DEMENTIA - DEFINITION, DIAGNOSIS, EPIDEMIOLOGY
Dementia can be defined as a decline in cognition and/or behavioral impairment coupled with progressive deterioration of daily functionality which cannot be explained in terms of delirium or major psychiatric disorders[1].
The worldwide prevalence of dementia for individuals who are 60 years and older is estimated to be between 5%-7% in most regions of the world[2] with estimated worldwide costs of $604 billion (in 2010), the majority of which (70%) incurred in western Europe and North America[3]. Moreover, dementia’s contribution (11.2%) to the years lived with disability (YLD) in people aged 60 years and over is higher than that of stroke, cardiovascular disease or cancer[4]. Dementia presents a considerable burden both at the micro (individual/patient, family, formal and informal caregivers) and macro levels (societal, governmental).
While several etiologies can lead to dementia, Alzheimer’s disease accounts for around 60% of all cases, and consequently is considered the leading cause of dementia[5]. Besides disabling behavioral and motor disturbances, cognitive dysfunctions are considered to be a major source of difficulties for patients, caregivers and practitioners. Moreover, cognitive impairments are not only observed in early stages of Alzheimer’s disease (AD) but also in a number of other dysfunctions such as vascular dementia, frontotemporal dementia (FTD) and even Parkinson disease (PD).
Currently, options for treating dementia include pharmacological and non-pharmacological therapies. At present, pharmacological treatments provide viable but rather modest symptom control[6,7]. Also, disease-modifying therapies are currently under clinical testing (e.g., vaccination, passive immunization for AD; for a review please refer to Galimberti and Scarpini[8]) or in preclinical phases (e.g., compounds aiming to interfere with tau[9] or synuclein[10] deposition in tauopathies and synucleinopathies, respectively).
Non-pharmacological approaches have also been used to manage behavioral problems and compensate for cognitive impairments. However, evidence of their effectiveness and use remain scarce and controversial. In the following sections, we aim to provide a balanced evidence-based perspective of cognitive intervention (CI) approaches to cognitive impairments in elderly populations with no cognitive pathology as well as with pathological cognitive decline.
CI FOR DEMENTIA - FROM HEALTHY TO PATHOLOGICAL TRAJECTORIES OF COGNITIVE AGING
Conceptual framework/background
The conceptual framework introduced by Clare et al[11] will be adopted in this review. The framework consists of 3 main approaches in CI: cognitive training (computer-based or paper-and-pencil cognitive exercises); cognitive stimulation (cognitive and social group activities); and cognitive rehabilitation (individualized interventions addressing patients’ key difficulties and goals).
Cognitive training delivered in individual or a group format focuses on specific cognitive functions. Health professionals deliver paper-and-pencil as well as computer-based exercises with different difficulty levels (additionally, if not stressful to the patient, caregivers might participate in the intervention sessions or assist with exercise delivery at home). Traditionally, in older adults this intervention mostly focuses on memory training, but it can also target or include other cognitive functions aiming primarily at improving the trained functions/skills and/or learning compensatory techniques and secondary generalized gains to other tasks as well as to daily life. While there is some evidence for cognitive improvements resulting from training cognitive functions in all populations (i.e., healthy adults[12], pre-dementia[13], and mild-to-moderate dementia[14]), generalization of effects is not yet evident[15].
Cognitive stimulation, instead, adopts a more global and contextualized perspective on cognitive functioning, and assumes that cognitive functions work together and should be stimulated accordingly in a social setting. There is some evidence that this approach can benefit cognitive functioning in dementia patients[16]. However it is not yet clear whether the observed effects are due to cognitive or social components, since both are integral parts of cognitive stimulation.
Cognitive rehabilitation aims to identify and work on personally relevant difficulties and goals. These difficulties may include memory or performance in daily tasks. For this method, a holistic approach is adopted with health professionals working with the patient as well as a family member or caregiver if needed. Although there are only few studies investigating intervention in age related cognitive impairment with the holistic approach, there is initial evidence that people in fact do benefit from cognitive rehabilitation[17].
It is noteworthy that not only these non-pharmacological cognitive approaches have been explored as a complementary therapeutic approach to medication[18], but also other non-pharmacological multicomponent approaches such as combining different CIs (e.g., cognitive stimulation plus computer-based cognitive training[19]), physical exercise and cognitive training[20], and more recently transcranial magnetic stimulation with cognitive training[21] have been employed as well.
A selective narrative review was performed while recurring to representative articles, which were identified by authors and complemented with literature searches in PubMed/MEDLINE and ScienceDirect. Searches were performed for reviews (from which relevant references were extracted also) and trials of non-pharmacological CIs in healthy aging as well as several types of dementia. In our search, we used the expression “CI” and related terms (i.e., cognitive stimulation/training/remediation/rehabilitation) which were combined with the terms aging, mild cognitive impairment, AD, vascular dementia, PD, FTD, and Lewy body dementia. Relevant articles were selected following abstract inspection.
Healthy aging
Cognitive functioning and aging: A large number of scientists believe that aging does not have to encompass severe loss of memory or prevent healthy functioning. Many have spent their devoted life-long efforts on trying to understand how cognitive functioning changes across the human developmental span. A still popular theory, proposed nearly 30 years ago, assumes that the origin of differences between young and aged healthy humans lies in the speed of processing[22]. Indeed, reduced speed of processing could compromise many cognitive processes, such as overall memory and in particular working memory which is commonly found to be affected with increasing age[23]. Regardless of the origins of age-related cognitive decline, it is important to outline some of these specific age dependent changes. Besides memory and attention, language, and components of executive functioning may also be compromised with increasing age[24]. Deficits in perception are also reported but these could simply be due to functional decline of sensory organs.
Often times it is rather difficult to disentangle whether a certain patient’s complaint is part of a specific underlying disease process or if it is merely an age-normative complaint. Therefore, neuropsychological assessments are utilized as useful tools in assisting with diagnosis of disorders that imply cognitive impairment[25] and help with distinguishing a subjective complaint from cognitive dysfunction.
CI in healthy elderly: To overcome age-related cognitive decline several non-pharmacological approaches[26] have been developed. Exercise, for example, has been extensively studied with current evidence suggesting benefits for cognitive functioning and the promotion of functional mobility in healthy aging[27,28]. Moreover, to explore the hypothetical additive or synergistic effects of several interventions, exercise has been combined with other techniques. Up until now, findings suggest that interventions targeting multiple domains may be more effective than those that treat each domain independently[29]. These researchers believe that cognitive training should be offered in conjunction with physical activity[30] since findings point to greater effects of combined approaches than either intervention alone[29,31]. For instance, in a 4-mo randomized controlled trial (RCT), the combined training group (physical and cognitive) showed improvements in cognitive speed both immediately and at three months after intervention[20]. Notwithstanding these findings, the present work will strictly focus on evidence of various CIs.
Cognitive training has been extensively studied in healthy elderly individuals[12,32,33]. To quote Martin and colleagues[34], cognitive training is “an intervention providing structured practice on tasks relevant to aspects of cognitive functioning, using standardized tasks” and “intended to address cognitive function and/or cognitive impairment directly”. Building on these works, Gates and Valenzuela[32] have stated that cognitive training implies repeated practice and use of standardized tasks that target specific cognitive domains. Similarly, cognitive training teaches theoretically-driven strategies and skills aiming to improve cognitive functioning[35]. In order to determine if this approach is effective for healthy elderly, we briefly review current evidence from the literature.
In the ACTIVE study (Advanced Cognitive Training for Independent and Vital Elderly) large-scale RCTs were conducted to compare training in different cognitive domains[12]. They studied the effects of memory, reasoning, and speed-of-processing training while comparing these groups with no-training controls. When compared to baseline assessment, each of these interventions improved the cognitive domain of interest with sustained improvements visible at a 2-year post-intervention follow-up[12]. Furthermore, five years after training, it was observed that the groups with reasoning and memory training still maintained improvements in the targeted cognitive abilities[36].
Another example of this line of research includes reading aloud and solving simple arithmetic calculations, such as the one in an ongoing trial study by Nouchi et al[37].
Due to limited evidence of transfer effects of this training approach to everyday life functions, some have adopted a multimodal strategy[36]. Typically, these approaches involve lifestyle changes and social interactions. Quite different from standard cognitive training, multimodal programs engage older adults in “enjoyable or socially meaningful” activities. The underlying assumption is that this approach would increase the possibility of older adults maintaining their activity and skill levels even long after completing an intervention[36] (for an example of this approach see Tranter and Koutstaal[38]).
Several review studies have summarized the evidence in favor of CI. A review paper from Tardif and Simard[39] states that CI can result in improvements in at least one outcome in each of the studies they have analyzed. In a more recent systematic review[40], authors gathered evidence from thirty-five studies (most of them RCTs). Despite the diversity among employed interventions as well as methodological differences, their review presented evidence stating that cognitive training can be beneficial in improving several domains of cognitive functioning namely attention, memory, speed of processing and executive functioning. In a different review[41] with clinical trials comparing the effects of CIs between older adults and a wait-and-see control group, the authors found a strong effect size. The longitudinal RCTs included in this review presented relative effect sizes pointing to protective effects of CI. When authors computed these values for meta-analysis they found an integrated effect size estimated to be 1.07 (95%CI: 0.32-1.83; z = 2.78; n = 7; P = 0.006). Furthermore, the same review also noted that the effects of RCTs were maintained even at longer follow-up intervals. The authors conclude that CI in healthy older adults “produces strong and persistent protective effects on longitudinal neuropsychological performance” which might play a role in preventing the development of dementia[41]. Hence, there is some evidence that CI can boost cognitive functioning in healthy older adults.
Even though the debate over the generalization of the effects of CI to everyday life activities remains to be fully addressed[40], some have found evidence in support of this contention. For instance, improvements in cognitive outcomes were evident after CIs and were accompanied by more favorable scores of self-reported Instrumental Activities of Daily Living[42] and the Useful Field of View test. These benefits lasted for at least 2 years and were reflected in everyday activities including in safer driving performance[43].
Research has shown that adult brains preserve mechanisms that permit flexible change, such as neurogenesis and functional recruitment of neurons in the presence of a lesion[44]. Whether older human brains have these capabilities or not, needs to be further explored to potentiate successful aging while preventing cognitive decline. One such strategy involves training and exposure to novelty, which has been associated with human cortical reorganization and grey matter volumetric changes (e.g., changes in hippocampus[45]; for a review see Greenwood and Parasuraman[44]). Even short but yet intensive memory training, using for example the Method of Loci, yielded regional increases in cortical thickness (right fusiform and lateral orbitofrontal cortex), which were positively correlated with improvements in memory performance outcomes[46].
Mild cognitive impairment/pre-dementia
The “mild cognitive impairment” (MCI) concept has been traditionally most closely related to AD with the amnestic variant of MCI being the most common in patients progressing to a diagnosis of dementia of the Alzheimer type. However, current guidelines[47] explicitly include non-amnestic presentations in MCI (e.g., executive functioning, attention, language, and visuospatial skills; either with single or multi-domain deficits), whether due to AD or other etiologies. The National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for AD[47] refer to the following core criteria as features of MCI: evident change in cognition from a previous level of cognitive functioning; cognitive impairment in one or more domains (1 to 1.5 standard deviations below the mean for age and education matched peers); autonomously daily life functioning; no dementia. Biomarkers are also currently available (e.g., cerebrospinal fluid amyloid and tau, structural and functional imaging, etc.) for aiding in the differentiation of MCI due to AD or due to other causes (pseudodementia or other dementias)[47].
In order to ameliorate or reverse cognitive deficits characteristic of MCI and/or to prevent its progression to an overt stage of dementia, new CIs have been steadily developing. Currently, there is positive evidence from individual studies, such as described by Belleville et al[13] displaying improvements in memory after an episodic memory training program of 120 min per session for 6 weekly sessions (4-5 participants per group) focusing on mnemonic techniques. The latter mentioned training included psychoeducational information regarding memory and ageing, and also memory techniques promoting encoding and retrieval (i.e., imagery, the method of loci, face-name associations, hierarchical organization and semantic organization techniques), delivered by trained neuropsychologists.
In another randomized controlled trial study with amnestic mild cognitive impairment patients and family partners, Kinsella et al[48] found beneficial effects after a 5 wk intervention training program in everyday memory, namely in prospective memory performance, use of memory strategies, as well as in patient and family knowledge of memory strategies.
In a study of a 6-mo long CI program that included a comparison with a late active control group (receiving intervention after 8-mo), Buschert et al[49] found a stable intervention effect on the primary outcome Alzheimer Disease Assessment Scale - Cognitive subscale (ADAS-Cog) in the early-intervention group. Additionally, only the later-intervention group participants (6 out of 12) progressed into AD during an entire 28 mo period of the study.
A recent systematic review concerning CI in amnestic MCI individuals[50], pointed out that evidence of improvements in neuropsychological measures after CI is in fact limited. However, the authors did state that patients were able to learn as well as benefit from memory strategies.
Moreover, aside from efficacy, feasibility parameters have rarely been evaluated, although there is evidence of CI being well received while meeting the needs of MCI patients[51]. Considering the fact that the latter opinion is from the patient’s perspective (i.e., whether their needs are being met) it should be regarded as an important future area of research that has thus far received little attention. MCI patients similarly show improved subjective perception of memory capacity after CI[52].
Recently, neuroimaging has also been incorporated as an outcome measure assessing potential brain changes related to CI. Clare et al[53] provide evidence from a case report where a patient with MCI underwent a cognitive rehabilitation program showing post-treatment reduction in activation of areas such as fusiform gyrus and increased activation in prefrontal areas as well as the temporal-parietal junction. Furthermore, when using verbal encoding and retrieval tasks, Belleville et al[13] report increased post-intervention activation in MCI patients’ large fronto-temporo-parietal network, while at the same time healthy controls show decreased activation. Interestingly, the authors note the activation of specialized areas (already activated in pre-intervention) and “new” alternative areas, which we could interpret both as restorative or compensatory processes resulting from CI. However, despite the observed changes, only right inferior parietal lobule activation correlated with performance.
Dementia
AD: As AD is the most prevalent cause of dementia, and since research on CI is comparatively abundant for this cause of dementia, the present section will largely focus on AD. Moreover, cognitive deficits are one of the key features of AD dementia and are a relevant target for pharmacological as well as non-pharmacological therapies. While memory deficits have been traditionally considered the hallmark of AD, it is now widely accepted that other deficits are present even during early stages[54]; with AD pathology often leading to quite different symptomatic presentations such as prominent language or visual deficits[1,55]. Due to the progressive and unstoppable nature of AD, interventions complementing pharmacological treatments have also been developing worldwide. One of these psychosocial approaches is CI. In 1982 Brinkman et al[56] conducted one of the first trials of cognitive training in Alzheimer patients. In this lecithin trial study, using a double-blind crossover, patients received memory training in addition to the lecithin condition, and “placebo training” during the placebo drug condition. Follow-up trials suggested that memory training may have led to small immediate improvements in list-learning ability. More recently, Tárraga et al[57] conducted a randomized controlled trial with 3 arms, comparing standard treatment (stable treatment with cholinesterase inhibitors) vs psycho-stimulation vs combined psycho-stimulation and internet-based cognitive training. The authors found that both CI programs lead to improvements in ADAS-Cog and Mini Mental State Examination (MMSE) after 12 wk of intervention, which were maintained at 24 wk post-intervention. Also, Clare et al[17] conducted a cognitive rehabilitation trial for people with AD or mixed AD and vascular dementia, comparing an 8 weekly individual cognitive rehabilitation session program vs relaxation program vs no treatment (all participants were receiving a stable dose of acetylcholinesterase inhibitors). The individual cognitive rehabilitation program consisted of eight weekly 1-h sessions tackling personal relevant goals and including learning (e.g., face-name learning), attention and concentration maintaining techniques. In the end, the cognitive rehabilitation group did in fact display improvements in goal performance and satisfaction. A subgroup of patients in this study also performed pre and post-intervention functional Magnetic Resonance Imaging face-name association task, exhibiting increased brain activity at the right fusiform face area, right parahippocampal cortex, right temporal parietal junction, and right medial prefrontal cortex. The authors interpreted these changes as being related to deeper encoding of faces and functional restoration of this face-name association learning network.
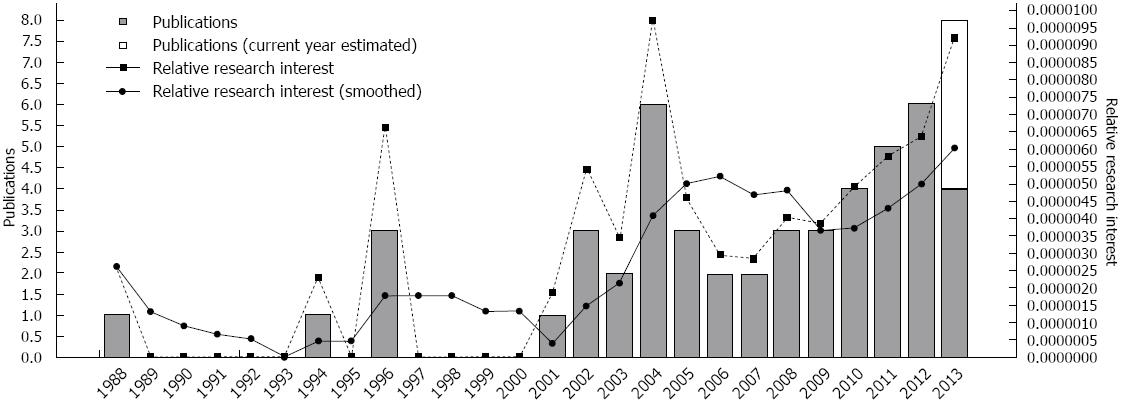
Despite these promising findings and the steady increase of research in this area (Figure 1), a recent systematic review[58] states the need for additional well-controlled and high-quality trials. Despite the limited amount of evidence, the aforementioned review suggests that CI for AD might lead to improvements in global cognitive functioning (i.e., 0.83 points in MMSE, as estimated). Moreover, high rates of completion and adherence to the intervention procedures were observed which point to the feasibility of this approach for AD patients (and caregivers) and health professionals. Interestingly, when examining costs associated with treatment, we found preliminary evidence in support of cost-effectiveness of CIs in dementia. Importantly, we should note that over a 4-mo period, a 1-point decline in the MMSE is estimated to add £56 (£74.74 as of 2011) to direct health and social care costs)[59].
Figure 1 Temporal evolution of cognitive intervention research in Alzheimer's disease (Source: GoPubmed).
Although CIs in non-AD dementias are comparatively scarce, some of these studies will nonetheless be reviewed in the following sections.
Vascular dementia: Being the second cause of dementia and accounting for about 30% of dementia cases[60], Vascular Dementia is the center of a considerable amount of research that includes its characterization and etiopathology. Still, there are only a few trial studies that are directed at investigations of the efficacy of CI for this type of dementia. One of these studies[61] has specifically investigated the possible role of CI in vascular dementia. The authors addressed this issue in a patient with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and found post-intervention improvements in processing speed as well as executive functioning and functionality. Nonetheless, given potentially conflicting findings, more detailed and comprehensive evidence concerning the efficacy of CI in this area is warranted. For example, although a recent systematic review[62], including both vascular dementia and AD, reported no effect of cognitive training, a cognitive rehabilitation trial, including people diagnosed with AD, mixed AD or vascular dementia[17], found improvements in goal performance and satisfaction.
PD: Although CI research in PD is scarce, a cognitive remediation study[63] for attention skills with non-demented PD patients showed this type of intervention to be feasible and well accepted by patients. Another cognitive-motor intervention (including computer-based cognitive training) study with early stage non-demented PD patients[64] found verbal fluency, memory (logic memory) and reasoning (Raven’s matrices) improvements.
In addition, a neuroimaging study[65] of a 6 mo daily cognitive training program found post-intervention improved performance in reaction time and hit rate in a fMRI modified Stroop task. The authors also observed a reduction in cortical activation when making comparisons with untrained patients.
FTD: To the best of our knowledge, despite CI being considered a therapeutic option for FTD[66], such intervention trials in FTD are almost nonexistent. However, a speech therapy pilot trial, for Primary Progressive Aphasia, showed beneficial effects in language performance and naming skills[67], providing preliminary evidence that additional studies in this area are warranted.
Other types of dementia: So far, research on CI has not focused on other types of dementias, such as Lewy bodies’, for which we found no studies during our literature search.
One should consider the sensorial or perceptive (cortical) disruption and severe behavioral disturbances that make the implementation of CI approaches in some of these dementia patients difficult (e.g., FTD) which somewhat explains the scarcity of practice and research in some of these types of dementia.
CONCLUSION
General conclusion and future directions
In the present manuscript, we narratively reviewed the current state of CI approaches for healthy aging, pre-dementia, and different types of dementia.
Taken as a whole, studies show evidence for small but consistent effects of CI in improving cognition in both healthy and unhealthy populations of aging adults. Preliminary studies also point to the feasibility, adequacy, patient involvement, and cost-effectiveness of these approaches.
Although research on aging and dementia in general is a dynamic and a rapidly changing field[68], the CI sub-field of this research is still in its infancy and in spite of the growing evidence of its effectiveness, is still lacking recognition among health professionals as well as caregivers. With disease-modifying therapies still in preclinical or clinical trial stages, research in this field warrants a well deserved attention. While we hope for the development and assessment of new pharmacological therapies for cognitive deficits[69], the positive role of non-pharmacological approaches should be considered more carefully, both in research and in practice.
To establish high quality evidence-based standard practices in the field, research on various CI approaches needs to increase considerably. In this regard, the following systematic steps may be considered: (1) Expert meetings should provide a comprehensive perspective ranging from healthy to impaired cognitive aging and allow standardization of methods of practice and research (e.g., defining whether or not, if and when, Randomized Controlled Trials should be the gold standard for CI research, and establishing what constitutes a clinically significant change for a dementia patient); (2) Affirmation of the importance of multidisciplinary clinician/practitioner-researcher roles in the field of CI, as facilitators of advances in the field by rapid translation between research and practice and vice-versa; (3) Establishment of the optimal (the most efficacious and most cost-effective) intervention parameters such as frequency (i.e., which is the most cost-effective number of sessions per week and per month?), duration (i.e., which is the optimal session duration and program duration taking into account patient fatigue, costs and benefits?), and intensity (which is the optimal level of difficulty? What are the effects of different interval schedules?), which are still unclear, and in need of systematic evaluation by researchers; (4) Clarification of what constitutes an “active substance” in CI, and identification of global vs specific effects. For example, CI might exert its effects through tackling cognitive deficits related to brain dysfunction (e.g., hippocampal atrophy), or it might improve overall activity and arousal levels; (5) Exploring the existence of secondary negative effects for each type of dementia, such as fatigue, depression, frustration, “burnout”/overtraining which could hypothetically lead to faster progression of cognitive decline; (6) Standardization of outcome protocols taking into account different types and stages of dementia, including neuropsychological, non-cognitive (e.g., quality of life, subjective experience), and neuroimaging measures. Clarification of the relation between cognitive, experiential and neuroimaging outcomes is also needed. For example, should we focus on cognition or on experiential components of the intervention? What is the meaning of brain activation changes? - Is it a possible restoration of function[70]? Are neurophysiological markers more sensitive than behavioral ones? Can and should biomarkers (e.g. CSF) be incorporated as an outcome measure? (7) Although, there is some evidence for the immediate effects of CI in healthy and impaired cognitive aging, long-term effects and a possible preventive or protective role[41] of systematic CI needs to be thoroughly assessed (either in the progression from healthy aging to MCI, or from MCI to different types of dementia). Can it delay or slow disease progression? Can it delay or prevent dementia for people with genetic risks? These are critical issues since efficacious preventive interventions might considerably lower the incidence of dementia[2] with positive repercussions for individuals, and a reduced burden on society; (8) Focus on macro-issues (e.g., systemic, organizational and societal issues); allowing one to assess and modify the variables hindering the adoption and implementation of CI by different professionals and in different settings (e.g., hospital, health centers, clinics); and (9) Awareness of the comparative relevance, importance, efficacy and cost-effectiveness of new technologies (vs traditional approaches) not only as a mean of intervention delivery but also for an interaction between professionals, scientists and the public (see for example, the International Non-pharmacological Therapies Project - http://nptherapies.org/). New technologies not only can facilitate the delivery of interventions but may also help in scientific research, allowing maximization of resources and sharing of ideas/protocols, as well as dividing tasks in an organized way. For example, it may facilitate the concerted, effective, and timely translation of neuropsychological instruments into several languages.
In summary, we hope that tackling the aforementioned issues will allow the field of CI to move into an evidence-based and patient-centered multidisciplinary personalized approach. However, before this goal is accomplished there is a need for additional evidence concerning the efficacy of these approaches for each type of dementia and each of its deterioration stages. This information may then be implemented based on individual patterns of dysfunction. Attaining this goal would positively impact cognitive functioning of healthy and impaired elderly and mitigate individual and societal impact of cognitive decline.