the preoperative carbohydrate load of type 2 diabetes (T2D) patients 2 h before anesthesia induction was found by ultrasonic gastric volume assessment and blood glucose monitoring. 200 mL does not increase the risk of reflux, aspiration, and hyperglycemia, but 300 mL glucose load may cause hyperglycemia in T2D patients before induction of anesthesia.
- Citation: Lin XQ, Chen YR, Chen X, Cai YP, Lin JX, Xu DM, Zheng XC. Impact of preoperative carbohydrate loading on gastric volume in patients with type 2 diabetes. World J Clin Cases 2022; 10(18): 6082-6090
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6082.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6082
Enhanced recovery after surgery (ERAS) is a set of perioperative protocols to reduce complications, facilitate recovery, and decrease the length of hospitalization[1,2]. Insulin resistance is a critical complication of injury or stress. Most patients receiving surgery may develop postoperative insulin resistance. The resulting hyperglycemia is often associated with an increased risk of morbidity and mortality. ERAS recommends preoperative carbohydrate loading to decrease postoperative hyperglycemia by 50%, optimizing recovery[3-5].
More than 10% of the world population is reported to have diabetes[6], and nearly 15% of surgical patients have type 2 diabetes (T2D)[7]. Complications and hospital stays are greater in these patients than in non-diabetic patients[8,9]. Delayed gastric emptying (gastroparesis) is also more frequent in patients with diabetes[10,11]. Therefore, preoperative carbohydrate loading may adversely affect gastric volume (GV) in diabetic patients. Moreover, carbohydrate loading-induced hyperglycemia may outweigh the potential benefits of ERAS protocols in such patients. Laffin et al[12] found no significant difference in the hyperglycemic incidence between the groups with and without carbohydrate loading[12]. However, other studies have reported high rates of adverse outcomes, such as postoperative wound infections, cardiac events, and other complications caused by hyperglycemia, in diabetic patients receiving preoperative carbohydrate loading[13]. It is important to further study the change in blood glucose levels in diabetic patients receiving preoperative carbohydrate loading.
Perlas used ultrasound to grade GV, which was measured in the right decubitus and supine positions to assess the risk of aspiration, The visualization of gastric antrum content was scored using the Perlas grading system: Grade 0, no content visible in the supine or right lateral (RLD) position; grade 1, clear gastric fluid content only in the RLD position, but not in the supine position; and grade 2, clear gastric fluid content visible in both supine and RLD positions[14]. Perlas grade II and GV > 1.5 mL/kg have been reported to be associated with a high risk of reflux and aspiration[15,16]. Therefore, ultrasonography is used to evaluate GV both qualitatively and quantitatively. It is an economical, safe, non-invasive, and repeatable technique to assess the risk of anesthesia before surgery[17]. Data on preoperative carbohydrate loading in patients with T2D are limited[18,19]. In this study, we assessed GV, the incidence of hyperglycemia, and the risk of gastric reflux and aspiration using ultrasonography. We also evaluated the time and dose of preoperative carbohydrates using stratified analysis. These assessments allowed us to determine the safety and feasibility of preoperative carbohydrate loading in patients with T2D.
Data of adult patients (age: 40-80 years) who received surgery under general anesthesia were enrolled according to the following inclusion criteria: (1) American Society of Anesthesiologists Physical Status Classification System (ASA) classified as II-III; (2) Definite diagnosis of T2D for > 2 years; (3) Preoperative blood glucose < 10 mmol/L; (4) Glycosylated hemoglobin (HbA1c) < 8.5%; and (5) Body mass index (BMI) of 18-35 kg/m2. Patients were excluded from the study if they had any of the following: (1) Pregnancy; (2) Cardiac or renal dysfunction; (3) Hypothyroidism; (4) Obesity (BMI > 35 kg/m2); (5) Digestive system diseases, including gastroesophageal reflux, peptic ulcer, digestive system tumors, cholelithiasis or history of upper gastrointestinal surgery; (6) Receiving antiemetic drugs or other drugs affecting gastrointestinal motility before operation; (7) Preoperative gastrointestinal decompression or nutrition; or (8) Unwilling to participate in the study.
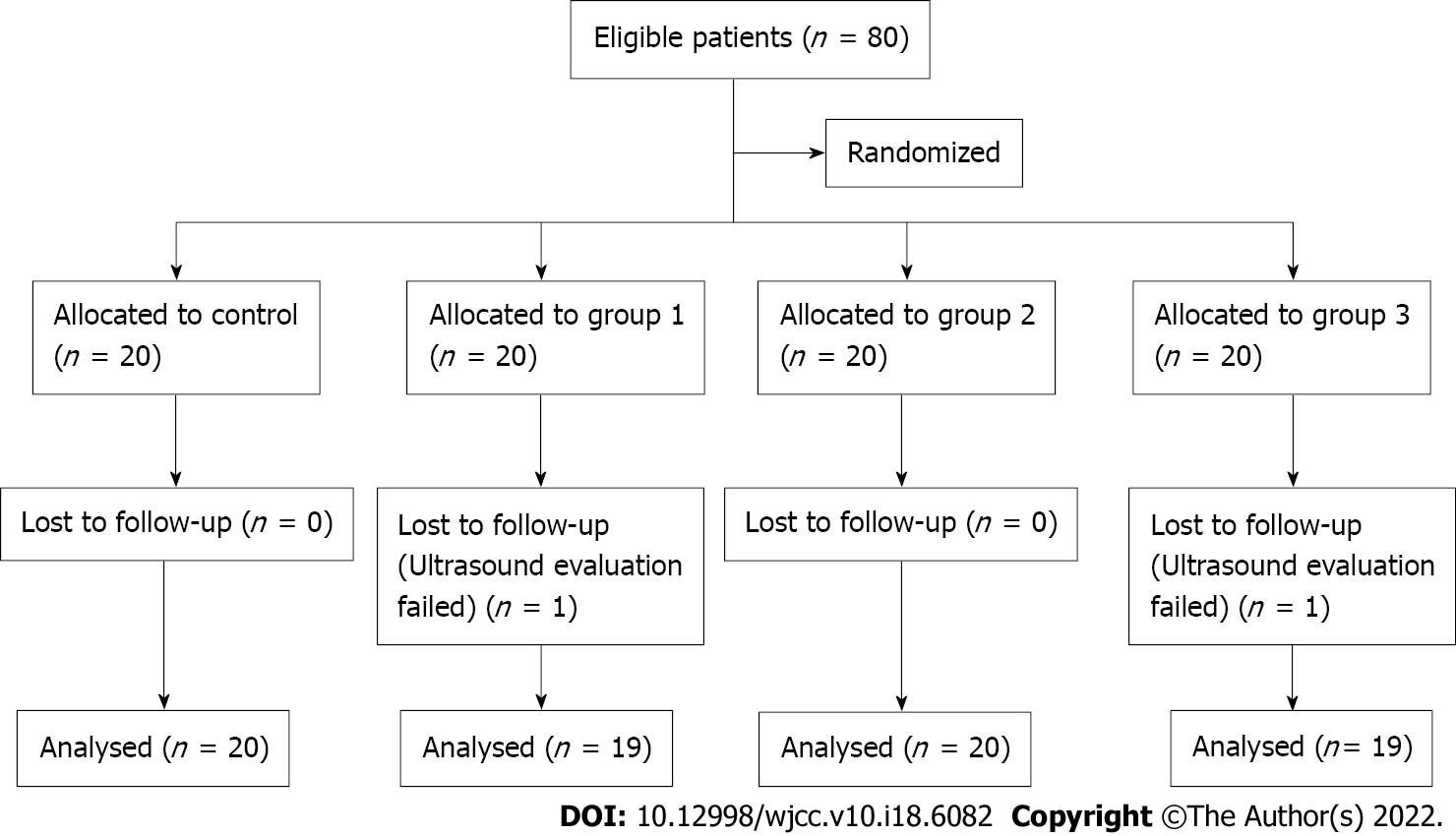
Overall, 80 patients with T2D who received surgery under general anesthesia from December 2019 to December 2020 were enrolled in the study. Of them, 2 patients were excluded due to unclear images of the gastric antrum. Finally, 78 patients with complete follow-up data were included in the study. The flow chart of the study is presented in Figure 1. The day before surgery, patients who fulfilled the study criteria and provided written consent were randomly allocated to 4 groups. Randomization was performed using computer-generated random numbers indicating different volumes of carbohydrate loading. Patients received a clear carbohydrate drink (0, 100, 200, or 300 mL) 2 h before anesthesia induction on the day of surgery. Each group uses the same concentration of carbohydrate drink that contains 14.2 g of carbohydrate per 100 mL (Yichang Human Medical Food Co., Ltd.). Randomization was performed using computer-generated four-digit random numbers indicating the treatment, which were kept in sealed envelopes. An envelope was opened according to the random number from small to large based on the time sequence of inclusion of each subject. The ultrasound examiner was blinded by the study protocol, as was the staff involved in the medical procedures and data collection process. All patients received surgery under general endotracheal anesthesia. Intraoperative fluid management was limited to a glucose-free solution, and no exogenous insulin was administered. Postoperative care was standardized as clinically indicated.
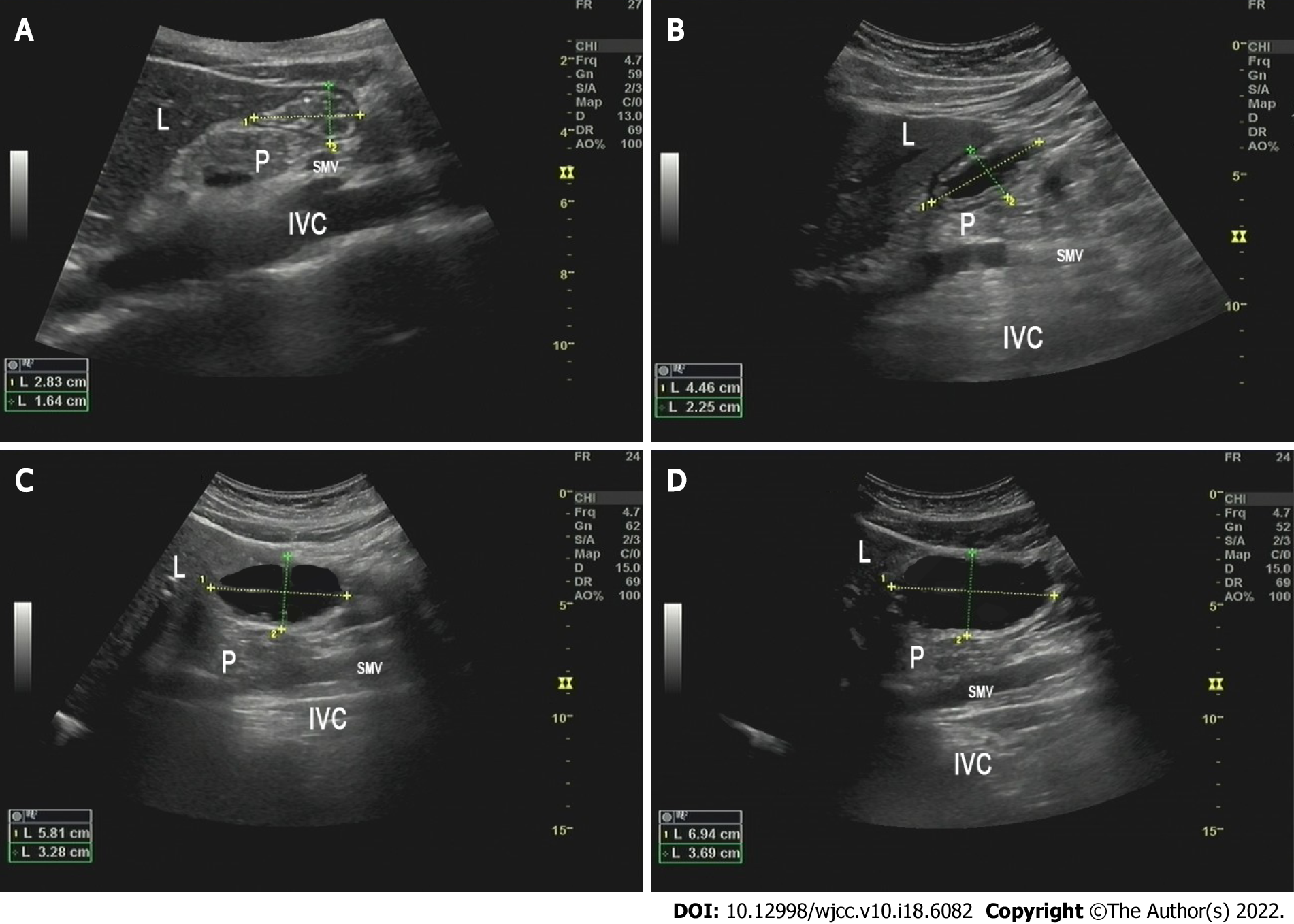
Ultrasonography was performed by an experienced investigator certified by the Chinese Health Commission. GV was assessed on the day of surgery before carbohydrate loading (T0, basal value), 2 min after carbohydrate loading (T1), and before anesthesia induction (T2). A standard convex ultrasound probe was used to scan the gastric antrum in the sagittal plane between the liver and pancreas, at first in the supine position and then in the RLD position. The gastric antrum content visualization was scored using the Perlas grading system: Grade 0, no content visible in the supine or RLD position; grade 1, clear gastric fluid content only in the RLD position, but not in the supine position; and grade 2, clear gastric fluid content visible in both supine and RLD positions[14]. The longitudinal (D1) and anteroposterior (D2) diameters of the antrum were determined, which were repeated 3 times and averaged (Figure 2). The gastric antral area (CSA) was calculated using the following formula: CSA = π × D1 × D2/4. A mathematical model was used to measure GV: 27 + 14.6 × CSA - 1.28 × age. In addition, blood glucose levels were monitored before carbohydrate loading (T0) and anesthesia induction (T2). Patients with GV per unit weight (GV/W) > 1.5 mL/kg were regarded as having a high risk of reflux and aspiration. Gastrointestinal decompression was performed before anesthesia induction in these patients. If the blood glucose level was > 10 mmol/L at T2, the surgery was delayed until it normalized.
The sample size was determined on the basis of the GV/W at different time periods. The average GV/W at T0, T1 and T2 in the control group was 0.66, 0.64, and 0.70 mL/kg, respectively, in our preliminary study. The values at T0, T1, and T2 in groups receiving 100 mL, 200 mL, and 300 mL carbohydrate drink were 0.45, 1.2, and 0.53 mL/kg; 0.70, 3.20, 0.85 mL/kg; and 0.65, 4.67, 0.8 mL/kg, respectively. Based on these values, we found that a sample of at least 20 patients in each group and 80 patients in total would ensure 80% power for the study to evaluate the effect of preoperative carbohydrate loading on GV. The 80% power was calculated considering a two-sided type I error of 0.05 by log-rank test and 20% loss to follow-up.
All statistical analyses were performed in SPSS (version 24.0, IBM, New York, United States). Normally distributed continuous data are presented as mean ± SD. Categorical data are presented as frequency or rate. Age, height, weight, BMI, course of the disease, HbA1c (%), and fasting blood glucose were compared using one-way ANOVA. The ASA scores, gender, and control of blood glucose were the Chi-square test or Fisher exact test. The GV per unit body weight and peripheral capillary blood glucose were examined with the repeated measures analysis of variance. The Bonferroni method was applied for pairwise comparisons in the repeated measures analysis of variance. All statistical analyses were two-sided tests. A P < 0.05 indicated a statistically significant difference.
A total of 78 patients with T2D were randomly allocated to 4 groups, with the control group receiving 0 mL, group 1 receiving 100 mL, group 2 receiving 200 mL, and group 4 receiving 300 mL carbohydrate drink. All groups were well balanced for characteristics, including gender, age, BMI, height, weight, ASA grade, disease course, HbA1c, fasting blood glucose level, and control of blood glucose (Table 1).
| Variables | Control | Group 1 | Group 2 | Group 3 | χ2 | F | P value |
| ASA grade (II/III) | 18/2 | 18/1 | 18/2 | 17/2 | 1.000 | ||
| Gender (M/F) | 12/8 | 10/9 | 8/12 | 12/7 | 2.83 | 0.422 | |
| Age (yr) | 62.0 ± 8.8 | 65.6 ± 9.5 | 57.4 ± 9.6 | 62.1 ± 10.9 | 1.79 | 0.209 | |
| BMI (kg/m2) | 23.8 ± 2.3 | 24.3 ± 3.2 | 23.9 ± 2.0 | 25.3 ± 3.1 | 0.91 | 0.443 | |
| Height (cm) | 162.5 ± 7.8 | 163.1 ± 6.9 | 165.1 ± 6.1 | 164.1 ± 7.4 | 0.39 | 0.759 | |
| Weight (kg) | 63.5 ± 11.3 | 64.5 ± 8.2 | 65.5 ± 9.9 | 68.1 ± 9.9 | 0.60 | 0.623 | |
| Course of disease (yr) | 9.50 ± 4.26 | 9.42 ± 3.91 | 9.15 ± 4.34 | 8.74 ± 4.25 | 0.13 | 0.942 | |
| HbA1c (%) | 7.34 ± 0.37 | 7.34 ± 0.50 | 7.49 ± 0.46 | 7.46 ± 0.56 | 0.53 | 0.663 | |
| Fasting blood glucose (mmol/L) | 7.35 ± 2.13 | 7.08 ± 1.21 | 6.95 ± 0.80 | 6.56 ± 1.20 | 1.03 | 0.386 | |
| Control of blood glucose (oral/injection of insulin) | 16/4 | 14/5 | 16/4 | 17/2 | 1.76 | 0.568 |
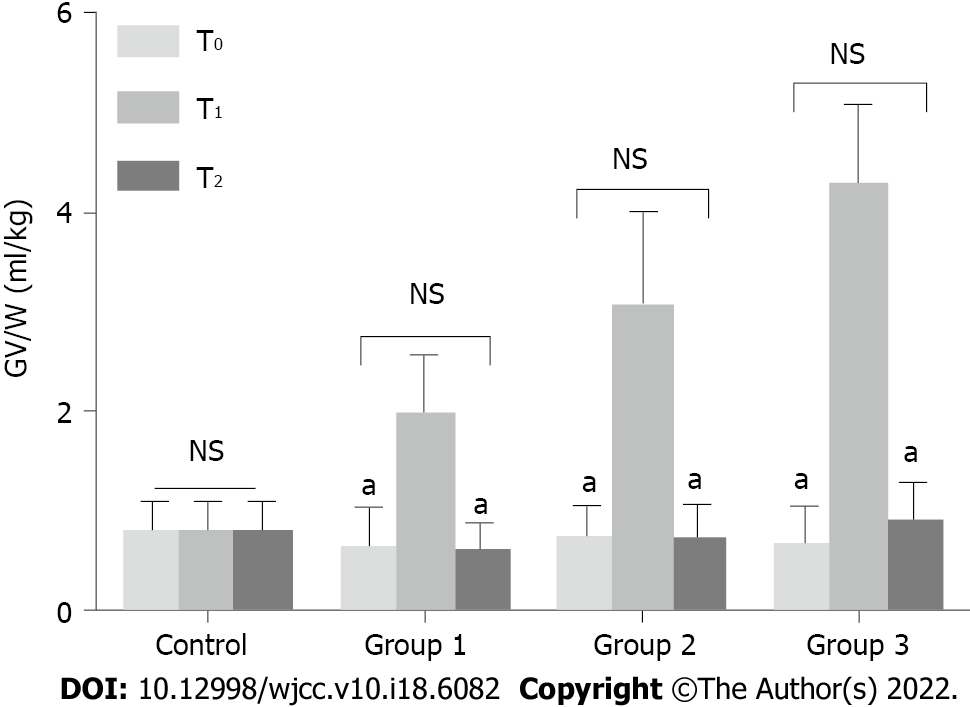
GV was assessed at 3 different time points as described above. Gastric content was first evaluated using the Perlas A scale. No difference was observed in patients with Perlas grade II at T0 and T2 among the groups (P > 0.05). GV/W was increased significantly at T1 in groups 1, 2, and 3. At T2, GV/W decreased significantly, with no statistical difference observed between T0 and T2 in all the groups (P > 0.05) (Figure 3). Moreover, the number of patients with GV/W > 1.5 mL/kg was similar among the groups (P > 0.05) (Figure 3).
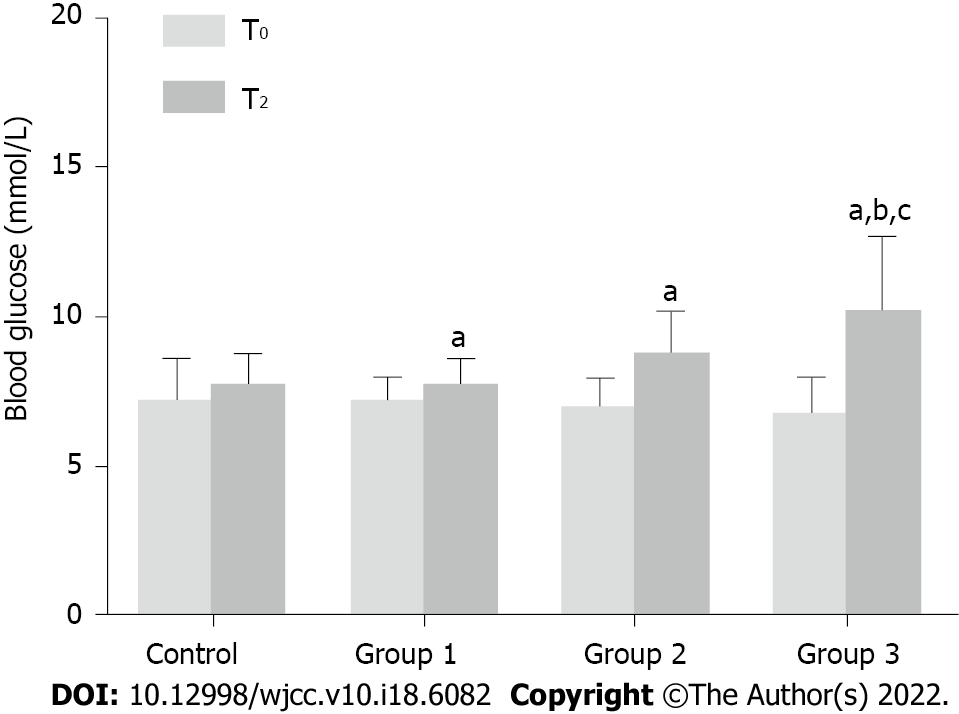
The blood glucose level in all patients was tested before carbohydrate loading (T0) and anesthesia induction (T2). In groups 1, 2, and 3, blood glucose levels increased significantly at T2 compared with that at T0 (P < 0.05). In patients receiving 300 mL of the carbohydrate drink (group 3), the blood glucose level at T2 increased by > 2 mmol/L, which was significantly higher than that in groups 1 and 2. This finding indicates that a 300 mL carbohydrate load may increase the blood glucose level in patients with T2D before anesthesia induction (Figure 4).
Preoperative carbohydrate loading improves glycemic control and postoperative recovery in nondiabetic patients[3,4]. However, the practice of carbohydrate loading in patients with T2D is controversial because of reflux and aspiration concerns due to increased GV and delayed emptying. In our study, no difference was found in GV/W between T0 and T2 in all groups. This finding indicates that GV does not increase with a carbohydrate loading of < 300 mL. Our results are in line with those of previous studies, which reported no delay in gastric emptying in patients with T2D compared with healthy control subjects[20]. Our patients drank 14.2% liquid carbohydrates with low osmotic pressure. Delayed gastric emptying seems to affect solids rather than liquids in patients with diabetes, which can possibly explain the similar GV between T0 and T2 in our study[10,21]. Furthermore, in our study, the preoperative fasting blood glucose level was controlled less than 10 mmol/L, which may reduce the incidence of delayed gastric emptying in T2D. Previous studies have shown that severe acute hyperglycemia may lead to delayed gastric emptying[22]. In summary, carbohydrate loading of < 300 mL 2 h before anesthesia induction does not significantly affect GV in patients with T2D.
The risk of reflux and aspiration was further evaluated using Perlas grading determined by ultrasonography. Patients with Perlas grade I had < 100 mL gastric content, whereas those with grade II had obvious gastric content in both supine and RLD positions[23]. Moreover, GV/W > 1.5 mL/kg helps determine the risk of reflux and aspiration[24-26]. In our study, the number of patients with Perlas grade II and GV/W > 1.5 mL/kg did not differ among the groups. This finding further confirms that preoperative carbohydrate loading does not increase the risk of reflux and aspiration in patients with T2D. However, it should be noted that all our groups had patients with Perlas grade II and GV/W > 1.5 mL/kg. This indicates the importance of performing routine preoperative GV ultrasonography in patients with diabetes.
Change in blood glucose level was another focus of our study. In the control group, group 1 and group 2, blood glucose level increased by < 2 mmol/L after carbohydrate loading. However, in patients receiving 300 mL of the carbohydrate drink (group 3), blood glucose levels increased by 3.4 mmol/L after 2 h. Studies have shown that a change in blood glucose level of < 2 mmol/L after carbohydrate loading does not increase perioperative complications[12,27,28]. Therefore, our results support a preoperative carbohydrate loading of < 200 mL in patients with T2D, although the optimal time for preoperative carbohydrate loading remains unaddressed. Carbohydrate loading 3 h before surgery does not pose a risk for hyperglycemia or aspiration in diabetic patients[12,20]. However, some researchers do not recommend the 2-h interval between carbohydrate loading and surgery due to concerns of delayed gastric emptying[29]. In our study, carbohydrate loading 2 h before anesthesia induction did not affect GV or increase the risk of reflux and aspiration. Future studies are warranted to confirm our results.
Our study has certain limitations. First, the blood glucose level of the enrolled patients was well controlled, and their preoperative FPG was < 10 mmol/L. Further stratified analysis must be performed in patients with different levels of blood glucose and HbA1c. Second, data about primary diseases in our patients were lacking. Because primary diseases may affect GV and gastric emptying, the lack of such data could have introduced a bias in result interpretation. Finally, single-center study design and insufficient data limit further application of our results. Prospective, large-scale, randomized, and multi-centered studies are needed to further validate our results.
Preoperative carbohydrate loading < 300 mL 2 h before anesthesia induction in patients with T2D did not affect GV or increase the risk of reflux and aspiration. Blood glucose level did not significantly change with preoperative carbohydrate loading of < 200 mL. However, 300 mL carbohydrate loading may increase blood glucose levels in patients with T2D before anesthesia induction. In conclusion, it is safe for patients with T2D to drink 200 mL 14.2% carbohydrate 2 h before surgery. In the future, we will study whether preoperative consumption of 200 mL of 14.2% carbohydrate can reduce postoperative insulin resistance and promote recovery of patients.
More than 10% of the world's population and almost 15% of surgical patients are reported to have type 2 diabetes (T2D). Diabetic patients are prone to delayed gastric emptying due to the risk of reflux, aspiration and hyperglycemia.
Different guidelines for preoperative carbohydrate loading in diabetic patients are still controversial.
This study is conducted to evaluate the safety and feasibility of preoperative carbohydrate loading on gastric volume (GV) before anesthesia induction in T2D patients.
Patients with T2D were randomly allocated to 4 groups receiving 0, 100, 200, or 300 mL of carbohydrate loading 2 h before anesthesia induction. Gastric volume per unit weight (GV/W), Perlas grade, changes in blood glucose level, and risk of reflux and aspiration were evaluated before anesthesia induction.
No significant difference was found in GV/W among the groups before anesthesia induction (P > 0.05). The number of patients with Perlas grade II and GV/W > 1.5 mL/kg did not differ among the groups (P > 0.05). Blood glucose level increased by > 2 mmol/L in patients receiving 300 mL carbohydrate drink, which was significantly higher than that in groups 1 and 2 (P < 0.05).
Preoperative carbohydrate loading < 300 mL 2 h before anesthesia induction in patients with T2D did not affect GV or increase the risk of reflux and aspiration. Blood glucose levels did not change significantly with preoperative carbohydrate loading of < 200 mL. However, 300 mL carbohydrate loading may increase blood glucose levels in patients with T2D before induction of anesthesia.
Our study illustrates the safety and recommended volume of preoperative carbohydrate loading in patients with T2D.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Harsini PA, Iran; Herold M, Hungary S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104:187-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Ljungqvist O, de Boer HD, Balfour A, Fawcett WJ, Lobo DN, Nelson G, Scott MJ, Wainwright TW, Demartines N. Opportunities and Challenges for the Next Phase of Enhanced Recovery After Surgery: A Review. JAMA Surg. 2021;156:775-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 3. | Wang S, Gao PF, Guo X, Xu Q, Zhang YF, Wang GQ, Lin JY. Effect of low-concentration carbohydrate on patient-centered quality of recovery in patients undergoing thyroidectomy: a prospective randomized trial. BMC Anesthesiol. 2021;21:103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Li L, Wang Z, Ying X, Tian J, Sun T, Yi K, Zhang P, Jing Z, Yang K. Preoperative carbohydrate loading for elective surgery: a systematic review and meta-analysis. Surg Today. 2012;42:613-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Tsutsumi R, Kakuta N, Kadota T, Oyama T, Kume K, Hamaguchi E, Niki N, Tanaka K, Tsutsumi YM. Effects of oral carbohydrate with amino acid solution on the metabolic status of patients in the preoperative period: a randomized, prospective clinical trial. J Anesth. 2016;30:842-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Bi Y, Xu Y, Ning G. Prevalence of diabetes in Chinese adults--reply. JAMA 2014; 311: 200-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Albalawi Z, Laffin M, Gramlich L, Senior P, McAlister FA. Enhanced Recovery After Surgery (ERAS®) in Individuals with Diabetes: A Systematic Review. World J Surg. 2017;41:1927-1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Darwiche G, Almér LO, Björgell O, Cederholm C, Nilsson P. Delayed gastric emptying rate in Type 1 diabetics with cardiac autonomic neuropathy. J Diabetes Complications. 2001;15:128-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Watson LE, Phillips LK, Wu T, Bound MJ, Jones KL, Horowitz M, Rayner CK. Longitudinal evaluation of gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2019;154:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Horowitz M, O'Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med. 2002;19:177-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Horváth VJ, Izbéki F, Lengyel C, Kempler P, Várkonyi T. Diabetic gastroparesis: functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep. 2014;14:527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Laffin MR, Li S, Brisebois R, Senior PA, Wang H. The Use of a Pre-operative Carbohydrate Drink in Patients with Diabetes Mellitus: A Prospective, Non-inferiority, Cohort Study. World J Surg. 2018;42:1965-1970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Sabry R, Hasanin A, Refaat S, Abdel Raouf S, Abdallah AS, Helmy N. Evaluation of gastric residual volume in fasting diabetic patients using gastric ultrasound. Acta Anaesthesiol Scand. 2019;63:615-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113:93-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 16. | Tacken MCT, van Leest TAJ, van de Putte P, Keijzer C, Perlas A. Ultrasound assessment of gastric volumes of thick fluids: Validating a prediction model. Eur J Anaesthesiol. 2021;38:1223-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kruisselbrink R, Arzola C, Jackson T, Okrainec A, Chan V, Perlas A. Ultrasound assessment of gastric volume in severely obese individuals: a validation study. Br J Anaesth. 2017;118:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126:376-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 19. | Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Søreide E, Spies C, in't Veld B; European Society of Anaesthesiology. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 20. | Gustafsson UO, Nygren J, Thorell A, Soop M, Hellström PM, Ljungqvist O, Hagström-Toft E. Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand. 2008;52:946-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | He XD, Guo YM, Goyal RK. Effect of Hyperglycemia on Purinergic and Nitrergic Inhibitory Neuromuscular Transmission in the Antrum of the Stomach: Implications for Fast Gastric Emptying. Front Med (Lausanne). 2018;5:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Halland M, Bharucha AE. Relationship Between Control of Glycemia and Gastric Emptying Disturbances in Diabetes Mellitus. Clin Gastroenterol Hepatol. 2016;14:929-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, Cubillos J, Chan V. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 24. | Chang JE, Kim H, Won D, Lee JM, Jung JY, Min SW, Hwang JY. Ultrasound assessment of gastric content in fasted patients before elective laparoscopic cholecystectomy: a prospective observational single-cohort study. Can J Anaesth. 2020;67:810-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ohashi Y, Walker JC, Zhang F, Prindiville FE, Hanrahan JP, Mendelson R, Corcoran T. Preoperative gastric residual volumes in fasted patients measured by bedside ultrasound: a prospective observational study. Anaesth Intensive Care. 2018;46:608-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Van de Putte P, Vernieuwe L, Bouvet L. Gastric ultrasound as an aspiration risk assessment tool. Indian J Anaesth. 2019;63:160-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Tang X, Li S, Wang Y, Wang M, Yin Q, Mu P, Lin S, Qian X, Ye X, Chen Y. Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin Chim Acta. 2016;461:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Voss AC, Maki KC, Garvey WT, Hustead DS, Alish C, Fix B, Mustad VA. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition. 2008;24:990-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Chen L, Chen YJ, Dong HL, Feng Y, Gu XP, Huang YG, Jiang ZW, Lou WH, Liu LX, Mi WD, Ma ZL, Min S, Peng SL, Tian XD, Wang TL, Xu ZK, Xue ZG, Yao HW, Yang YM, Zhang KC, Zhu SM. Chinese expert consensus and pathway management guide for Chinese accelerated rehabilitation surgery (2018 Edition). Zhongguo Shiyong Waike Zazhi. 2018;38:1-19. [DOI] [Cited in This Article: ] |