Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4294
Peer-review started: November 20, 2021
First decision: December 27, 2021
Revised: January 5, 2022
Accepted: March 7, 2022
Article in press: March 7, 2022
Published online: May 6, 2022
Hypertrophic neuropathy of the cauda equina (HNCE) is a rare disease, especially in children. It can be caused by different etiological agents such as inflammation, tumor or hereditary factors. Currently, there is no uniform standard for clinical treatment of HNCE. Furthermore, it is unclear whether spinal canal decom
We report the case of a 13-year-old boy with enlargement of the cauda equina. The onset of the disease began at the age of 6 years and was initially marked by radiating pain in the buttocks and thighs after leaning over and weakness in the lower limbs when climbing a ladder. The child did not receive any medical treatment. As the disease slowly progressed, the child needed the help of others to walk, and he had a trendelenburg gait. He underwent spinal canal decompression and a nerve biopsy during his hospital stay. A diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy was made based on electrophysiological findings and pathological examination results. Immunoglobulin or hormone therapy was recommended during hospitalization, but his mother refused. After discharge, the boy’s mother helped him carry out postoperative rehabilitation training at home. His lower-limb muscle strength gradually increased, and he could stand upright and take steps. Six mo after surgery, the child was readmitted and began immunoglobulin therapy. Long-term oral steroid treatment was initiated after discharge. The movement and sensation of the lower limbs were further improved, and the boy could walk normally 1 year after surgery.
Spinal canal decompression can improve the clinical symptoms of HNCE caused by inflammation, even in children. When combined with specific etiological interventions, spinal cord decom
Core Tip: The rare case reported here thoroughly illustrates that chronic inflammatory demyelinating polyradiculoneuropathy can result in hypertrophic neuropathy of the cauda equina (HNCE) in children. This case provides new insights into the management of childhood chronic inflammatory demyelinating polyradiculoneuropathy. We demonstrated that laminectomy, followed by adjunct therapy, is a safe and effective treatment of HNCE. At the same time, etiological diagnosis is very important.
- Citation: Ye L, Yu W, Liang NZ, Sun Y, Duan LF. Spinal canal decompression for hypertrophic neuropathy of the cauda equina with chronic inflammatory demyelinating polyradiculoneuropathy: A case report. World J Clin Cases 2022; 10(13): 4294-4300
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4294.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4294
Hypertrophic neuropathy (HN) is very uncommon in clinical settings and has been described as a form of hereditary motor and sensory neuropathy or acquired demyelination neuropathy in the literature[1]. It can be characterized as a chronic progressive clinical process that mainly affects the peripheral nerve. HN involving the whole cauda equina is even more uncommon and is exceedingly rarely reported in children. There is no uniform standard for clinical treatment of HN of the cauda equina. We report a case of childhood-onset HN of the cauda equina caused by chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
A 13-year-old Asian boy presented to our facility with reduced muscular strength of both lower limbs, abnormalities in gait, and paresthesia.
The patient had shown progressive reduced muscular strength of both lower limbs, abnormalities in gait, and paresthesia from 6 years old. He initially presented with radiating pain in the buttocks and thighs after bending over and weakness in both lower limbs when climbing steps. Intermittent symptoms gradually developed into a persistent decline in muscle strength of the lower limbs (particularly in the distal lower extremities) and gait instability.
There was no associated history of trauma.
There was no inherited disorders in the patient’s family history.
The patient’s level of consciousness, neurological physical examination, and upper limb activities were within normal limits. The muscle strength of both lower extremities was grade IV. The patient needed the help of others to walk, and he had a trendelenburg gait. He had numbness after acupuncture below the hip, and hypoalgesia and hypoesthesia were more obvious in the distal extremities. Additional presentation symptoms included bilateral abolished tendon reflexes, extension contracture of the ankles, and restricted toe movement. Other symptoms such as pathological reflexes, incontinence, scoliosis, atrophy of the muscles of the lower extremities, and rashes were not noted.
Examination of the cerebrospinal fluid (CSF) revealed normal cell numbers. The patient’s CSF protein level was 0.55 g/L (reference range 0.1-0.4 g/L).
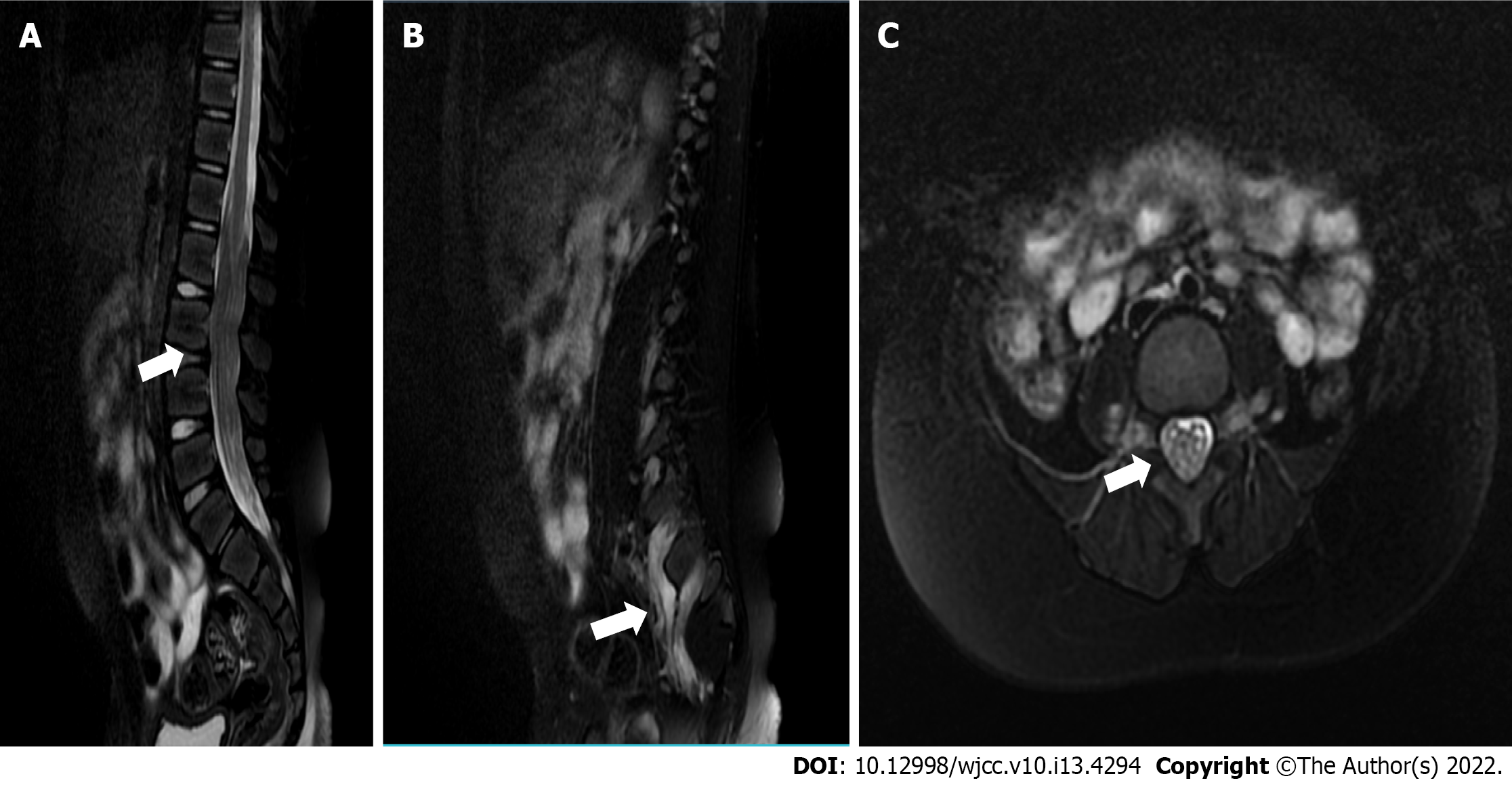
Magnetic resonance imaging (MRI) of the lumbosacral spine was performed 7 years after symptom onset. The patient underwent MRI with T1- and T2-weighted imaging and T1-contrast-enhanced sequences. Findings demonstrated that diffuse thickening of the cauda equina nerve roots filled the entire spinal canal, even extending to the intervertebral foramen and the paravertebral space (Figure 1). The cauda equina nerve roots also showed a low T1 signal and a slightly higher T2 signal, with mild enhancement. Brain MRI findings were within normal limits.
Electrophysiological testing demonstrated that nerve conduction velocity in both lower limbs was decreased. The compound muscle action potential of the femoral nerve, tibial nerve, common peroneal nerve, the sensory nerve action potential, and the F wave were not detected. When multiple lower-limb muscles were lightly contracted, electromyography showed increased motor unit potentials (MUP), long-duration MUP, and increased polyphasic potentials.
Whole-exome sequencing was performed on deoxyribonucleic acid extracted from the patient’s peripheral blood, with no disease-causing variant identified.
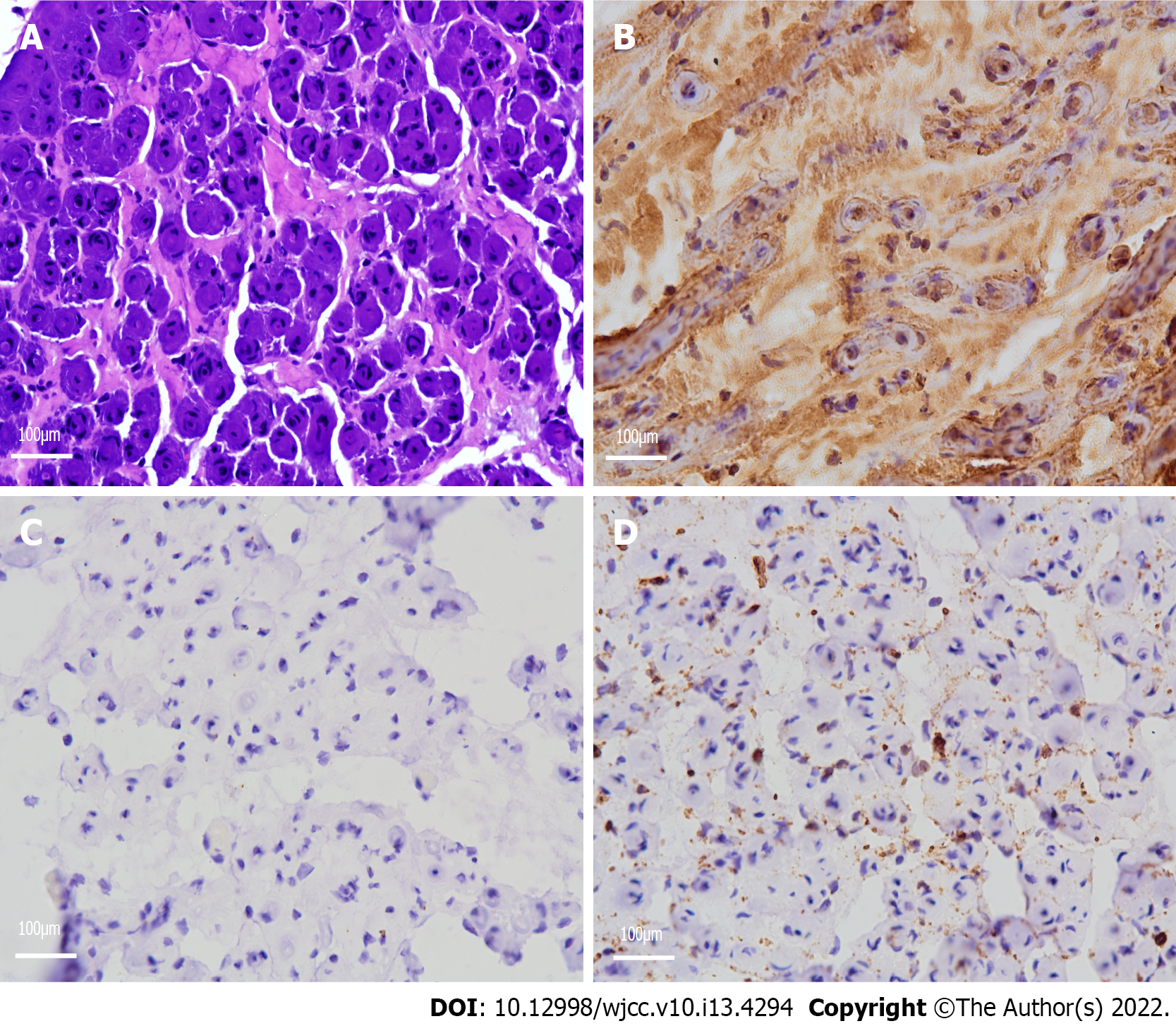
Neuropathological examination demonstrated a remarkable reduction in the density of myelinated fibers and hyperplasia of the schwann cells, forming onion bulb wrappings of myelinated fibers and blood vessels, and a small population of perivascular macrophages. In addition, immunohistochemical analysis showed positivity for the S-100 protein antibody and negativity for epithelial membrane antigen (EMA) (Figure 2).
Based on the combination of findings, CIDP was finally diagnosed.
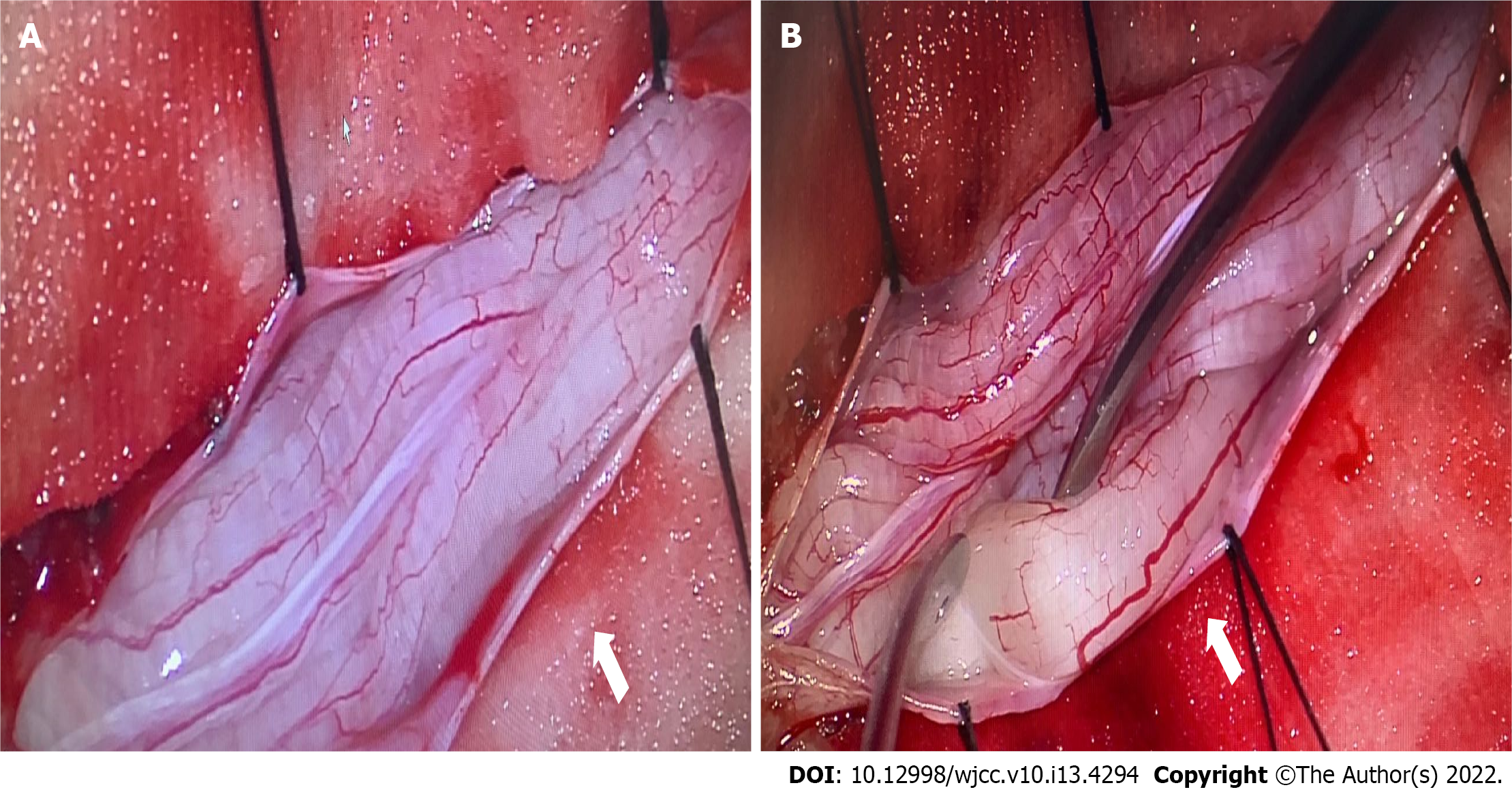
With the consent of his parents, the child underwent spinal canal decompression at vertebrae L1-L5 and biopsy of a nerve root of the cauda equina. The surgical procedure was performed with intraoperative neuromonitoring (motor and somatosensory evoked potentials). The bilateral tibialis anterior muscles, quadriceps femoris, gastrocnemius, and sphincter were selected for electromyography. The spinal dural sac was expanded, the local pressure of the spinal dural sac was increased, and the cauda equina nerve roots were hypertrophic and extruding from the spinal dural opening (Figure 3). A cauda equina nerve root that did not elicit a motor response was selected through nerve stimulation and was surgically resected for pathological examination. We enlarged the spinal dural sac and removed the spinous processes and laminae of L1-L5. The patient recovered well after surgery, and no surgical complications occurred. Early postoperative neurological function remained stable. Immunotherapy and hormone therapy were recommended for the patient, however, his family refused them.
When the patient returned for a follow-up visit 6 mo after the surgery, he could limp, and his walking posture had improved, however, he still complained of numbness in the distal lower limbs. He was re-admitted and administered intravenous immunoglobulin treatment at 6 mo post-surgery, and long-term oral steroid treatment was initiated after discharge. Oral prednisone was initially prescribed at 1 mg/kg/d. After 2 mo, the dosage was gradually reduced to 5 mg/kg/d with 2 wk intervals between doses. The patient continued maintenance therapy using prednisone (10 mg/d) for 1 year. Liver and kidney function and electrolytes were examined regularly during prednisone treatment, but no abnormal results were found. The symptoms further improved by the follow-up visit 1 year after the operation, including near-normal muscle strength of both lower limbs and partial recovery of sensory function. The boy could walk upright independently, however, he still complained of numbness of the distal lower extremities.
CIDP is an immune-mediated peripheral neuropathy characterized by progressive or recurrent sensory and motor dysfunctions[2]. The estimated incidence of CIDP is approximate 0.33 per 100000 population, and the prevalence of CIDP is approximate 2.81 per 100000[3], which is significantly higher than in children, at less than 1 per 2000000[4]. For several years, elements of macrophage-mediated demye
HN frequently manifests as thickening of the peripheral nerves, accompanied by neurological dysfunction. It is generally a slowly progressive disease and is rare in children. HN can be caused by a variety of diseases, such as CIDP, intrathecal schwannoma, hereditary motor and sensory neuropathy type I (charcot-marie-tooth disease) and type III (dejerine sottas disease), neurofibromatosis, amyloidosis, lymphoma, and leprosy[8]. Although cranial nerve involvement is rare, it has been reported to affect the trigeminal, oculomotor, facial, and auditory nerves[9-12]. HN of the cauda equina is exceedingly rare and can cause symptoms similar to those of spinal stenosis. Hypertrophic nerve roots occupy and narrow the lumbar spinal canal, resulting in symptoms similar to those of typical lumbar spinal stenosis[13].
The signal abnormality of HN can be observed via B-mode ultrasound and MRI. Hypoechoic masses in B-mode ultrasound images could represent neural edema and acute inflammatory response, while hyperechoic enlargement could indicate fibrosis, onion bulb formation, and remyelination after chronic demyelination[14]. However, B-mode ultrasound is usually more sensitive to isolated peripheral HN. Considering the patient’s clinical symptoms, we initially suspected that the lesion might have been located in the spinal cord, hence, we chose MRI instead of B-mode ultrasound imaging. MRI may demonstrate hypertrophic nerve roots as multifocal and asymmetrical or diffuse. The different patterns of HN likely reflect the unique mechanisms of nerve demyelination of different etiologies[15]. Histopathological sections of hypertrophic nerve caused by CIDP usually display characteristic changes such as “onion bulbs”. Onion bulbs are mainly formed by the proliferation of schwann cells, which are positive for the S-100 protein anti-body[16]. Other diseases that cause hypertrophic nerve roots can present with changes similar to onion bulbs, for example, in the case of intrasheath schwannomas, the proliferation of peripheral nerve cells is the main cause of hypertrophic nerve roots. These cells surround one or more axons and their schwann cell sheaths, therefore, EMA is positive[17]. This distinguishes between perineuroma and schwannoma, as well as inflammatory and acquired polyneuropathy[18]. In this case, the immunohistochemical result exhibited positivity for S-100 proteins, and inflammatory cells were visible under the electron microscope; hence, CIDP was diagnosed. The etiology of HN is crucial and sometimes difficult to diagnose by non-invasive examination. Nerve biopsy examination is a useful method for discriminating between inflammatory and non-inflammatory neurological disorders[19].
Alternative treatments for CIDP include intravenous immunoglobulin, corticosteroids, or plasmapheresis[20]. Patients with IgG4 subclass antibodies may not tolerate standard CIDP treatment, however, they may show significant improvement after treatment with rituximab (a monoclonal anti-CD20 antibody that can eliminate B cells)[21]. Neurological decompression surgery has been reported as a treatment for some patients with HN. Lee et al[22] reported that an adult patient with cauda equina hypertrophy caused by CIDP received intravenous immunoglobulin injection and spinal canal decompression at segments L2-L5. The patient’s symptoms were significantly improved after surgery. Lyons[19] reported another case with HN of the cauda equina who showed clinical symptom improvement after spinal canal decompression and intravenous immunoglobulin treatment. Conversely, O'Ferrall et al[23] reported a patient with aggravated symptoms who was treated with laminar decompression combined with intravenous immunoglobulin and eventually improved after plasmapheresis. All these patients with CIDP underwent spinal canal decompression with disparate results. Our case demonstrates that symptoms can improve even if only spinal canal decompression is performed. This may result from cauda equina nerve edema and inflammatory response to local pressure relief. We consider that neurological decompression is effective for HN with high signal on T2 MRI sequence images. However, neurological decompression did not alleviate the degree of inflammatory demyelination. Our patient achieved the greatest improvement in clinical symptoms after receiving hormone therapy. Thus, we think that surgical treatment combined with interventions targeted at a specific etiology is more suitable for treating patients with HN of cauda equina.
Spinal canal decompression can improve sensory and motor functions in HN of the cauda equina caused by inflammation, even in children. Surgery combined with specific etiology intervention is the best way to achieve the optimal outcome.
We are deeply indebted to Professor Yang L who guided us throughout our writing of this report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Ojukwu DI, United States S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Landrieu P, Baets J, De Jonghe P. Hereditary motor-sensory, motor, and sensory neuropathies in childhood. Handb Clin Neurol. 2013;113:1413-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Allen JA. Chronic Demyelinating Polyneuropathies. Continuum (Minneap Minn). 2017;23:1310-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Broers MC, Bunschoten C, Nieboer D, Lingsma HF, Jacobs BC. Incidence and Prevalence of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2019;52:161-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol. 1999;46:910-913. [PubMed] [Cited in This Article: ] |
| 5. | Vallat JM, Mathis S, Vegezzi E, Richard L, Duchesne M, Gallouedec G, Corcia P, Magy L, Uncini A, Devaux J. Antibody- and macrophage-mediated segmental demyelination in chronic inflammatory demyelinating polyneuropathy: clinical, electrophysiological, immunological and pathological correlates. Eur J Neurol. 2020;27:692-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Stratton JA, Holmes A, Rosin NL, Sinha S, Vohra M, Burma NE, Trang T, Midha R, Biernaskie J. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 2018;24:2561-2572.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 7. | Jessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 8. | Pinto MV, Spinner RJ, Staff NP. A treatable hypertrophic neuropathy. Pract Neurol. 2019;19:80-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lim JJ, Clark HB, Grande AW. Isolated Hypertrophic Neuropathy of the Oculomotor Nerve. World Neurosurg. 2017;98:880.e1-880.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Jordan JE, Lane B, Marks M, Chang Y, Weinberger M. Cranial hypertrophic interstitial neuropathy. AJNR Am J Neuroradiol. 1992;13:1552-1554. [PubMed] [Cited in This Article: ] |
| 11. | Kania RE, Cazals-Hatem D, Bouccara D, Cyna-Gorse F, Lisovoski F, Hénin D, Sterkers O. Hypertrophic neuropathy of the facial nerve. Ann Otol Rhinol Laryngol. 2001;110:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Neely JG, Armstrong D, Benson J, Neblett C. "Onion bulb" formation associated with a solitary neoplasm of the eighth nerve sheath. Am J Otolaryngol. 1981;2:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Ishida K, Wada Y, Tsunemi T, Kanda T, Mizusawa H. Marked hypertrophy of the cauda equina in a patient with chronic inflammatory demyelinating polyradiculoneuropathy presenting as lumbar stenosis. J Neurol. 2005;252:239-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Grimm A, Schubert V, Axer H, Ziemann U. Giant nerves in chronic inflammatory polyradiculoneuropathy. Muscle Nerve. 2017;55:285-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Yoshii S, Shibuya K, Yokota H, Ikehara H, Shiohama T, Sawada D, Kuwabara S, Fujii K. Magnetic resonance neurography in diagnosing childhood chronic inflammatory demyelinating polyradiculoneuropathy. Brain Dev. 2021;43:352-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Koszyca B, Jones N, Kneebone C, Blumbergs P. Localized hypertrophic neuropathy: a case report and review of the literature. Clin Neuropathol. 2009;28:54-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Norris B, Gonzales M, Drummond KJ. Solitary localised hypertrophic neuropathy of the cauda equina. J Clin Neurosci. 2011;18:712-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Boyanton BL Jr, Jones JK, Shenaq SM, Hicks MJ, Bhattacharjee MB. Intraneural perineurioma: a systematic review with illustrative cases. Arch Pathol Lab Med. 2007;131:1382-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Lyons MK. Nerve rootlet and fascicular biopsy in patients with hypertrophic inflammatory neuropathy. Neurologist. 2009;15:40-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;1:CD010369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Querol L, Rojas-García R, Diaz-Manera J, Barcena J, Pardo J, Ortega-Moreno A, Sedano MJ, Seró-Ballesteros L, Carvajal A, Ortiz N, Gallardo E, Illa I. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm. 2015;2:e149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 22. | Lee SE, Park SW, Ha SY, Nam TK. A case of cauda equina syndrome in early-onset chronic inflammatory demyelinating polyneuropathy clinically similar to charcot-marie-tooth disease type 1. J Korean Neurosurg Soc. 2014;55:370-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 23. | O'Ferrall EK, Gendron D, Guiot MC, Hall J, Sinnreich M. Lower motor neuron syndrome due to cauda equina hypertrophy with onion bulbs. Muscle Nerve. 2013;48:301-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |