Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3268
Peer-review started: November 11, 2021
First decision: December 27, 2021
Revised: January 11, 2022
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: April 6, 2022
Emphysematous pyelonephritis (EPN) is a severe acute necrotizing infection of the renal parenchyma and surrounding tissues that causes the presence of gas in the renal parenchyma, collecting system, or perinephric tissue and has a poor prognosis. EPN occurs primarily in people with diabetes mellitus (DM), but can occur in those without DM when the associated renoureteral unit is obstructed.
We describe our experience with six patients who developed EPN. Five patients had DM, including one with diabetic ketoacidosis, one with multisystem involvements, including eye, lung and brain. Bilateral urolithiasis was present in one case, along with emphysematous cystitis. Unilateral kidney stones were present in one patient. One patient was an older man in poor general health. Five individuals survived and underwent surgical procedures including ureteral stent installation (Double J stent placement), percutaneous nephrostomy and perinephric abscess puncture drainage, while one died because the patient’s family chose to terminate therapy. Klebsiella pneumoniae and Escherichia coli were the microorganisms implicated.
We conclude that EPN is a potentially fatal illness. A positive outcome neces
Core Tip: Emphysematous pyelonephritis (EPN) is a life-threatening kidney infectious disease. The incidence is not high. We report 6 cases of different types of EPN. Once diagnosed as EPN, we should take active and effective treatment according to the patient's condition at that time, so that patients can gain the greatest benefit.
- Citation: Ma LP, Zhou N, Fu Y, Liu Y, Wang C, Zhao B. Emphysematous pyelonephritis: Six case reports and review of literature. World J Clin Cases 2022; 10(10): 3268-3277
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3268.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3268
Emphysematous pyelonephritis (EPN) is an acute life-threatening infection characterized by gas generation in renal tissues. Bacteria are common pathogens, particularly Gram-negative facultative anaerobic bacteria like Escherichia coli (E. coli) and Klebsiella pneumonia (K. pneumonia). Fungi have been proven in certain recent studies to be pathogens[1-5]. Bacterial EPN is associated with a rapid progression of gas formation and an overall mortality rate ranging from 11% to 42%[6-9]. There is currently no agreement on the best treatment for EPN. The majority of experts recommend a vigorous medical and interventional approach[7]. The development of minimally invasive surgery, such as double J (DJ) stent placement, percutaneous drainage (PCD) and percutaneous nephrostomy (PCN), has resulted in a significant reduction in mortality when compared to traditional medical treatment and nephrectomy. However, several writers report a favorable prognosis when this condition is treated conservatively[10,11]. We present our experience of six EPN patients, including disease classification, complications, and treatment methods.
Case 1: A 70-year-old female patient with type 2 diabetes mellitus (T2DM) was admitted to the Emergency Department of Jishuitan Hospital in Beijing, China in July 2020, after experiencing fever and left back pain for 10 d.
Case 2: An 81-year-old female patient was admitted to the Department of Urology at Jishuitan Hospital in Beijing, China in October 2020, after suffering from right back pain for 4 d.
Case 3: A 47-year-old female patient with T2DM was admitted to the Emergency Department of Jishuitan Hospital in Beijing, China in November 2015, after suffering from a fever and right back pain for 7 d.
Case 4: A 70-year-old male patient with T2DM who had been experiencing fever for 6 h was admitted to the Emergency Department, Jishuitan Hospital, Beijing, China in July 2021.
Case 5: A 62-year-old female patient with T2DM who had been experiencing fever and left back pain for 3 d was admitted to the Emergency Department, Jishuitan Hospital, Beijing, China in September 2016.
Case 6: A 90-year-old female patient who had been experiencing anorexia and abdominal pain for 7 d was admitted to the Emergency Department, Jishuitan Hospital, Beijing, China in July 2021.
Case 1: She had T2DM for 20 years, with diabetic retinopathy and a fasting glucose level of 10 mmol/L.
Case 2: She had a puncture and drainage procedure 2 mo ago due to a hematoma on the right side of her kidney caused by previous lumbar trauma. She was diagnosed with hypertension, stroke, atrial fibrillation, and lung cancer, but she maintained she did not have T2DM.
Case 3: She had been suffering from T2DM for a long time without receiving proper treatment.
Case 4: He had been suffering from T2DM without systematic treatment.
Case 6: She had been suffering from anemia.
Case 1: The following was discovered during a physical examination: temperature 37.5 °C, heart rate 120 beats/min and blood pressure 154/83 mmHg. Pain was elicited by percussion in the left flank region.
Case 2: The following was discovered during physical examination: temperature as 37.9 °C, heart rate 98 beats/min, and blood pressure 105/82 mmHg. Pain was elicited by percussion in the right flank region.
Case 3: The following was discovered during a physical examination: temperature 38.4 °C, heart rate 120 beats/min, and blood pressure 90/60 mmHg. Pain was elicited by percussion in the right flank region.
Case 4: A physical examination revealed the following: temperature, 38.4 °C, heart rate 130 beats/min; and blood pressure 102/75 mmHg. Percussion in the right flank region elicited pain.
Case 5: A physical examination revealed the following: temperature, 38 °C; heart rate, 120 beats/min; and blood pressure, 136/58 mmHg. Percussion in the left flank region elicited pain.
Case 6: A physical examination revealed the following: temperature, 37.5 °C; heart rate 92 beats/min; and blood pressure 114/51 mmHg. Percussion in the hypogastric region elicited pain.
Case 1: White blood cell count, 11.17 × 109/L; absolute neutrophil count, 10.69 × 109/L; hemoglobin, 81 g/L; platelets, 129 × 109/L; C-reactive protein, 244.90 mg/L; procalcitonin (PCT) 4.5 ng/mL; albumin, 26.5 g/L; serum creatinine, 155 mmol/L; glucose, 26.7 mmol/L; fibrinogen, 429.1 mg/dL.
Case 2: The following were the results of routine laboratory tests: white blood cell count, 19.06 × 109/L, absolute neutrophil count, 16.98 × 109/L; hemoglobin, 94 g/L; platelets, 292 × 109/L; C-reactive protein, 124 mg/L; PCT 3.43 ng/mL; albumin, 25.5 g/L; serum creatinine, 79 mmol/L; glucose, 6.8 mmol/L; fibrinogen, 539.9 mg/dL.
Case 3: The following were the results of routine laboratory tests: white blood cell count, 29.41 × 109/L; absolute neutrophil count, 25.24 × 109/L; hemoglobin, 115 g/L; platelets, 520 × 109/L, C-reactive protein, 323mg/L; PCT 26.86 ng/mL; albumin, 31 g/L; serum creatinine, 148 mmol/L; glucose, 28.8 mmol/L; carbon dioxide binding capacity, 4 vol%; fibrinogen, 489.8 mg/dL. Blood gas analysis revealed pH 7.285; carbon dioxide partial pressure, 9.7 mmHg; oxygen partial pressure, 128 mmHg; bicarbonate, 4.7 mmol/L; anion gap, 20.5 mmol/L; base excess, -18.1 mmol/L; lactose, 1.2 mmol/L. Urinalysis measurements were glucose, 4+; ketone body, 4+.
Case 4: Routine laboratory studies revealed the following: white blood cell count, 17.41 × 109/L; absolute neutrophil count, 12.66 × 109/L; hemoglobin, 101 g/L; platelets, 105 × 109/L; C-reactive protein, 318.8 mgL; PCT, 5.33 ng/mL; albumin, 31.2 g/L; serum creatinine, 188 mmol/L; glucose, 16.8 mmol/L; fibrinogen, 802.4 mg/dL.
Case 5: Routine laboratory studies revealed the following: white blood cell count, 15.25 × 109/L; absolute neutrophil count, 13.21 × 109/L; hemoglobin, 121 g/L; platelets, 324 × 109/L; C-reactive protein, 391 mg/L; PCT, 12.82 ng/mL; albumin, 32.6 g/L; serum creatinine, 72 mmol/L; glucose, 13.3 mmol/L; fibrinogen, 732 mg/dL.
Case 6: Routine laboratory studies revealed the following: white blood cell count, 10.25 × 109/L; absolute neutrophil count, 9.22 × 109/L; hemoglobin, 44 g/L; platelets, 367 × 109/L; C-reactive protein, 124.83 mgL; albumin, 30.3 g/L; serum creatinine, 188 mmol/L; glucose, 4.7mmol/L; fibrinogen, 623.7 mg/dL.
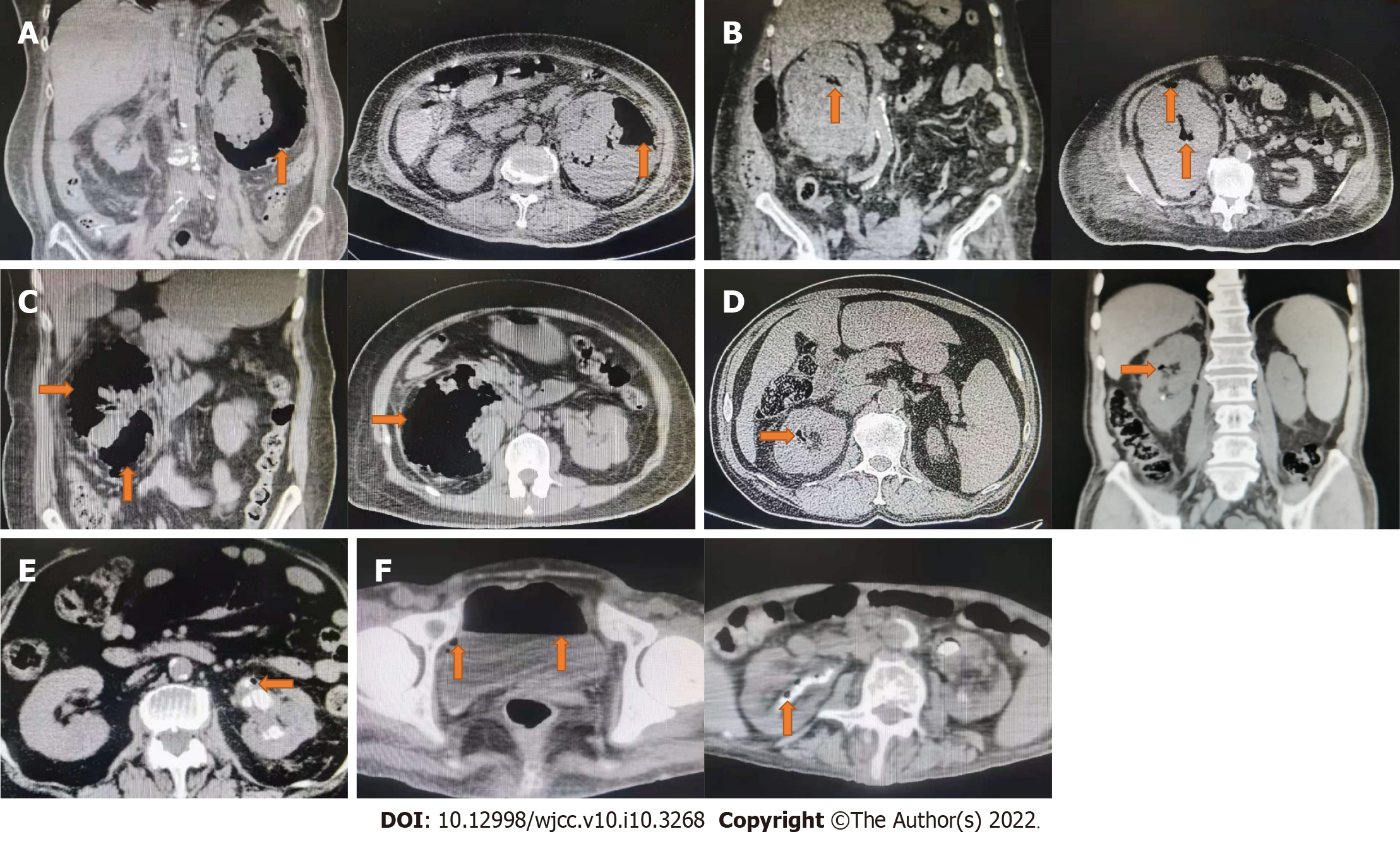
Case 1: Computed tomography (CT) detected gas in the left renal parenchyma following that (Figure 1A).
Case 2: Following that, CT revealed gas in the right renal parenchyma (Figure 1B).
Case 3: Following that, CT revealed gas in the right renal parenchyma (Figure 1C).
Case 4: CT showed gas in the right renal parenchyma (Figure 1D).
Case 5: CT showed gas in the left renal parenchyma (Figure 1E).
Case 6: CT showed gas in bilateral renal parenchyma and bladder (Figure 1F).
Diagnosis of EPN combined with imaging results.
The patient was finally diagnosed with emphysema pyelonephritis.
The finally diagnosed was EPN.
The final diagnosis was EPN and emphysema cystitis.
Empirical antibiotics, fluid resuscitation, blood glucose control, and supportive treatment were administered. PCN and perinephric abscess puncture drainage of the left kidney was performed, and K. pneumoniae was isolated from culture of blood, puncture fluid, sputum and urine. Cerebrospinal fluid culture was not positive.
Empirical antibiotics and supportive care were administered. Right kidney PCN and perinephric abscess puncture drainage were performed. K. pneumoniae was isolated using a parallel puncture fluid culture. Blood culture did not come out positive.
Empirical antibiotics, fluid resuscitation, intravenous infusion of small doses of insulin to correct ketoacidosis, blood glucose control, and supportive treatment were all administered following diagnosis of EPN. We performed PCN, abscess incision and drainage of the right kidney, as well as perinephric expansion several times, and isolated E. coli from parallel puncture fluid culture. On the other hand, the blood culture was not positive.
Empirical antibiotics, fluid resuscitation and supportive treatment were all administered following diagnosis of EPN. Ureteral DJ stenting was performed on the right kidney and purulent fluid was seen flowing out of the ureteral orifice under ureteroscopy. We isolated K. pneumoniae from parallel puncture fluid culture. On the other hand, the blood culture was negative.
Empirical antibiotics and supportive care were administered. PCN of the left kidney was performed. Parallel puncture fluid culture isolated E. coli. On the other hand, the blood culture was negative.
Empirical antibiotics, blood transfusion and supportive treatment were all administered following diagnosis of EPN and emphysematous cystitis. Pyuria and air bubbles were seen after catheterization.
Unfortunately, the patient experienced septic shock, severe pneumonia, right-sided endogenous panophthalmitis, left-sided endogenous endophthalmitis, epilepsy, meningitis stimulation, meningitis, and kidney puncture drainage. The patient was finally released 113 d after being admitted, but the right eye was already blind.
The patient improved and was discharged 15 d after being admitted.
Clinically, the patient recovered and was discharged 39 d after admission.
The patient recovered and was discharged 10 d after admission.
The patient recovered and was discharged 8 d after admission.
The family gave up treatment and the patient died 2 d later (Table 1).
| Demographic and clinical characteristics of patients and outcomes | ||||||
| Patient | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| Age, yr | 70 | 81 | 47 | 70 | 62 | 90 |
| Gender | Female | Female | Female | Male | Female | Female |
| Past history | DM | HP, AF, lung cancer, puncture and drainage on right side kidney | DM | DM | DM | Anemia |
| Clinical feature | Left back pain, fever | Right back pain, fever | Right back pain, fever | Fever | Fever and left back pain | Anorexia and abdominal pain |
| Clinical finding | T 37.5 °C, HR 120 bpm | T 37.9 °C, HR 98 bpm | T 38.4 °C, HR 120 bpm, BP 90/60 mmHg | T 38.4 °C, HR 130 bpm | T 38 °C, HR 120 bpm | T 37.5 °C, HR 92 bpm |
| Class | IIIb | II | II | I | I | IV |
| Blood test | ||||||
| WBC (× 109/L) | 11.17 | 19.06 | 29.41 | 17.41 | 15.25 | 10.25 |
| Absolute neutrophil count (× 109/L) | 10.69 | 16.98 | 25.24 | 12.66 | 13.21 | 9.22 |
| Hemoglobin (g/L) | 81 | 94 | 115 | 101 | 121 | 44 |
| Platelets (× 109/L) | 129 | 292 | 520 | 105 | 324 | 367 |
| CRP (mg/L) | 244.90 | 124 | 323 | 318.8 | 391 | 124.83 |
| PCT (ng/mL) | 4.5 | 3.43 | 26.86 | 5.33 | 12.82 | NA |
| Albumin (g/L) | 26.5 | 25.5 | 31 | 31.2 | 32.6 | 30.3 |
| Serum creatinine (μmol/L) | 155 | 79 | 148 | 188 | 72 | 188 |
| Glucose (mmol/L) | 26.7 | 6.8 | 28.8 | 21.7 | 13.3 | 4.7 |
| Fibrinogen (mg/dL) | 429.1 | 539.9 | 489.8 | 802.4 | 732 | 623.7 |
| Other | NA | NA | pH 7.285, AG 20.5 mmol/L, BE 18.1 mmol/L, Lac 1.2 mmol/L, urine acetone bodies, 4+ | NA | Unilateral urolithiasis | Bilateral urolithiasis; emphysematous cystitis |
| Complication | Septic shock, severe pneumonia, right-sided endogenous panophthalmitis, left-sided endogenous endophthalmitis, epilepsy, meningitis stimulation, meningitis | NA | NA | NA | NA | NA |
| Etiology | Blood culture, puncture fluid culture, sputum culture, urine culture: K. pneumoniae | Parallel puncture fluid culture: K. pneumoniae | Parallel puncture fluid culture: E. coli | Parallel puncture fluid culture: K. pneumoniae | Parallel puncture fluid culture: E. coli | NA |
| Intervention | PCN and perinephric abscess puncture drainage | PCN and perinephric abscess puncture drainage | PCN and abscess incision and drainage | DJ stenting | PCN | - |
| LOS | 113 d | 15 d | 39 d | 10 d | 8 d | - |
| Outcome | Recovered | Recovered | Recovered | Recovered | Recovered | Died |
EPN is an infectious kidney and perirenal illness that can be fatal. Gas generation occurs inside the renal parenchyma, collecting system, or perinephric tissues. Kelly and MacCullum initially described a case of renal illness with gas build-up in 1898. Schultz and Klorfein[12] coined the term EPN in 1962. Since then, several forms of kidney infection associated with gas accumulation have been reported.
EPN is most prevalent in patients with DM[13], but it can also occur in patients with urinary tract blockage (usually due to urinary tract stones, but also due to giant fecaloma[14] and severe uterine prolapse[15]) or who are immunocompromised. At the moment, all EPN patients mentioned in the literature were adults, with women outnumbering men[9]. However, this disease has been seen in newborns and young children[16,17]. Almost all previous literature indicates that the left kidney is more susceptible to infection than the right; however, in our research, three patients were affected in the right kidney, two in the left kidney, and one in both. Five of the patients were female, and four had T2DM. E. coli is the most prevalent pathogenic agent, having been linked to 62.7% of cases[18]. Additionally, K. pneumoniae, Proteus mirabilis[1], Pseudomonas aeruginosa[19], Enterobacter cloacae[16], Bacteroides fragilis[3], Clostridium septicum[4], Aspergillus fumigatus[20], Candida[2,20,21], Enterococcus[22] and Entamoeba histolytica[5] have been found. K. pneumoniae was detected in three cases and E. coli in two.
The pathophysiology of EPN is still unknown. Stones in the urinary system can obstruct urine flow, promoting bacterial growth and reproduction, leading to urinary tract infections and sepsis. Increased glucose concentrations in the tissues, impaired tissue perfusion, impaired immunity, and a hypoxic environment in the renal medulla are thought to predispose patients with DM with associated microvascular disease to tissue ischemia and necrosis, thereby facilitating the growth of gas-forming organisms[23]. Huang and Tseng[24] proposed a mechanism for the generation of gas in EPN. Hydrogen and CO2 were detected in the gas samples. Only mixed acid fermentation (which occurs in the majority of Enterobacteriaceae, e.g., E. coli, K. pneumoniae and Proteus) and butyric fermentation (which occurs in Clostridium) may produce hydrogen. They hypothesized that persons with diabetes may have a more favorable environment for gas-forming bacteria to grow and catabolize rapidly, resulting in massive generation of gas.
The diagnosis is confirmed with radiography. Wan et al[25] based diagnosis on CT findings in 1996. Four years later, in 2000, a new classification technique, Huang and Tseng[24]’s, was produced (Table 2). It was based on the CT findings but was more thorough than the previous one. Two were classified as class 1, two as class 2, one as class 3B and one as class 4 in our study, according to this classification.
| Classification | Radiological basis | Class |
| Wan et al[25] | CT | I: Renal necrosis with presence of gas but no fluid; II: Parenchymal gas associated with fluid in renal parenchyma, perinephric space or collecting system |
| Huang and Tseng[24] | CT | 1: Gas in collecting system only; 2: Gas in the renal parenchyma without extension to extrarenal space; 3A: Extension of gas or abscess to perinephric space; 3B: Extension of gas or abscess to pararenal space; 4: Bilateral EPN or solitary kidney with EPN |
Fever/chills, flank pain, tenderness at the renal angle, vomiting, and dysuria are common symptoms of EPN. Some individuals may exhibit mental abnormalities, tachycardia, or hypotension[26-28]. Pneumomediastinum, emphysematous osteomyelitis, psoas abscesses, pneumorachis, and spondylodiscitis are all uncommon presentations[29-31]. EPN can progress to septic shock and multiorgan failure, which both have a significant mortality rate. Seven trial groups were included in the meta-analysis, totaling 175 patients with EPN[9]. The overall mortality rate was 25%. According to this meta-analysis, conservative treatment alone, type I (according Wan radiological classification), bilateral EPN, and thrombocytopenia are all associated with a fatal result in patients with EPN. Tsu et al[22] demonstrate that significant hyperglycemia at presentation and the EPN radiological CT class (both the Wan and Huang and Tseng systems) are the only predictors of mortality. Kaiser and Fournier[32] demonstrated that patients with acute kidney failure, shock, confusion, and thrombocytopenia frequently have a bad prognosis. Krishnamoorthy et al[13] discovered that low serum albumin levels, low serum sodium levels, high absolute leukocyte count, and high hemoglobin A1c level are all substantially related with poor prognosis. In our investigation, we discovered that all of the patients had fever, an elevated absolute leukocyte count, and hypoalbuminemia, and that four of the six had acute renal injury. Fortunately, just one patient died as a result of their family members refusing treatment.
Fluid resuscitation, antibiotics, strict glucose control, surgical or PCD, and nephrectomy are among the treatment options for EPN. When compared to medical treatment alone, early aggressive surgical surgery resulted in a successful outcome, according to early investigators[33]. Kuzgunbay et al[34] categorized the patients into three risk groups (Table 3). It is widely acknowledged that nephrectomy saves lives in severe EPN patients with septic signs and widespread kidney involvement. In mild/moderate EPN with septicemia, broad-spectrum antibiotic treatment and PCD should be combined, while monotherapy with broad-spectrum antibiotics may be required for lesions that are too small to drain.
| Risk group | Definition |
| Mild | No septicemia; CT: Emphysematous lesion < 1 cm2 in the kidney, solitary or multiple; Percutaneous drainage is necessary/inapplicable |
| Moderate | No septicemia; CT: Emphysematous lesion involving equal or less than half of the kidney; Amenable to percutaneous drainage |
| Severe | Septicemia; CT: Emphysematous lesion involving more than half of the kidney and/or perinephric extension |
A recent study[26] found that medical therapy with antibiotics was effective in 10% of patients. Ninety percent of patients were treated medically and surgically, with DJ stenting, PCN and PCD among the procedures used. None of the patients required emergency nephrectomy, and none died. Rahim et al[28] discovered that only 20% of patients required surgical intervention, none required PCD or DJ stenting, and all patients survived. Olvera-Posada et al[18] found that mortality rates were comparable across definitive care modalities when comparing drug, minimally invasive and surgical therapy, as well as between those who underwent nephrectomy and those who received alternative treatment. Treatment of EPN is evolving. In recent times, minimally invasive approaches in the management strategy of EPN have gained momentum. Somani et al[35] discovered a 25% mortality rate associated with emergency nephrectomy, compared to 13.5% mortality with medical management with PCD alone and 6.6% mortality with PCD and elective nephrectomy. A single-center study[36] of 18 patients found that all underwent minimally invasive surgery (DJ stenting placement, PCN and PCD), that 94% of patients responded well, that one patient underwent nephrectomy, and that there were no deaths. There appears to be no definitive rule governing the selection of a conservative or aggressive surgical strategy. The starting approach will be determined by the clinical presentation. In severe situations, extreme procedures such as surgical draining or even nephrectomy may be required. In our study, five patients were treated aggressively, including anti-infective treatment and DJ stent placement and PCN, but one of them presented with multiple organ involvement, including blood, lung, brain and eye. Due to the presence of shock, the high surgical risk and family refusal of surgical treatment, early nephrectomy was not selected, but this did have a possibility of a different outcome. The last patient died due to old age, poor general condition and family refusal of surgery.
Because of the rarity of EPN, most studies are retrospective. We recommend prospective randomized studies to better evaluate the treatment strategy and outcomes.
EPN necessitates prompt diagnosis and therapy. The type of intervention is determined by the severity of the lesions and the patient’s clinical status. Fluid resuscitation as soon as possible, with hard hammering and strong anti-inflammatory agents, nutritional support, and a race against time to dislodge the obstruction (such as DJ stent placement, PCN, PCD or nephrectomy). Any intervention deemed necessary should not be postponed. If the patient’s progress is not sufficient, therapeutic modification may be indicated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balakrishnan DS, Kotelevets SM S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Uruc F, Yuksel OH, Sahin A, Urkmez A, Yildirim C, Verit A. Emphysematous pyelonephritis: Our experience in managing these cases. Can Urol Assoc J. 2015;9:E480-E483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Mohamed AH, Mohamud HA. Emphysematous pyelonephritis caused by candida species: A case report and outcome of 1 year follow-up. Urol Case Rep. 2020;30:101113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Liao HW, Chen TH, Lin KH, Lin HH, Hsu YH, Hou CC, Sue YM. Emphysematous pyelonephritis caused by Bacteroides fragilis. Nephrol Dial Transplant. 2005;20:2575-2577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Christensen J, Bistrup C. Case report: emphysematous pyelonephritis caused by clostridium septicum and complicated by a mycotic aneurysm. Br J Radiol. 1993;66:842-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Guvel S, Kilinc F, Kayaselcuk F, Tuncer I, Ozkardes H. Emphysematous pyelonephritis and renal amoebiasis in a patient with diabetes mellitus. Int J Urol. 2003;10:404-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Eswarappa M, Suryadevara S, John MM, Kumar M, Reddy SB, Suhail M. Emphysematous Pyelonephritis Case Series From South India. Kidney Int Rep. 2018;3:950-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Aboumarzouk OM, Hughes O, Narahari K, Coulthard R, Kynaston H, Chlosta P, Somani B. Emphysematous pyelonephritis: Time for a management plan with an evidence-based approach. Arab J Urol. 2014;12:106-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Li S, Wang J, Hu J, He L, Wang C. Emphysematous pyelonephritis and cystitis: A case report and literature review. J Int Med Res. 2018;46:2954-2960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Falagas ME, Alexiou VG, Giannopoulou KP, Siempos II. Risk factors for mortality in patients with emphysematous pyelonephritis: a meta-analysis. J Urol. 2007;178:880-5; quiz 1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Irfaan AM, Shaikh NA, Jamshaid A, Qureshi AH. Emphysematous Pyelonephritis: A single center review. Pak J Med Sci. 2020;36:S83-S86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kolla PK, Madhav D, Reddy S, Pentyala S, Kumar P, Pathapati RM. Clinical profile and outcome of conservatively managed emphysematous pyelonephritis. ISRN Urol. 2012;2012:931982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Schultz EH Jr, Klorfein EH. Emphysematous pyelonephritis. J Urol. 1962;87:762-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 106] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Krishnamoorthy S, Zumla A, Sekar H, Muneer A, Thiruvengadam G, Kumaresan N. Prognostic scoring system and risk stratification in patients with emphysematous pyelonephritis: an 11-year prospective study at a tertiary referral centre. BJU Int. 2021;127:418-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Abi Abdallah M, Raad N, Yarak N, Noujeim JP, Noujeim A. Emphysematous Pyelonephritis Caused by a Giant Fecaloma. Case Rep Urol. 2019;2019:8743525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Iwamoto H, Anno T, Takenouchi H, Takahashi K, Horiya M, Kimura Y, Kawasaki F, Kaku K, Tomoda K, Uehara S, Kaneto H. Case Report: Emphysematous Cystitis and Pyelonephritis Induced by Uterine Prolapse in a Subject With Untreated Diabetes Mellitus. Front Med (Lausanne). 2021;8:658682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Siddique K, Seikaly MG. Emphysematous pyelonephritis in an infant. Pediatr Infect Dis J. 2013;32:1157-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Gross IT, Ford R. Emphysematous Pyelonephritis in a Child with Nephrolithiasis. J Pediatr. 2016;168:250-250.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Olvera-Posada D, Armengod-Fischer G, Vázquez-Lavista LG, Maldonado-Ávila M, Rosas-Nava E, Manzanilla-García H, Castillejos-Molina RA, Méndez-Probst CE, Sotomayor M, Feria-Bernal G, Rodríguez-Covarrubias F. Emphysematous pyelonephritis: multicenter clinical and therapeutic experience in Mexico. Urology. 2014;83:1280-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lu YC, Hong JH, Chiang BJ, Pong YH, Hsueh PR, Huang CY, Pu YS. Recommended Initial Antimicrobial Therapy for Emphysematous Pyelonephritis: 51 Cases and 14-Year-Experience of a Tertiary Referral Center. Medicine (Baltimore). 2016;95:e3573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Cases-Corona C, Shabaka A, Gonzalez-Lopez A, Martin-Segarra O, Moreno de la Higuera MA, Lucena R, Fernandez-Juarez G. Fulminant Emphysematous Pyelonephritis by Candida glabrata in a Kidney Allograft. Nephron. 2020;144:304-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Krishnasamy PV, Liby C 3rd. Emphysematous pyelonephritis caused by Candida tropicalis. Am J Med. 2010;123:e7-e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Tsu JH, Chan CK, Chu RW, Law IC, Kong CK, Liu PL, Cheung FK, Yiu MK. Emphysematous pyelonephritis: an 8-year retrospective review across four acute hospitals. Asian J Surg. 2013;36:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Yao J, Gutierrez OM, Reiser J. Emphysematous pyelonephritis. Kidney Int. 2007;71:462-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;160:797-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 415] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 25. | Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology. 1996;198:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 182] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Sengupta S, Basu S. Outcome of conservative and minimally invasive management in emphysematous pyelonephritis. Urol Ann. 2021;13:277-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Karthikeyan VS, Manohar CMS, Mallya A, Keshavamurthy R, Kamath AJ. Clinical profile and successful outcomes of conservative and minimally invasive treatment of emphysematous pyelonephritis. Cent European J Urol. 2018;71:228-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Rahim MA, Ananna MA, Iqbal S, Uddin KN, Latif ZA. Emphysematous pyelonephritis: experience at a tertiary care hospital in Bangladesh. J R Coll Physicians Edinb. 2021;51:19-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Neelakandan S, Viswanathan S, Selvaraj J, Pillai V, Sharma D, Chakkalakkoombil SV. Concurrent Presentation of Emphysematous Pyelonephritis, Emphysematous Osteomyelitis, and Psoas Abscesses. Cureus. 2021;13:e15908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Miller MA, Masullo LN, Miles EA, Coon TP. What happens when a person eats the entire "meals ready to eat? Am J Emerg Med. 2006;24:349-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Melgarejo-Segura MT, Morales-Martinez A, Arrabal-Polo MA. Pneumorachis and spondylodiscitis caused by emphysematous pyelonephritis. Int Urol Nephrol. 2021;53:91-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Kaiser E, Fournier R. [Emphysematous pyelonephritis: diagnosis and treatment]. Ann Urol (Paris). 2005;39:49-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Pontin AR, Barnes RD, Joffe J, Kahn D. Emphysematous pyelonephritis in diabetic patients. Br J Urol. 1995;75:71-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Kuzgunbay B, Turunc T, Tokmak N, Dirim A, Aygun C, Ozkardes H. Tailored treatment approach for emphysematous pyelonephritis. Urol Int. 2011;86:444-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Somani BK, Nabi G, Thorpe P, Hussey J, Cook J, N'Dow J; ABACUS Research Group. Is percutaneous drainage the new gold standard in the management of emphysematous pyelonephritis? J Urol. 2008;179:1844-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Gite VA, Shaw V, Agrawal M, Sankapal P, Maheshwari M. Minimally invasive techniques as a first line approach in the management of emphysematous pyelonephritis - A single centre experience. J Postgrad Med. 2021;67:146-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |