Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5854
Peer-review started: December 27, 2021
First decision: March 10, 2022
Revised: March 11, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Gastric tube formation and pull-up is the most common technique of recon
A 57-year-old man underwent sequential chemotherapy and radiotherapy for gastric MALT-lymphoma seven years prior to diagnosis of esophageal adenocarcinoma. Esophagectomy without neoadjuvant treatment was recommended by the multidisciplinary tumor board due to early tumor stage [uT1 (sm2) uN+ cM0 according to TNM-classification of malignant tumors, 8th edition] without lymph node involvement. Minimal invasive esophageal resection with esophagogastrostomy was performed. Due to gastric tube necrosis with anastomotic leakage on the twelfth postoperative day, diverting resection with construction of a cervical salivary fistula was necessary. Rapid recovery facilitated colonic interposition without any complications six months afterwards.
This case report may represent the start for further investigation to know if it is reasonable to refrain from esophagogastrostomy in patients with a long interval between gastric radiotherapy and surgery.
Core Tip: A patient with previous radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma underwent esophagectomy and esophagogastrostomy for esophageal cancer more than seven years later. Gastric tube necrosis, made diversion surgery with salivary fistula necessary. Six months later, interposition of the transverse colon was performed without occurrence of any complications. The patient fully recovered with unlimited oral intake capability and remains free of tumor recurrence at date of publication. In patients with a long interval between gastric radiotherapy and surgery esophagogastrostomy should be avoided.
- Citation: Yurttas C, Wichmann D, Gani C, Bongers MN, Singer S, Thiel C, Koenigsrainer A, Thiel K. Beware of gastric tube in esophagectomy after gastric radiotherapy: A case report. World J Clin Cases 2022; 10(17): 5854-5860
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5854.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5854
Esophagectomy, combined with neoadjuvant chemo(radio)therapy in the locally advanced situation, is considered standard treatment with curative intention for carcinomas of the esophagus and the esophagogastric junction[1]. Most commonly, anastomosis of the remnant esophagus to a gastric tube is performed[2]. Whether prior chemoradiotherapy for gastric mucosa-associated lymphoid tissue lymphoma limits the stomach’s suitability for reconstruction is unknown. With this case report we provide first evidence for pretreated stomach usage for esophagogastrostomy in esophagectomy.
Due to asymptomatic gastro-esophageal reflux disease with Long Segment Barrett’s esophagus C9M13 according to Prague Classification, a 64-year-old patient underwent repetitive esophagogastroduodenoscopy.
In 2020, biopsy of the distal esophagus 34 cm from row of teeth revealed invasive moderately differentiated (G2) adenocarcinoma. Moreover, erythema and atrophy of the gastric mucosa were detected. However, the patient had no disease-specific complaints when he first presented to our department. Oral intake of standard western-diet was unrestricted and body weight was constant at a BMI of 29.1 kg/m².
In 2012, the 57-year-old man was diagnosed with diffuse large B-cell lymphoma (DLBCL) of the stomach in the course of endoscopic treatment of gastric bleeding (2a according to the Forrest Classification of gastrointestinal bleedings). Although there was no detection of Helicobacter pylori, eradication therapy was performed. Endosonography proved localization at the posterior gastric wall without infiltration of neighboring tissues, whereas computed tomography (CT) scan and bone marrow biopsy were without evidence of disease equivalent to stage IE according to the Ann Arbor staging system. Following four courses of rituximab, cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate (Oncovin), and prednisone (R-CHOP) with curative intention percutaneous normofractionated radiotherapy of the stomach with a total of 39.6 Gray (Gy) in 20 fractions weekly was performed as consolidating therapy. Both systemic and radiation therapy were well tolerated. Due to herpes zoster of the left thorax antiviral therapy with aciclovir was introduced.
The patient had a history of herniated vertebral disc, struma nodosa, chronic-venous insufficiency and endoscopic resection of a low-grade adenoma of the sigmoid colon and regularly took metformin, thyroxine and sitagliptin for type 2 diabetes mellitus and hypothyroidism respectively. Hepatic and renal function were not impaired. Follow-up examinations up to five years were without any peculiarities or evidence of tumor recurrence. The patient had skipped drinking and smoking after intake of 60 pack-years.
Family history was unremarkable and not related to the present case.
The patient was in a normal general state without any evidence of disease or restriction of normal activities.
Preoperative blood examinations were unremarkable. Tumor markers CEA, CA19-9 and CA72-4 were within reference range.
Whereas CT scan showed no signs of distant metastases or involvement of locoregional lymph nodes, endosonography described uT1 (sm2) uN+ according to TNM classification of malignant tumors, 8th edition. Positron emission tomography-CT was performed for further clarification, which ruled out involvement of locoregional lymph nodes.
Surgery for esophageal cancer and gastric tube necrosis: Surgery was performed in minimally invasive technique of Ivor Lewis esophagectomy. Access to the abdominal cavity and capnoperitoneum was established with the help of a Veress needle. An optic trocar was introduced under vision with a 30° camera (KARL STORZ SE & Co. KG, Tuttlingen, Germany). The abdominal cavity was inspected to rule out injuries during access and also peritoneal or hepatic metastases. Then, gastric mobilization was performed with an electrosurgical vessel sealer, left gastric artery was clipped whereas the right gastric artery as well as the right gastroepiploic arcade were preserved. Complete D2-lymphadenectomy was performed followed by stapled gastric tube formation of approximately 5 cm in diameter. Esophagectomy including mediastinal lymphadenectomy was operated thoracoscopically with four right-sided intercostal trocars. The resection was completed with formation of a stapled circular end-to-side-esophagogastrostomy.
Emergency thoracotomy was necessary for resection of the necrotic gastric tube, hemithyroidectomy and creation of the salivary glandula. A jejunal feeding tube was inserted after laparotomy. Continuous intestinal passage was reconstructed by colonic interposition. Following laparotomy, the transverse colon was prepared for retrosternal pull-up and formation of an end-to-end esophagocolostomy and an end-to-side colojejunostomy. A side-to-side ascendodescendostomy was created.
Endoscopy and endoscopic negative-pressure therapy: Endoscopy was performed with a standard gastroscope with 9.8-mm outer caliber and 3.2-mm working channel (PENTAX Medical, Tokyo, Japan). A thin open-pore film wrapped around a drain (Medicoplast, Illingen, Germany) and fixed with a suture was constructed prior to endoscopically controlled insertion and positioning of the device. Negative pressure of -125 mmHg was established with the use of a vacuum therapy system (KCI medical, Wiesbaden, Germany).
Moderately differentiated adenocarcinoma of the distal esophagus with infiltration of the submucosal layer without locoregional lymph node metastases [TNM: pT1b, pN0 (0/17) L0, V0, Pn0, R0, Grading: G2].
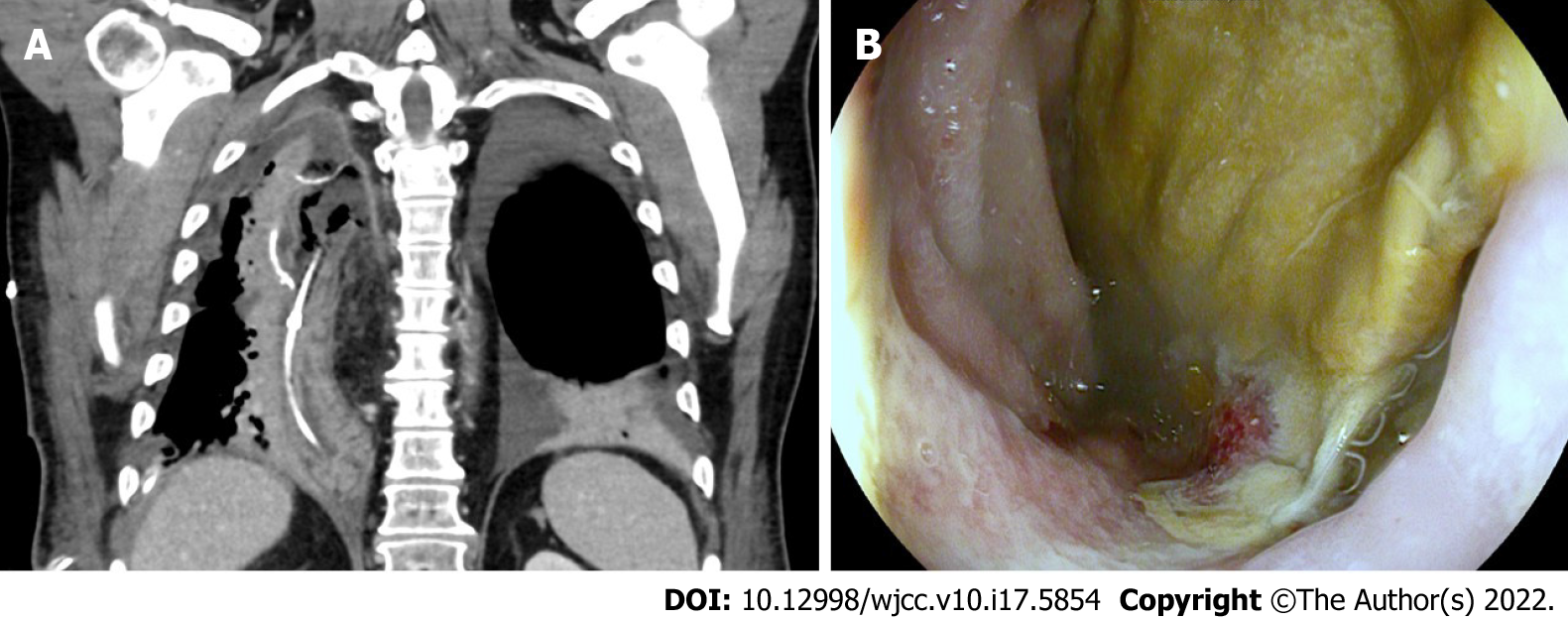
The multidisciplinary tumor board consequently recommended surgical resection without neoadjuvant treatment. Thoracoscopic and laparoscopic abdominal right thoracic esophagectomy with two-field lymphadenectomy (Ivor Lewis) and stapled end-to-side esophagogastrostomy was performed. Histopathological examination confirmed the diagnosis and staging results and complete resection of a moderately differentiated adenocarcinoma of the distal esophagus. The gastric mucosa showed signs of erosive gastritis with denuded surface epithelium, subepithelial and interstitial hemorrhage, but no recurrent lymphoma infiltrates. The initial postoperative course was regular and without any pathologic findings. Following extubation immediately after surgery, the patient was monitored at the intermediate care unit for one day without requiring cardiocirculatory or respiratory support before transfer to the general ward. Low-dose anticoagulation with unfractionated heparin was initiated six hours after surgery. Amount and quality of drain output were unsuspicious. Seven days after surgery the patient’s general state was seen to deteriorate and elevated leukocytes and C-reactive protein were observed, which required endoscopic assessment of the esophagogastrostomy to rule out anastomotic leakage. The gastric interposition showed compromised perfusion without evidence of anastomotic insufficiency. Endoscopic negative-pressure therapy was therefore introduced. After vomiting with aspiration during anaesthetization the patient was transferred to the intensive care unit. Despite initiation of calculated antibiotic therapy with meropenem, vancomycin and anidulafungin there was no observable improvement. On day 12 postoperative, endoscopy revealed necrosis of the gastric interposition with a pronounced anastomotic insufficiency prompting surgical resection of the gastric tube interposition, creation of a cervical fistula and insertion of a jejunal feeding catheter (Figure 1). Histopathology confirmed ischemic necrosis of the proximal gastric tube with anastomotic leakage. There was no evidence of residual adenocarcinoma or recurrent lymphoma in the resected esophagogastrostomy or gastric tube. Postoperative pleural effusion was treated with a thoracic drain and central venous line-associated blood-stream infection, while paroxysmal tachycardia and delirium necessitated respective therapy. The patient slowly recovered until he was discharged 40 d after esophageal resection. Follow-up care was recommended by the multidisciplinary tumor board.
Six months later, the patient underwent colonoscopy and CT scan in preparation for colonic interposition without any contraindications or signs of tumor recurrence. Retrosternal interposition of the transverse colon creating an end-to-end esophagotransversostomy, end-to-side transversojejunostomy and a side-to-side ascendotransversostomy was performed. Postoperative course was normal. Oral intake of food and liquids was without difficulty. Supportive enteral feeding was continued. The patient was discharged home on day 12 postoperative. Nine weeks later, the patient was in an unrestricted general condition with stable body weight so that the jejunal feeding catheter was removed. Table 1 shows information from this case report organized in a time table.
| Date | Diagnosis | Intervention |
| September, 2012 | Gastric MALT lymphoma | R-CHOP |
| February, 2013 | Gastric MALT lymphoma | Intensity-modulated radiation therapy up to 39.6 Gy |
| July, 2020 | Barrett’s carcinoma | Endoscopic biopsy |
| November, 2020 | Barrett’s carcinoma | Abdominal right-thoracic esophagectomy with two-field lymphadenectomy (Ivor Lewis) |
| December, 2020 | Gastric tube necrosis | Diverting resection with creation of cervical salivary fistula |
| June, 2021 | Presence of a cervical salivary fistula | Colonic interposition and insertion of a jejunal feeding catheter |
| August, 2021 | Needless jejunal feeding catheter | Extraction of jejunal feeding catheter |
When the patient first presented to our out-patient clinic, the suitability of the pretreated stomach for construction of an esophagogastrostomy was uncertain because evidence was missing. In the literature, complications of esophagogastrostomy in general are reported to occur in 12% and mortality in 4% of all cases[3]. According to the present literature, small bowel or colonic interposition may be considered alternative grafts. Compared to the colon, small bowel grafts require fewer anastomoses, are rarely affected by malignancies and have good peristalsis, but provide no reservoir function. Colonic interposition is complicated by the need for three to four anastomoses and potential metachronous development of adenoma and carcinoma. Nevertheless, longer grafts are available offering reservoir-like function and less reflux[4,5]. However, a retrospective cohort study comparing complex esophageal reconstruction including 44.7% of patients with other than gastric tube formation to non-complex esophagectomy with direct gastric pull-up reported higher morbidity and longer length of stay for patients in the complex therapy group[6]. Jejunal grafts are described as suitable primary alternatives for any scope of esophageal replacement, but are accompanied by up to 36% anastomotic leakage and 10% mortality[7]. In colonic interposition, higher overall morbidity of 45.0%-64.0% and increased risk of anastomotic leakage occurring in 13.0%-30.0% of patients is shown[8-11]. Alternatively, construction of a cervical salivary fistula with secondary gastric tube formation could be an option, but especially patients with cancer were shown to have poor outcome after primary diversion and secondary reconstruction in esophagectomy[12]. Considering our experience with gastric tubes and the lower complication rates as compared to small bowel and colonic interposition, the decision for esophagogastrostomy was therefore made together with the patient.
Despite expectable poor outcome following resection of the necrotic gastric tube with diversion[12], creation of a cervical fistula and secondary colonic interposition, our patient fully recovered, has sufficient oral intake capacity and to date remains without signs of any tumor recurrence.
Neoadjuvant radiochemotherapy prior to esophagectomy has been shown to improve overall survival compared to surgery alone with a very favourable toxicity profile. In particular, no increase in anastomotic leakage was reported in the CROSS trial[13], whereas in-field creation of anastomosis following neoadjuvant radiochemotherapy and esophagectomy was identified as a risk factor for anastomotic leakage in a retrospective analysis of 285 patients treated for esophageal cancer[14]. Especially in distal esophageal cancer the celiac lymph nodes and the ones at the lesser gastric curvature are frequently irradiated in the preoperative setting with doses that are comparable to the dose given in the current case presentation resulting in a considerable dose burden to the stomach without causing an excessive rate of anastomotic leakage. A major difference however between preoperative radiotherapy for esophageal cancer and the previous treatment with radiotherapy in the current case is the interval between radiotherapy and surgery. While surgery after planned neoadjuvant therapy is commonly scheduled within a couple of weeks, the interval was seven years in the present case. One can hypothesize that the tissue turned less “flexible” over the time due to fibrosis which might have contributed to anastomotic leakage. However, in the present case radiotherapy was applied to the specimen employed for reconstruction and not to the resected organ.
We therefore recommend that stomachs pretreated by radiotherapy should not be utilized for reconstruction in esophagectomy. Although this case report provides little evidence from a single patient only without proven causality, further investigations as to whether stomachs pretreated by radiotherapy in general should not be utilized for reconstruction in esophagectomy are required.
The authors thank Margreiter MH for her kind contribution to preparation of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Di Meglio L, Italy; Ding X, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Low DE. Evolution in surgical management of esophageal cancer. Dig Dis. 2013;31:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G, Davies A, D'Journo XB, Gisbertz SS, Griffin SM, Hardwick R, Hoelscher A, Hofstetter W, Jobe B, Kitagawa Y, Law S, Mariette C, Maynard N, Morse CR, Nafteux P, Pera M, Pramesh CS, Puig S, Reynolds JV, Schroeder W, Smithers M, Wijnhoven BPL. Benchmarking Complications Associated with Esophagectomy. Ann Surg. 2019;269:291-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 432] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 3. | Butskiy O, Rahmanian R, White RA, Durham S, Anderson DW, Prisman E. Revisiting the gastric pull-up for pharyngoesophageal reconstruction: A systematic review and meta-analysis of mortality and morbidity. J Surg Oncol. 2016;114:907-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Watanabe M, Mine S, Nishida K, Kurogochi T, Okamura A, Imamura Y. Reconstruction after esophagectomy for esophageal cancer patients with a history of gastrectomy. Gen Thorac Cardiovasc Surg. 2016;64:457-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Sohn M, Agha A, Trum S, Moser C, Gundling F, Hochrein A, Pratschke J, Aigner F, Ritschl P. Frequency of Polyps and Adenocarcinoma in Colon Interposition After Esophagectomy in Adulthood - A Systematic Review. Anticancer Res. 2019;39:6419-6430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Moore JM, Hooker CM, Molena D, Mungo B, Brock MV, Battafarano RJ, Yang SC. Complex Esophageal Reconstruction Procedures Have Acceptable Outcomes Compared With Routine Esophagectomy. Ann Thorac Surg. 2016;102:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Gaur P, Blackmon SH. Jejunal graft conduits after esophagectomy. J Thorac Dis. 2014;6 Suppl 3:S333-S340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 8. | Hamai Y, Hihara J, Emi M, Aoki Y, Okada M. Esophageal reconstruction using the terminal ileum and right colon in esophageal cancer surgery. Surg Today. 2012;42:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Mine S, Udagawa H, Tsutsumi K, Kinoshita Y, Ueno M, Ehara K, Haruta S. Colon interposition after esophagectomy with extended lymphadenectomy for esophageal cancer. Ann Thorac Surg. 2009;88:1647-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Klink CD, Binnebösel M, Schneider M, Ophoff K, Schumpelick V, Jansen M. Operative outcome of colon interposition in the treatment of esophageal cancer: a 20-year experience. Surgery. 2010;147:491-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg. 2003;138:303-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | DiPierro FV, Rice TW, DeCamp MM, Rybicki LA, Blackstone EH. Esophagectomy and staged reconstruction. Eur J Cardiothorac Surg. 2000;17:702-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3288] [Cited by in F6Publishing: 3652] [Article Influence: 304.3] [Reference Citation Analysis (0)] |
| 14. | Juloori A, Tucker SL, Komaki R, Liao Z, Correa AM, Swisher SG, Hofstetter WL, Lin SH. Influence of preoperative radiation field on postoperative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol. 2014;9:534-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |