Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.177
Peer-review started: March 12, 2021
First decision: July 28, 2021
Revised: July 30, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: January 7, 2022
Blink and masseter reflexes provide reliable, quantifiable data on the function of the central nervous system: Delayed latencies have been found in patients with neurocognitive disorder (ND) and type 2 diabetes mellitus (T2DM), but this has not been studied in patients with both pathologies.
To investigate if older adults with ND plus T2DM have prolonged latencies of blink and masseter-reflex and if they were associated with disease progression.
This cross-sectional study included 227 older adults (> 60 years) from Colima, Mexico. Neurocognitive disorder was identified by a neuropsychological battery test, and T2DM identified by medical history, fasting glucose, and glycosylated hemoglobin. Latencies in the early reflex (R1), ipsilateral late (R2), and contralateral late (R2c) components of the blink reflex were analyzed for all subjects, and 183 subjects were analyzed for latency of the masseter reflex.
In 20.7% of participants, ND was detected. In 37%, T2DM was detected. Latencies in R1, R2, and R2c were significantly prolonged for groups with ND plus T2DM, ND, and T2DM, compared with the control group (P < 0.0001). The masseter reflex was only prolonged in older adults (regardless of T2DM status) with ND vs controls (P = 0.030). In older adults with ND and without T2DM, the more the cognitive impairment progressed, the more prolonged latencies in R2 and R2c presented (P < 0.01).
These findings suggest that blink and masseter reflexes could be used to evaluate possible changes in brainstem circuits in older adults with ND and T2DM.
Core Tip: Delayed latencies were found in patients with neurocognitive disorder (ND) and type 2 diabetes mellitus (T2DM), but they have not been reported before for patients with both pathologies. We report, through blink and masseter reflex techniques, reliable and quantifiable data of the central nervous system function at the level of brainstem. The clinical implication is that brainstem reflexes could be linked with ND progression in the presence of T2DM in older adults. Older adults with ND and T2DM had longer latencies of the blink reflex components compared with healthy controls. In older adults with ND vs controls, the masseter reflex latency was prolonged. Age, sex, education, and dependence altered blink reflex latency in ND patients, while T2DM control, depression, and renal damage did not alter blink reflex latency.
- Citation: Bricio-Barrios JA, Ríos-Bracamontes E, Ríos-Silva M, Huerta M, Serrano-Moreno W, Barrios-Navarro JE, Ortiz GG, Huerta-Trujillo M, Guzmán-Esquivel J, Trujillo X. Alterations in blink and masseter reflex latencies in older adults with neurocognitive disorder and/or diabetes mellitus. World J Clin Cases 2022; 10(1): 177-188
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/177.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.177
In Mexico, the prevalence of type 2 diabetes mellitus (T2DM) is high and among individuals over 60 years of age, it is 25.1% (22.4% for males and 27.1% for females)[1]. It is also known that lack of metabolic control can lead to macrovascular and microvascular complications, and possibly neurocognitive alterations[2]. Neurocognitive disorder (ND) affects between 6% and 6.5% of the population in Latin America: Age is a risk factor for developing the disease, and the risk doubles every 5 years after reaching 65 years of age[3]. Early diagnosis of ND in diseases like Alzheimer’s disease (AD) would allow for timely and optimal treatment. In addition, early diagnosis of ND would enable clinicians to identify and treat concomitant physical diseases, to detect and treat problematic behavioral and psychological symptoms, and to provide information and long-term support to caregivers[4].
In the disease process, ND has been associated to the presence of tangles and amyloid plaques in cortical areas[5], and evidence shows that neurocognitive damage begins at the subcortical level, including the brainstem[6,7]. Electrophysiological techniques provide reliable and quantifiable data about the function of the central nervous system[8], and latencies of the blink and masseter reflexes, obtained by electrical stimulation, are useful to evaluate the brainstem function[9]. Similar to the clinical elicited corneal reflex, the evoked blink reflex is comprised of two components with different latencies: An early response (R1) that detects the integrity function of the involved central pathway in the pons, and a late ipsilateral (R2) and contralateral (R2c) response that locates the afferent pathways from their entry to the pons, descending along the trigeminal spinal complex at the level of the inferior olive and caudal pole of the hypoglossal nucleus and efferent pathways in the pons, and pontomedullary interneuronal pathways[8,10]. In T2DM and/or ND, there could be changes to latencies of the blink reflex responses as a consequence of demyelination and loss of synapses.
On the other hand, the masseter reflex (also referred to as the mandibular or ‘jaw jerk’ reflex) usually evaluates the jaw’s functional activities such as chewing, biting, drinking, and speaking. It is the only monosynaptic reflex of the cranial and facial muscles that involves neural circuits of the lower brainstem; however, several aspects of the functional behavior of this reflex are still unclear[10,11].
Previously, Trujillo-Hernández et al[12] found alterations in the blink reflex in patients with T2DM, and Mohammadian et al[13] detected differences of the blink reflex by type of ND (AD, vascular, or mixed) in R2 and R2c latencies of the blink reflex. Therefore, we consider it possible to establish electrophysiological changes related to the presence of ND in diabetic patients through evaluation of the brainstem reflexes. Thus, the objective of this study was to investigate possible alterations in the latencies of blink and masseter reflexes in older adults in the presence or absence of ND and/or T2DM, in addition to assessing the possible role of intervening variables such as depression, functional dependence, schooling, sex, renal function, and T2DM control.
This cross-sectional study evaluated adults of both sexes who were 60 years of age or older. All participants were from the state of Colima in Mexico and belonged to groups of recreational assistance or care homes. Each participant underwent a clinical history to detect the following exclusion criteria: Chronic alcohol consumption, previous history of major head trauma, trigeminal neuralgia or facial paralysis, current treatment for dementia (for example, the medication Memantine), administration of neuroleptics, pain narcotics, and previous medical diagnosis of low prevalent dementias (Parkinsonian diseases, Lewy body dementia, frontotemporal neurocognitive disorder, neurocognitive disorder due to human immunodeficiency virus infection, neurocognitive disorder induced by substances, Huntington’s disease, prion disease, progressive supranuclear palsy, parathyroid disease or hypothyroidism). Finally, those participants who reported having intolerable pain to electric currents were also excluded. There were 254 older adults evaluated, but 22 of them did not perform the electromyographic recording, and 5 did not undergo blood sampling; therefore, 227 older adults were finally assessed. The subjects were divided into four study groups to compare latencies of the blink and masseter reflexes: Control group (with neither T2DM nor ND), T2DM group (with T2DM, without ND), ND group (without T2DM, with ND), and ND plus T2DM group (with both ND and T2DM).
The identification of ND was based on the criteria of the DSM-5[14], which considers the performance of neuropsychological tests, the autonomy of the patient, and concern of the individual, or by a knowledgeable informant or the clinician, for the individual’s current cognitive state[15]. Neuropsychological batteries included the Mini-Mental State Examination (MMSE)[16] and the Brief Neuropsychological Assessment in Spanish (NEUROPSI)[17]. The NEUROPSI test evaluates nine cognitive domains and has different cut-off points depending on age and schooling of the person being assessed; and according to the score obtained, it can be categorized as follows: Normal, mild, moderate, and severe deterioration. It was designed and applied in the Spanish language and had an estimated length of application time of 25 to 30 min. In addition, the applied MMSE was a version adapted to the Spanish language. Both tests have been validated in the Mexican population.
To evaluate participant autonomy, the Barthel Scale/Index was performed. The Barthel Index evaluates the performance of activities of daily living such as self-care (changing, going to the bathroom, and bathing), and displacement (walking and climbing stairs). Study participants were considered independent when they did not present any disability in the test (score 100/100)[18], and were considered to have neurocognitive disorder when they presented < 24 points in the MMSE, two or more cognitive domains altered in the NEUROPSI test, and any degree of dependence.
T2DM was detected in accordance with the criteria of the American Diabetes Association[19]: Fasting glucose ≥ 126 mg/dL on two different days and a measurement of glycated hemoglobin (HbA1c) ≥ 6.5% (≥ 48 mmol/mol), in addition to a previous T2DM diagnosis. Fasting glucose was measured from capillary blood with an automatic glucometer (AccuCheck Performa), and HbA1c was measured through a venous blood sample collected in an Ethylenediaminetetraacetic acid (EDTA) tube with a latex turbidimetry technique (Spinreact). Participants with T2DM with values less than 7% were considered to have good T2DM control.
Renal function was evaluated by calculating the glomerular filtration rate (GFR) using the Cockcroft Gault equation ([(140-age in years) ×body weight in kg/(serum creatinine in mg/dL × 72)] [× 0.85 if female]), and serum creatinine was measured in a venous blood sample collected in a dry tube using an enzymatic colorimetric technique (Spinreact).
Hypertension was determined according to clinical history of each participant and/or established when blood pressure was ≥ 140/90 mmHg (measured with an integrated aneroid sphygmomanometer kit with stethoscope), with the patient seated and resting for least 5 min.
The presence of depression was evaluated with the Geriatric Depression Scale, which is composed of 15 questions with responses "yes" or "no". From them, 10 items indicated the presence of depression when responses were affirmative, while others indicated depression when responses were negative. Participants were classified with depression with a score ≥ 5 points[20].
The electrophysiological test was performed in the neuromuscular physiology laboratory at the University Center for Biomedical Research at the University of Colima. All subjects were examined in a supine position in a quiet room (room temperature 24°C), with their eyes gently closed.
The latencies of the blink reflex were recorded based on the technique described by Kimura[21] with a Nicolet Viking Quest 4 EMG and EP channels electromyography. Two channels were used, connected with silver cup electrodes of 10 mm diameter. Ten20 conductive paste was used to ensure adherence with the skin, and NuPrep was applied to each subject’s skin to reduce impedance on the skin. The EMG activity was recorded from the orbicularis oculi muscle. Surface electrodes were placed as follows: The active electrode was placed over the inferior portion of the orbicularis oculi muscle 1 cm lateral of the outer canthus, the reference electrode was placed on the lateral aspect of the nose bilaterally, and the ground electrode was placed on the forehead at the midline level. An electrode gel (Signagel) was used as a highly conductive electrolyte on the bipolar stimulation probe; then, the supraorbital nerve was stimulated in the superior orbital fissure with supramaximal stimulation. To avoid habituation to the stimulus, pauses of 15 s were made. The intensity of the stimulus ranged from 5 to 30 mA, increasing in 3-mA increments. The duration of the stimulus was 0.1 to 1 ms, and bandpass filter was 8 Hz to 8 kHz. The latencies for R1, R2, and R2c were recorded on both sides of the face.
The same equipment and surface electrodes were used to register masseter reflex latency: The active electrode was placed below to the lower third of the muscle belly, the reference electrode was placed just below the mandible (5 cm from the active electrode), and the ground was placed on the midline of the forehead. The electrical stimulus was applied in the position of the mental foramen at the mentalis nerve[22]. The intensity of the stimulus started at 10 mA, with incremental pulses of 5 mA to a maximum of 50 mA. Test concluded when a clear recording of two latencies was obtained. For the blink reflex recording the scanning speed was 5 ms/division, although latency of the masseter reflex was obtained only in 82.3% (n = 183) of the participants who agreed to evaluation. For those who discontinued the study, the main reason was the perception of a painful sensation from stimulation. The onset latencies and response durations were measured in the recorded tests by visual inspection of response on the computer screen and using the cursors of the analysis software. Both reflexes were recorded for each participant on the same day, and no participant presented any adverse effects after the electrophysiological test.
All subjects or legal caregivers provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki II and the Good Clinical Practice guidelines. The study was approved by the Bioethics Committee of the State Institute of Cancerology (CEICANCL131216-BIOALZR-11). In addition, each participant and their caregiver received an individual report with the main results, as well as general recommendations for specialized care.
For quantitative results, mean and standard deviation are used when the distribution is parametric, and medians with percentiles 25-75 are used if the distribution of the data is non-parametric. The qualitative variables are expressed as frequencies and percentages. Intergroup comparisons were performed using the Student t test, the Mann-Whitney U test, or a one-way analysis of variance (ANOVA), and/or the Kruskal Wallis test, depending on the number of groups included in the analysis and the distribution of the data. The latencies for the right and left R2, and the R2c blink reflexes were averaged. The Chi-squared test was used for categorical variables. Analysis of covariance (ANCOVA), executed in the study group (fixed factor), involved the statistically significant variables between the groups (covariates): Age, sex, education, dependence, and hypertension, and the inter-subject variability test was also performed. To calculate the effect size, the eta-squared (η2) value was used, which evaluates the proportion of variance. Values from 0.04 to ≤ 0.36 were considered a moderate effect and values > 0.36 considered a size of strong effect[23]. Results were considered statistically significant with a value of P < 0.05. The data were analyzed using IBM SPSS version 22 software.
Two hundred and twenty-seven elderly adults living in a nursing home (n = 29) or from recreational assistance groups (n = 198) were included in this study. Neurodegenerative disorder was detected in 20.7% (n = 47) of participants, and 37% (n = 84) had T2DM. Participants were categorized in the following groups: Control (n = 122), T2DM (n = 58), ND (n = 21), and ND plus T2DM (n = 26). This categorization revealed a significant association between the presence of ND and T2DM; we found that older adults with T2DM have a 2.67-fold greater risk of developing ND (95% confidence interval: 1.37-5.17, P = 0.004).
The demographic data collected in the study population is summarized in Table 1. Age presented the greatest difference among the study groups: On average, the group with ND was oldest, and the Control group was youngest. The T2DM group had the highest proportion of subjects with hypertension. There was no difference in the presence of depression between study groups. There was also no difference in the GFR between groups of study, and no association was found when groups were analyzed according to GFR cut-off points of < 90 mL/min or < 60 mL/min. We compared the mean values of clinical and biochemical variables by study group, and statistically significant differences were shown in glucose, HbA1c, and serum creatinine, with higher values presenting in the T2DM groups. There were no significant differences in blood pressure noted between groups (Table 1).
| Items | Total (n = 227) | Control (n = 122) | T2DM (n = 58) | ND (n = 21) | ND plus T2DM (n = 26) |
| Age, yr | 72.1 ± 8.4 | 70.0 ± 6.9 | 70.8 ± 7.7 | 80.4 ± 11.3 | 77.9 ± 7.5b |
| Women | 168 (74.0% ) | 93 (76.2%) | 40 (69.0%) | 18 (85.7%) | 17 (65.4%) |
| Neuropsy score | 92.5 ± 26.2 | 104.2 ± 15.0 | 99.2 ± 18.1 | 56.0 ± 16.7 | 49.3 ± 19.3 b |
| Systolic blood pressure (mmHg) | 121.5 ± 17.4 | 120.0 ± 17.8 | 125.0 ± 17.3 | 121.0 ± 12.3 | 120.9 ± 19.0 |
| Diastolic blood pressure (mmHg) | 67.5 ± 8.0 | 67.5 ± 8.0 | 68.3 ± 8.4 | 66.6 ± 7.6 | 66.6 ± 7.5 |
| Hypertension | 102 (44.9%) | 53 (43.4%) | 37 (63.8%) | 5 (23.8%) | 7 (26.9%)b |
| Illiterate | 38 (16.7%) | 18 (14.8%) | 4 (6.9%) | 7 (33.3%) | 9 (34.6%)b |
| Dependent | 51 (22.5%) | 16 (13.1) | 13 (22.4%) | 16 (76.2) | 17 (65.4%)b |
| Depression | 51 (22.4%) | 22 (18.0%) | 14 (24.1%) | 9 (42.8%) | 6 (23.0%) |
| Glucose (mg/dL) | 116.0 ± 40.9 | 101.4 ± 14.9 | 147.4 ± 62.5 | 98.5 ± 19.9 | 130.0 ± 34.9b |
| Glycosylated hemoglobin (%) | 5.5 ± 1.9 | 4.6 ± 0.8 | 7.0 ± 2.5 | 4.5 ± 1.3 | 6.2 ± 2.8b |
| Glycosylated hemoglobin (mmol/L) | 37 ± 21 | 27 ± 9 | 53 ± 27 | 26 ± 14 | 45 ± 24b |
| Creatinine (mg/dL) | 1.0 ± 0.4 | 0.9 ± 0.2 | 1.1 ± 0.5 | 0.9 ± 0.2 | 1.2 ± 0.5a |
| Glomerular filtration (< 90 mL/min/1.73 m2) | 200 (88.1%) | 104 (85.2%) | 51 (87.9%) | 19 (90.4%) | 26 (100) |
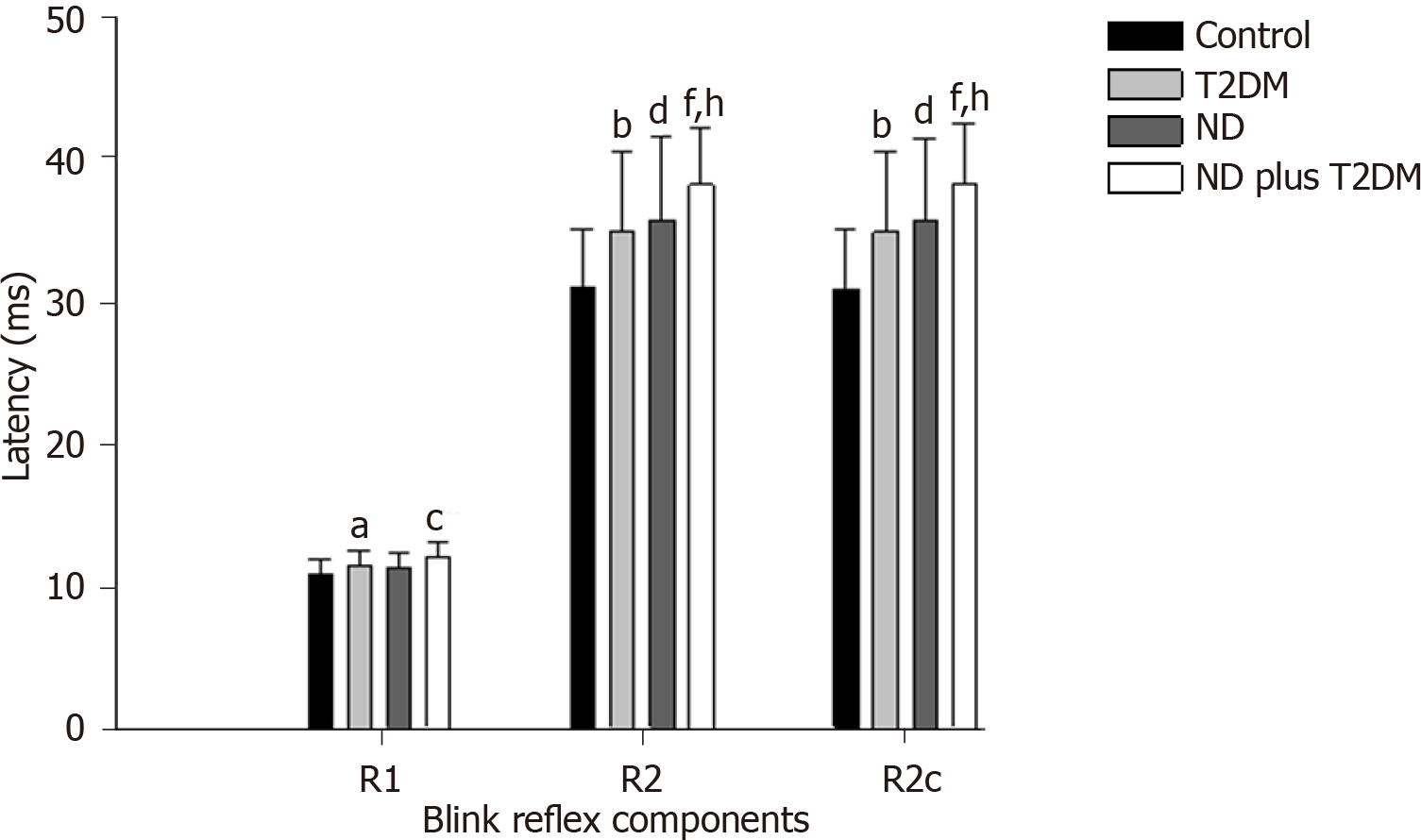
Regarding the blink reflex, after comparing latencies between the study groups, we found that R1, R2, and R2c were statistically different between groups (Figure 1), with prolonged latencies in the ND plus T2DM group, followed by the ND group, and then the T2DM group. The Control group had the shortest latencies (detailed values of the means and standard deviations of each group and post hoc analysis are presented in Supplementary Table 1 and Supplementary Table 2).
Subsequently, we sub-classified only the ND participants (n = 47) according to their NEUROPSI test score classification, showing the following classification: Mild 29.8%, moderate 40.4%, and severe 29.8%. When we compared the latencies of the components of the blink reflex between the ND categories, it is possible to appreciate that the group without T2DM presented with prolonged latencies in R2 and R2c when the cognitive impairment more progressed. The R1 latency of the blink reflex and the masseter reflex did not show statistical differences when comparing the ND status (Table 2).
| Latency | Subgroup | ND | |||
| Mild (n = 14) | Moderate (n = 19) | Severe (n = 14) | |||
| Left | R1 (ms) | All (n = 47) | 12.0 (10.4-12.8) | 12.0 (10.8-12.5) | 11.6 (10.5-12.8) |
| With T2DM (n = 21) | 12.1 (10.4-12.4) | 12.2 (10.8-12.6) | 10.9 (10.3-11.7) | ||
| Without T2DM (n = 26) | 12.4 (10.1-13.8) | 12.0 (11.2-12.4) | 12.8 (11.1-14.3) | ||
| R2 (ms) | All (n = 47) | 36.3 (28.5-38.0) | 37.7 (35.6-41.4) | 39.0 (37.9-41.2) | |
| With T2DM (n = 21) | 28.5 (26.3-35.2)1,2 | 40.6 (36.0-43.8) | 39.1 (38.4-41.2)b | ||
| Without T2DM (n = 26) | 37.8 (37.5-41.4) | 37.3 (34.5-40.1) | 38.1 (37.4-43.8) | ||
| R2c (ms) | All (n = 47) | 36.4 (30.8-39.3) | 37.8 (36.7-40.9) | 39.0 (37.4-41.3) | |
| With T2DM (n = 21) | 30.8 (24.3-35.2)2 | 38.0 (37.1-40.9) | 39.3 (37.7-41.3)a | ||
| Without T2DM (n = 26) | 39.3 (37.7-44.8) | 37.0 (35.6-40.0) | 38.1 (37.4-43.4) | ||
| Right | R1 (ms) | All (n = 47) | 11.5 (10.8-11.7) | 12.3 (11.5-12.8) | 38.4 (3.6-40.2) |
| With T2DM (n = 21) | 11.0 (10.3-11.5) | 11.7 (10.2-12.4) | 11.1 (10.4-12.2) | ||
| Without T2DM (n = 26) | 11.7 (11.5-12.3) | 12.5 (11.5-12.8) | 11.9 (11.1-12.3) | ||
| R2 (ms) | All (n = 47) | 36.0 (29.4-37.1)2 | 38.4 (34.7-41.3) | 38.0 (37.6-40.9)a | |
| With T2DM (n = 21) | 29.4 (24.6-34.4)1,2 | 38.4 (36.7-41.3) | 37.6 (37.2-39.9)b | ||
| Without T2DM (n = 26) | 37.1 (36.3-38.6) | 38.5 (33.2-40.8) | 39.8 (37.9-43.2) | ||
| R2c (ms) | All (n = 47) | 35.9 (27.9-39.0) | 38.3 (35.7-40.4) | 38.0 (37.6-40.9) | |
| With T2DM (n = 21) | 27.9 (26.4-33.5)2 | 38.7 (36.7-40.4) | 37.7 (36.9-40.9)a | ||
| Without T2DM (n = 26) | 38.3 (36.4-39.0) | 37.5 (33.1-40.4) | 38.0 (37.7-42.8) | ||
| Masseter reflex (ms) | All (n = 30) | 4.3 (3.1-4.8) | 4.5 (3.4-5) | 4.5 (3.0-5.1) | |
| With T2DM (n = 21) | 4.2 (4.1-4.4) | 4.5 (2.8-5.2) | 4.5 (3.0-5.1) | ||
| Without T2DM (n = 9) | 4.5 (3.1-4.8) | 4.3 (3.5-4.9) | - | ||
Based on Kimura’s criteria, clinically altered latency of the blink reflex was defined as the mean latency of the Control group plus 2.5 standard deviations. The group with ND plus T2DM presented a greater proportion of older adults with altered R2 and R2c reflexes on both sides of the face (Table 3).
| Latencies of blink reflex | R1 | R2 | R2c | R2 plus R2c |
| Control (n = 122) | 1.6% | 0.8% | 0.8% | 0.8% |
| T2DM (n = 58) | 1.7% | 8.6% | 6.8% | 6.8% |
| ND (n = 21) | 0 | 9.5% | 14.2% | 4.8% |
| ND plus T2DM (n = 26) | 11.5% | 19.2% | 23.0% | 19.2% |
| P value1 | 0.032 | < 0.0001 | 0.007 | 0.007 |
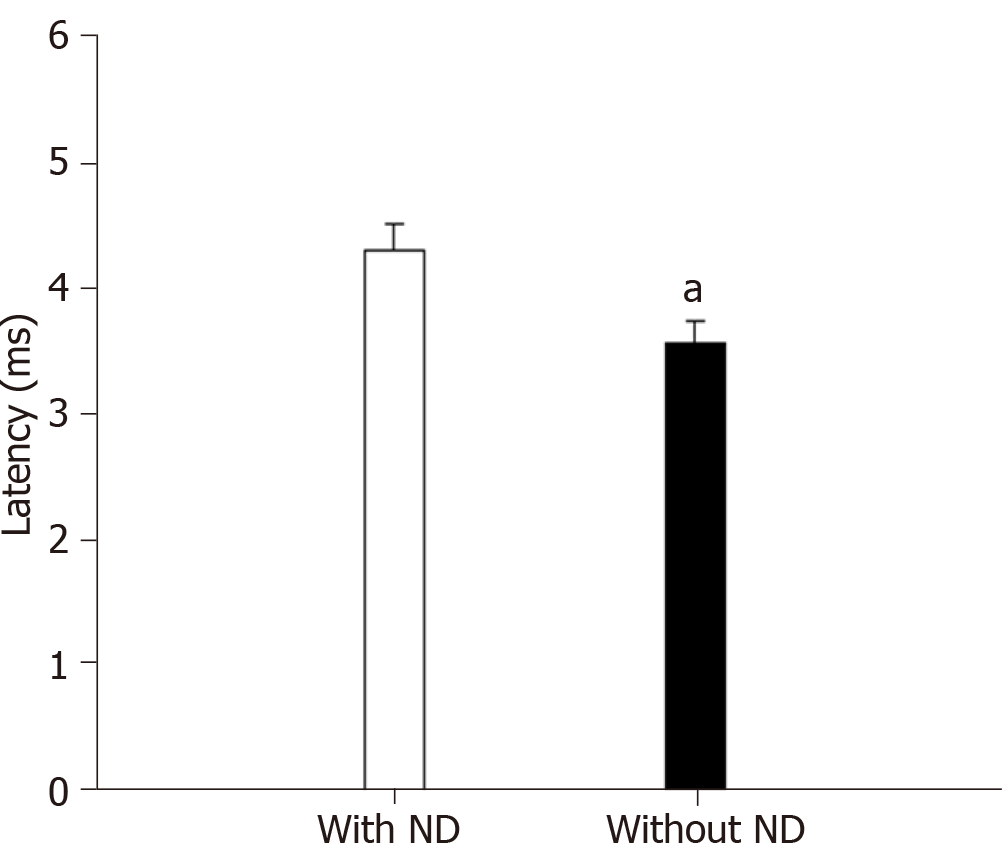
Regardless of T2DM status, subjects with ND (n = 47) had statistically greater right R2c latencies compared with subjects without ND (n = 180) [37.70 (35.40-39.30) ms vs 32.95 (27.15-35.35) ms, respectively (P < 0.0001)]. The masseter reflex was also prolonged in older adults with ND (with and without T2DM) (n = 30) vs controls (with and without T2DM) (n = 153) (Figure 2) [4.38 (3.10-4.47) ms vs 3.49 (2.73-4.47) ms (P = 0.03)].
Blink and masseter reflex latencies were compared with respect to other clinical parameters (Supplementary Table 3). Males presented slightly higher latencies in R2 and R2c on the left and the right sides of the face. The illiterate population presented significantly longer latencies only in the R2 and R2c on both sides. Finally, the independent group showed shorter latencies on both sides too. The masseter reflex did not show significant differences with respect to any clinical parameter.
The main findings of this study were that latencies of the masseter reflex are longer in patients with ND (with or without T2DM) vs patients without ND (with or without T2DM); R1, R2, and R2c latencies of the blink reflex are prolonged in older adults with T2DM and ND; and clear differences were found in the blink reflex when the older adult presented with both pathologies. The relationship of T2DM with dementia has been evidenced in numerous studies including many about AD. This ND is characterized by memory loss and cognitive impairment, and it is only confirmed by a postmortem neuropathological brain analysis showing the presence of senile plaques of amyloid-β protein and neurofibrillary tangles of hyperphosphorylated Tau protein[24]. Even in the Braak system, it has been proposed that stage 1 includes lesions in the region of the brainstem, while patients remain asymptomatic[25]. Neuronal phospho-tau cytoskeletal changes have been found in the dorsal raphe nucleus and then take an ascending cortical and descending course to the brainstem[6]. Evidence of damage at the level of the brain stem in the early stages of AD has also been demonstrated in a voxel-based morphometry study, showing a bilateral loss in the pons and the left part of the midbrain in patients with mild AD compared with controls[7]. Although clinical evaluation of brain stem function is possible, small changes cannot be identified, so the electrophysiology techniques offer an objective evaluation of this portion of the central nervous system. Thus, the simultaneous evaluation of different reflexes that are integrated in the brain stem have shown to be useful to locate the damage at this site[26], including the masseter and blink reflexes. There are different ways to evaluate the reflexes of the masseter muscle, and the techniques frequently involve the ability to strongly tighten the jaws against each other, a capacity that is often reduced in older adults[26]. However, the evaluation of this reflex is important because several studies[27] describe the relationship between alterations of masticatory function and ND. Regarding diabetes, there are few studies that analyze this reflex, and their results are controversial: A difference between people with diabetes vs controls can probably only be found in the presence of severe neuropathy. Yet despite this, there are no reported latencies above normal limits[28,29]. This is in line with our results, because it was observed that study participants had adequate metabolic control, which makes them less likely to suffer from severe neuropathy.
Several studies propose the blink reflex as a complementary tool to diagnose neurological diseases; for example, longer latencies have been observed in patients with dementia with Lewy bodies vs control subjects[30,31]. Mohammadian et al[13] assessed the blink reflex in different types of dementia; however, they did not include patients with long-term dementia or those who presented with chronic non-transmissible degenerative diseases, such as hypertension and T2DM. Our study provides complementary data because we found prolonged latencies in R1, R2, and R2c in the study groups of interest, with multivariate adjustments made for the presence of dependence, hypertension, sex, and education. Likewise, alterations in the blink reflex have been observed in patients with diabetes. Trujillo-Hernández et al[12] compared the blink reflex in patients with recently diagnosed T2DM vs a control group, and because T2DM affects both the central nervous system and the peripheral nervous system, they found alterations in the blink reflex even though T2DM had been recently diagnosed; and results were consistent in patients who already had symptoms of diabetic neuropathy[32,33]. Elkholy et al[34] found that the R2c component was the most sensitive parameter of the blink reflex related to subclinical cranial neuropathy in T2DM patients, suggesting a loss of sensory function and neuronal hyperexcitability[35]; however, Costa et al[36] did not find differences in patients with diabetic peripheral neuropathy compared with controls.
The delayed latencies and amplitude of the blink reflex have been demonstrated in older adults[37]. Trigeminal-facial stimulation is influenced by the action of dopamine produced by dopaminergic neurons of the substantia nigra, which decreases with age[38]; in fact, Ladas et al[39] found increased eye blink rate in patients with mild cognitive impairment, which has been related with dopamine activity. The difference in latencies between sexes could be related to the greater sensitivity of nociceptive trigeminal primary afferents in females[40]. In humans, ipsilateral R1 and R2 components were slightly larger in males than in females[41], as was shown by Peddireddy et al[42], who studied these in participants with closed eyes.
Observations and experimental data on insulin signaling in the brain are an important feature in the development of AD and similar dementias[2]. It is important to note that in the case of the blink reflex, the presence of T2DM and ND in the same patient had an enhancing effect on the prolongation of latencies. Differences were more evident in R2 and R2c. Since these responses involve interneurons at the central level, we consider that they are more associated with cognitive impairment. Although, as we found differences in R1, these could be associated with the presence of T2DM as previously described by Trujillo-Hernandez et al[12]; in addition, Lai et al[43] detected prolonged R1, R2, and R2c latencies of blink reflex in adults with diabetic distal symmetrical polyneuropathy (n = 60) vs controls (n = 49). In this way, we propose that in a particular evaluation of diabetic patients, all the components of the blink reflex must be registered, and, since T2DM is one of the most prevalent risk factors in patients with ND, our results suggest that longer latencies of both reflexes, the masseter and blink reflexes, in patients with T2DM may be an indicator of the presence of ND.
Dependence has also been related to ND: Formiga et al[44] found that only 15.3% of patients with AD were independent, in addition to an association between severe dementia and low scores on the Barthel Index. Aside from the cultural role of older adults with ND, and the lack of social occupation, general motor system dysfunction has been shown in AD, which could be relevant to the appearance of contractures, followed by immobility, and decreased muscular tone[45]. Illiteracy is a key factor for the development of cognitive deterioration: Deterioration may be based on the cognitive and brain reserve for which education, recreational activities, and social stimulation may be protective factors to avoid the threshold of ND[46].
Although neuropsychological tests such as NEUROPSI have the advantage of evaluating cognitive domains more precisely than commonly used tests such as the MMSE, one of the main limitations of our study is that the detection of ND in participants was based on tests. Imaging and biochemical techniques would allow for greater precision in the diagnosis of participants, and even to classify the type of ND[17]. Further studies considering the presence or absence of organ damage caused by T2DM would be interesting.
In conclusion, prolonged latencies in the masseter reflex were observed in older adults with ND. In addition, subjects with T2DM and ND had longer latencies of blink reflex components compared with controls. More studies must be done to demonstrate that the evaluation of brainstem reflexes could be complementary to the timely detection of ND in conditions such as: (1) When the neurocognitive battery tests are compromised by visual or auditory difficulties in the evaluated patient; and/or (2) If the patient is not a candidate for imaging tests or if facilities cannot be economically accessed, even in the presence of T2DM.
Blink and masseter reflex techniques provide reliable and quantifiable data of the central nervous system function: Delayed latencies have been found in patients with neurocognitive disorder (ND) and type 2 diabetes mellitus (T2DM), but this has not been studied in patients with both pathologies.
The clinical implication is that brainstem reflexes could be linked with ND progression in the presence of T2DM.
To investigate if older adults with ND plus T2DM have prolonged latencies of the blink and masseter reflexes, and therefore assess if the brainstem reflexes could be linked with ND progression in the presence of T2DM.
This cross-sectional study included 227 older adults (> 60 years old) from Colima, Mexico. Neurodegenerative disorder was identified by a neuropsychological battery test, and T2DM through medical history, fasting glucose, and glycosylated hemoglobin. Latencies in the R1, R2, and R2c components of the blink reflex were analyzed for all subjects, and 183 subjects were analyzed for latency of the masseter reflex.
Older adults with ND plus T2DM had delayed latencies of the blink reflex responses R1, R2, and R2c. In older adults with ND vs controls, the masseter reflex latency was prolonged. Additionally, age, sex, education, and dependence altered blink reflex latency in ND patients, while T2DM control, depression, and renal damage did not alter blink reflex latency.
Older adults with ND and T2DM have longer latencies of the blink reflex components compared with healthy controls.
Further studies could separate the study groups according to the presence or not of organic damage by T2DM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society for Neuroscience.
Specialty type: Geriatrics and gerontology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Osailan A S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Shamah-Levy T, Vielma-Orozco E, Heredia-Hernández O, Romero-Martínez M, Mojica-Cuevas J, Cuevas-Nasu L, Santaella-Castell JA, Rivera-Dommarco J. Encuesta Nacional de Salud y Nutrición 2018-19: Resultados Nacionales. Instituto Nacional de Salud Pública, 2020. [Cited in This Article: ] |
| 2. | Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 801] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 3. | American Speech-Language-Hearing Association. Dementia. [cited January 3, 2021] Available from: https://www.asha.org/Practice-Portal/Clinical-Topics/Dementia/. [Cited in This Article: ] |
| 4. | World Health Organization. Dementia. [cited January 3, 2021]. Available from: http://www.who.int/mediacentre/factsheets/fs362/es/. [Cited in This Article: ] |
| 5. | Murphy C. The chemical senses and nutrition in older adults. J Nutr Elder. 2008;27:247-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Grinberg LT, Rüb U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H; Brazilian Brain Bank Study Group. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer's disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;35:406-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 88] [Reference Citation Analysis (0)] |
| 7. | Ji X, Wang H, Zhu M, He Y, Zhang H, Chen X, Gao W, Fu Y; Alzheimer’s Disease Neuroimaging Initiative. Brainstem atrophy in the early stage of Alzheimer's disease: a voxel-based morphometry study. Brain Imaging Behav. 2021;15:49-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kimura J. Nerve conduction studies. In: Mills KR. Oxford textbook of clinical neurophysiology. Oxford University Press, 2017: 49-66. [Cited in This Article: ] |
| 9. | Téllez MJ, Ulkatan S. Bringing the masseter reflex into the operating room. Neurophysiol Neurosurg. 2020;223-228. [DOI] [Cited in This Article: ] |
| 10. | Cruccu G, Iannetti GD, Marx JJ, Thoemke F, Truini A, Fitzek S, Galeotti F, Urban PP, Romaniello A, Stoeter P, Manfredi M, Hopf HC. Brainstem reflex circuits revisited. Brain. 2005;128:386-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Farella M, Palla S, Erni S, Michelotti A, Gallo LM. Masticatory muscle activity during deliberately performed oral tasks. Physiol Meas. 2008;29:1397-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Trujillo-Hernández B, Huerta M, Pérez-Vargas D, Trujillo X, Vásquez C. Blink reflex alterations in recently diagnosed diabetic patients. J Clin Neurosci. 2003;10:306-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Mohammadian F, Noroozian M, Nafissi S, Fatehi F. Blink Reflex May Help Discriminate Alzheimer Disease From Vascular Dementia. J Clin Neurophysiol. 2015;32:505-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | American Psychiatric Association. Diagnostic criteria consultation guide for DSM-5®. 5th ed. American Psychiatric Association, 2013. [Cited in This Article: ] |
| 15. | López-Álvarez J, Agüera-Ortiz LF. Nuevos criterios diagnósticos de la demencia y la enfermedad de Alzheimer: una visión desde la psicogeriatría. Psicogeriatría. 2015;5:3-14. [Cited in This Article: ] |
| 16. | Ostrosky-Solís F, López-Arango G, Ardila A. Sensitivity and specificity of the Mini-Mental State Examination in a Spanish-speaking population. Appl Neuropsychol. 2000;7:25-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Ostrosky-Solis F, Esther Gomez-Perez M, Matute E, Rosselli M, Ardila A, Pineda D. NEUROPSI ATTENTION AND MEMORY: a neuropsychological test battery in Spanish with norms by age and educational level. Appl Neuropsychol. 2007;14:156-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Bertrán J, Pasarín A. La escala de Barthel en la valoración funcional de los ancianos. Rev Esp Geriatr Gerontol. 1992;27:135. [Cited in This Article: ] |
| 19. | American Diabetes Association. Understanding A1C. Diagnosis. [cited January 3, 2021]. Available from: https://www.diabetes.org/a1c/diagnosis. [Cited in This Article: ] |
| 20. | Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-172. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3109] [Cited by in F6Publishing: 3176] [Article Influence: 198.5] [Reference Citation Analysis (0)] |
| 21. | Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. 4th ed. Oxford University Press, 2013: 183-188. [Cited in This Article: ] |
| 22. | Cruccu G, Frisardi G, Pauletti G, Romaniello A, Manfredi M. Excitability of the central masticatory pathways in patients with painful temporomandibular disorders. Pain. 1997;73:447-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | IBM. Eta squared. [cited January 3, 2021]. Available from, https://www.ibm.com/support/knowledgecenter/en/. [Cited in This Article: ] |
| 24. | Ittner LM, Götz J. Amyloid-β and tau--a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci. 2011;12:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 918] [Cited by in F6Publishing: 975] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 25. | Del Tredici K, Braak H. To stage, or not to stage. Curr Opin Neurobiol. 2020;61:10-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Valls-Solé J. Neurophysiological assessment of trigeminal nerve reflexes in disorders of central and peripheral nervous system. Clin Neurophysiol. 2005;116:2255-2265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Watanabe Y, Hirano H, Matsushita K. How masticatory function and periodontal disease relate to senile dementia. Jpn Dent Sci Rev. 2015;51:34-40. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Cruccu G, Agostino R, Inghilleri M, Innocenti P, Romaniello A, Manfredi M. Mandibular nerve involvement in diabetic polyneuropathy and chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 1998;21:1673-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Urban PP, Forst T, Lenfers M, Koehler J, Connemann BJ, Beyer J. Incidence of subclinical trigeminal and facial nerve involvement in diabetes mellitus. Electromyogr Clin Neurophysiol. 1999;39:267-272. [PubMed] [Cited in This Article: ] |
| 30. | Anzellotti F, Bonanni L, Iorio E, Di Baldassarre F, D'Andreagiovanni A, Monaco D, Thomas A, Onofrj M. Delayed blink reflex in dementia with Lewy bodies is sensitive to cholinergic modulation. Clin Neuropharmacol. 2008;31:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Bonanni L, Anzellotti F, Varanese S, Thomas A, Manzoli L, Onofrj M. Delayed blink reflex in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2007;78:1137-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Nazliel B, Yetkin I, Irkeç C, Koçer B. Blink reflex abnormalities in diabetes mellitus. Diabetes Metab Res Rev. 2001;17:396-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Kazem SS, Behzad D. Role of blink reflex in diagnosis of subclinical cranial neuropathy in diabetic mellitus type II. Am J Phys Med Rehabil. 2006;85:449-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Elkholy SH, Hosny HM, Shalaby NM, El-Hadidy RA, Abd El-Rahim NT, Mohamed MM. Blink reflex in type 2 diabetes mellitus. J Clin Neurophysiol. 2014;31:552-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220-2224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 36. | Costa YM, Karlsson P, Bonjardim LR, Conti PCR, Tankisi H, Jensen TS, Nyengaard JR, Svensson P, Baad-Hansen L. Trigeminal nociceptive function and oral somatosensory functional and structural assessment in patients with diabetic peripheral neuropathy. Sci Rep. 2019;9:169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C. Aging of the trigeminal blink system. Exp Brain Res. 2001;136:351-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Basso MA, Powers AS, Evinger C. An explanation for reflex blink hyperexcitability in Parkinson's disease. I. Superior colliculus. J Neurosci. 1996;16:7308-7317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Ladas A, Frantzidis C, Bamidis P, Vivas AB. Eye Blink Rate as a biological marker of Mild Cognitive Impairment. Int J Psychophysiol. 2014;93:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Kofler M, Kumru H, Schaller J, Saltuari L. Blink reflex prepulse inhibition and excitability recovery: influence of age and sex. Clin Neurophysiol. 2013;124:126-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Peddireddy A, Wang K, Svensson P, Arendt-Nielsen L. Influence of age and gender on the jaw-stretch and blink reflexes. Exp Brain Res. 2006;171:530-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Lai YR, Huang CC, Chiu WC, Liu RT, Tsai NW, Wang HC, Lin WC, Cheng BC, Su YJ, Su CM, Hsiao SY, Wang PW, Chen JF, Ko JY, Lu CH. The role of blink reflex R1 latency as an electrophysiological marker in diabetic distal symmetrical polyneuropathy. Clin Neurophysiol. 2020;131:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Formiga F, Fort I, Robles MJ, Rodriguez D, Regalado P. Lower Barthel Index scores predict less prescription of pharmacological therapy in elderly patients with Alzheimer disease. Dement Geriatr Cogn Disord. 2010;29:198-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Souren LE, Franssen EH, Reisberg B. Contractures and loss of function in patients with Alzheimer's disease. J Am Geriatr Soc. 1995;43:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Pinto C, Tandel KY. Cognitive reserve: Concept, determinants, and promotion. J Geriatr Mental Health. 2016;3:44-51. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |