Published online Jun 26, 2017. doi: 10.5662/wjm.v7.i2.55
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: April 12, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: June 26, 2017
Targeted temperature management (TTM) shows the most promising neuroprotective therapy against hypoxic/ischemic encephalopathy (HIE). In addition, TTM is also useful for treatment of elevated intracranial pressure (ICP). HIE and elevated ICP are common catastrophic conditions in patients admitted in Neurologic intensive care unit (ICU). The most common cause of HIE is cardiac arrest. Randomized control trials demonstrate clinical benefits of TTM in patients with post-cardiac arrest. Although clinical benefit of ICP control by TTM in some specific critical condition, for an example in traumatic brain injury, is still controversial, efficacy of ICP control by TTM is confirmed by both in vivo and in vitro studies. Several methods of TTM have been reported in the literature. TTM can apply to various clinical conditions associated with hypoxic/ischemic brain injury and elevated ICP in Neurologic ICU.
Core tip: Two main purposes of targeted temperature management (TTM) in patients admitted in neurological intensive care unit are neuroprotective therapy and intracranial pressure (ICP) control. TTM is the most potent neuroprotective treatment due to its numerous methods of protection against ischemic/hypoxic injury. TTM provides capable ICP reductive action. Two most popular methods using in clinical practice and clinical trials are invasive endovascular technique and non-invasive surface cooling. Fast induction, smooth maintenance and slow rewarming are the important steps to achieve ideal TTM.
- Citation: Muengtaweepongsa S, Srivilaithon W. Targeted temperature management in neurological intensive care unit. World J Methodol 2017; 7(2): 55-67
- URL: https://www.wjgnet.com/2222-0682/full/v7/i2/55.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i2.55
Clinical benefit of therapeutic hypothermia in patients with post-cardiac arrest syndrome (PCAS) has been demonstrated by two randomized control trials since 2002[1,2]. However, the term ″therapeutic hypothermia″ has been replaced with ″targeted temperature management (TTM)″ since 2011 after the meeting of five major professional physician societies[3]. TTM defines as a type of treatment that reduces a subject’s core temperature until a specific target with the purpose in salvage or alleviate the tissue injury from deficiency of blood perfusion[4]. TTM is recognized as a only established neuroprotective therapy for hypoxic/ischemic brain injury, particularly in patients after cardiac arrest[5]. The clinical practice guidelines state that TTM should apply as a major treatment for patients following successful resuscitation from cardiac arrest[6-10].
Elevated intracranial pressure (ICP) is one of the common conditions found in patients admitted in neurologic intensive care unit (ICU)[11]. Many clinical and animal trials demonstrate that TTM effectively lowers ICP[12]. However, the application of TTM as ICP control in each particular disease, for examples in primary intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), traumatic brain injury (TBI) and cerebral infarct, needs to be proved by large randomized controlled trial[13].
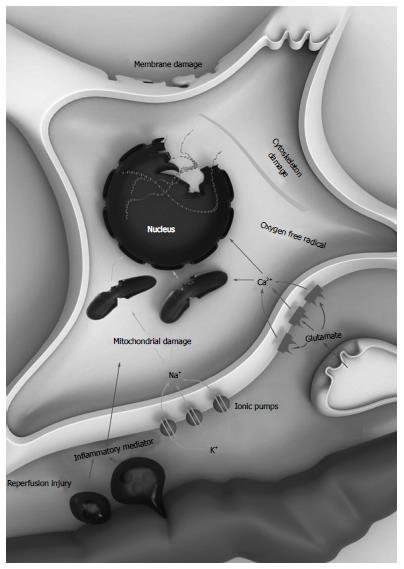
Hypoxic/ischemic brain damage is associated with the abruption of cerebral blood flow (CBF)[14]. Cessation of brain circulation leads to compound neurologic damages, the so-called hypoxic/ischemic cascade[15]. After the deficiency of oxygen and circulation supplying occur, adenosine triphosphate (ATP) manufacturing malfunction develops[16]. Neurons and glials change from aerobic to anaerobic process, resulting in accumulation of lactic acids[17]. Cells become depolarized due to the sodium-potassium ATPase pumps failure, letting ions, particularly calcium (Ca2+), to invade themselves[18]. Elevated intracellular Ca2+ stimulates the release of the well-known excitatory amino acid neurotransmitter such as glutamate[19]. Glutamate permits further Ca2+ influx the cells by activate the opening of Calcium-permeable NMDA receptors and AMPA receptors[20]. After excessive calcium Ca2+ influx, the production of deleterious substances including various free radicals, reactive oxygen species, phospholipases, ATPases, and endonucleases, the so-called excitotoxicity materials, initiates[21]. Membrane and mitochondria break down and lead to development of necrotic cells and apoptosis. Glutamate and other harmful materials are then released from these necrotic cells into the environment[22]. These materials cause further damage to adjacent cells. This continuous injury, the so-called reperfusion damage, usually starts when the cerebral tissue gets reperfused[23]. Inflammatory scavengers get accumulated to eat up the debris tissue and then generate many cytokines[24]. These toxic materials disrupt the blood-brain barrier (BBB). Destroyed BBB conducts to leakage of huge protein molecules particularly albumins into the environment causing brain edema[25]. Brain edema produces pressure effect of and further harm to adjacent brain tissue[26]. The hypoxic/ischemic cascade are shown in Figure 1.
The theory of ICP, the so-called Monro-Kellie doctrine, was first postulated by Alexander Monro in 1783 before George Kellie published the article support Monro’s idea in 1824[27,28]. This theory states that since the skull is a permanent volume and the brain is enclosed by rigid meninges, therefore, alterations in the volume of the intracranial components will affect ICP[29]. The intracranial components include blood, cerebrospinal fluid (CSF), and brain tissue, all of which are relatively constant. An enlargement in one component or development of a mass lesion will elevate ICP and require a diminishing in another component in order to preserve the permanent intracranial volume[30]. An expanding lesion can initially shift CSF and blood out of the cranium without much change in ICP. However, this capacity to compensate for changes in volume has limitation. If the lesion continues expansion, ICP will get elevated[31]. Elevated ICP leads to cerebral herniation[32]. Moreover, increased ICP harms CBF by depressed cerebral perfusion pressure (CPP), where CPP is calculated by subtraction of ICP from mean arterial pressure[33].
The multiple sites of actions are thought to be the protective effects of TTM on ischemic cascade[34]. These multiple sites of actions include prevention of BBB disruption, reduction of oxygen derivative free radical release, reduction of excitotoxic neurotransmitter production, anti-inflammatory action and delayed apoptosis[35]. The major neuroprotective effect of TTM in patients after cardiac arrest with restored of systemic circulation (ROSC) is apparently the protective effect on reperfusion damage[36]. Numerous effects resulted from reperfusion damage, including oxygen free radical production, excitotoxic neurotransmitter release, and calcium influx, are all diminished by TTM[5,6,34]. Moreover, TTM also reduces cerebral metabolic rate, protects mitochondrial break down and prevents cell membrane leakage[37,38]. The neurons and glials are finally prevented to turn apoptosis[38]. Protection of BBB damage is an important action of TTM[39]. Diminution of BBB disruption helps to reduce brain edema then lower ICP[38]. Effectiveness of ICP reduction by TTM in various brain disorders has been demonstrated in many clinical and experimental studies[12,40-43]. However, absolute profit of ICP control by TTM in diverse clinical features needs to be confirmed with large scale RCTs[3,13].
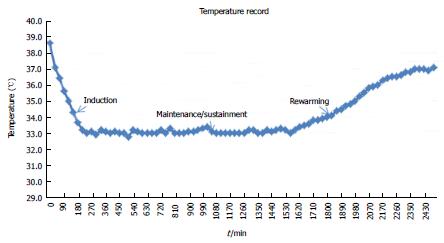
The course of TTM is divided into three phases[44]. The beginning of TTM is known as induction phase. The main idea of induction phase is to lower the current core temperature to the target as fast as possible[45,46]. Subsequently, that target temperature is smoothly maintained for certain duration (usually for 24 h), the so-called maintenance or sustainment phase[45,46]. The last part, the so-called rewarming phase, the core temperature is slowly raised to the ordinary point with actively control rate, usually at 0.2-0.5 °C/h[45,46]. Most of the important complications, particularly infection, usually happen during this last phase when the temperature is passively rewarmed with too rapid and out-of-control rate[4]. The ideal temperature curve of a patient with cardiac arrest treated with TTM is showed in Figure 2.
Many methods of TTM have been reported in the literature. Some methods are no longer utilized in clinical practice any more due to their unfeasibility or their ineffectiveness. The method with antipyretic drugs alone are, of course, not sufficient to achieve ideal TTM[47]. Under lack of electricity source circumstance, intravenous cold crystalloid solution may be helpful for initiation of TTM during pre-hospital period[48,49]. However, large volume is needed for induction phase. It is still not possible to achieve ideal TTM with intravenous cool fluid alone. Cooling helmets or hoods is effective to achieve selective cerebral TTM in infants however it seems to be ineffective in adults[6,50]. The two most accepted methods in clinical practice and major clinical trials are non-invasive surface technique and invasive endovascular technique[51,52].
The hallmark of invasive endovascular techniques is central venous catheter with extracorporeal cooling machine[4]. The central venous catheter can be sticked via femoral, jugular or subclavian vein. Of course, the auto-feedback temperature regulated system is integrated with the machine. Two commercial brands are obtainable in universal market: CoolGard 3000® and Celcius Control System®. The advantage of endovascular system is effective performance including rapid temperature reduction to the target, smoothly sustainment of target temperature and rewarm with actively controlled rate[53,54]. Application of non-pharmacologic shivering control with skin counter-warming is much convenient and more effective during endovascular cooling[55]. Without sedative effect from pharmacologic shivering control, intubation for airway protection can be avoided under skin counter-warming[56]. That’s why endovascular method is the recommend technique in several studies of TTM in subjects with acute ischemic stroke[57-59]. However, catheter-related complications and limitation of central venous access are disadvantage issues for endovascular method[51,60].
Compression of ice packs to neck, axilla and groin is the simplest way for surface cooling. Two landmark randomized-controlled-trials (RCT) for TTM in patients with PCAS demonstrated effectiveness of this ice packs application[1,2]. However, disadvantage of this technique is awkward, required strenuous staff effort, unreliable temperature control and high risk for complications[61]. The auto-feedback temperature regulated machine provides reliable temperature management and is favorable to perform in clinical practice[47]. The machine comes with circulatory cold water blankets/pads or cold air-flow blankets. Several trademarks of machine are commercially distributed in the worldwide market, including ArcticSun®, CritiCool® andBlanketrol®. The effective automatic cooling system with temperature feedback of the machine helps rapidly lower the temperature to the target and supports slowly rewarm back to the normal baseline temperature. Core temperature monitoring straight connected to the machine is the key for auto-feedback temperature regulated system. The temperature of water within the blankets or pads is automatically regulated by the machine upon target temperature setting and feedback data from core temperature measurement[52]. The surface method with cold water pads is showed in Figure 3.
EMCOOL® pads consist of graphite elements, the high heat conductivity, for cooling media which apply right to the superficial skin. This pads have to get frozen up in ordinary freezer to become 9 °C before application however do not require power supply while using[62]. Consequently, this system is extremely practical in pre-hospital situation for TTM induction[63].
The novel esophageal cooling device, the most recent non-invasive method, shows preliminary benefit of its use in PCAS patients[64]. The United States Food and Drug Administration has already approved this device[65].
Peripheral vasoconstriction is the initial physiologic response when temperature begins to go down[66]. When temperature declines to the certain point, shivering usually occurs[4]. Occurrence of shivering may represent intact physiologic response and indicate good neurologic outcomes[67]. Wonderful shivering control is a key of success to achieve ideal TTM and should be included in the treatment protocol[55,68]. Shivering is usually monitored with the Bedside Shivering Assessment Score during TTM (Table 1)[69]. Elevated peripheral vascular resistance during induction phase of TTM is usually transient and takes no effect to systemic blood pressure[70]. Sinus bradycardia with heart rate less than 50 beats per minute occurs in almost 50% of patients with PCAS during maintenance phase[71]. Nevertheless, this bradycardia should also indicate an intact physiologic response, does not require any treatment due to no hemodynamic effect and may predict good prognosis[72]. Platelets dysfunction and coagulation defect are hematologic abnormalities associated with hypothermia found in non-human experimental models[4,66]. However, abnormal bleeding associated with TTM is infrequently found in real world clinical practice[3,73]. Hypothermia also obliges kidneys to excrete water leading to volume reduction[74]. Serum potassium becomes lower during maintenance phase due to intracellular shift and renal loss however it is expected to be elevated once temperature goes up in rewarming period[4]. Serum amylase becomes elevated when temperature declines, nonetheless, this high serum amylase does not cause pathologic pancreatitis at all[75]. Although elevated blood sugar due to lower insulin level usually occurs during maintenance phase, supplementary insulin may worsen the pre-existing hypokalemia[76]. Infection, particularly pneumonia and sepsis, is a well-known adverse event in patients treated with TTM, however it is usually not associated with unfavorable outcomes[47,77].
Combination of complex pathophysiologic process after resuscitated from cardiac arrest, known as PCAS, attribute to multiple organs damage[78]. Global ischemic cascade occurs in the brain due to generalized and severe ischemia during cardiac arrest along with reperfusion process after return of spontaneous circulation (ROSC) leading to hypoxic/ischemic brain injury[79-81]. This global brain damage is responsible for a major cause of mortality in patients with PCAS pertaining to 68% of out-of hospital cardiac arrest (OHCA) and 23% of in-hospital cardiac arrest (IHCA)[82]. TTM is a well-known neuroprotective therapy for ischemic/hypoxic brain injury[83-85].
Two landmark (RCT show that induced mild hypothermia can reduce mortality rate and improve neurologic outcome in adult patients who remained comatose after resuscitated from out-of hospital cardiac arrest and had ventricular fibrillation (VF) or ventricular tachycardia (VT) as initial cardiac rhythm[1,2]. The benefit from these two RCTs is excellent with number-needed-to-treat (NNT) 7 for avoidance of mortality and NNT 6 for favorable neurological/clinical outcomes[86]. The summary of the two landmark RCTs is revealed in Table 2. Base on the results from these two RCTs, International Liaison Committee on Resuscitation and American Heart Association declared, in 2003 and 2010 respectively, that unconscious adults who become ROSC following OHCA with VT/VF or shockable rhythm should be treated with TTM under target temperature between 32 °C and 34 °C for 12 to 24 h[6,7].
| Australian trial | European trial | |
| Sample size | n = 77 | n = 275 |
| TTM vs untreated | 43 TTM vs | 137 TTM vs |
| 34 untreated | 138 untreated | |
| Initial rhythm | VT/VF | VT/VF |
| Method of TTM | Surface with ice packs | Surface with cooling blankets/pads and ice packs |
| Place of initiation | Emergency department | Prehospital setting |
| Target temperature | 33 °C | 32 °C-34 °C |
| Duration of TTM | 12 h | 24 h |
| Time of Follow up | 30 d | 6 mo |
| Outcomes | NNT of 7 to avoid death | NNT of 6 to improve neurological outcomes |
The appropriate target temperature for TTM in adult patients with PCAS then becomes an important dilemma. TTM Trial is a landmark RCT for comparing benefit of TTM in adult patients after OHCA with any initial rhythm at 33 °C vs 36 °C[87]. In November 2013, TTM Trial concludes the same benefit of neurologic outcomes and survival at six months in adult patients with OHCA treated with TTM at 33 °C vs which of 36 °C[88]. Furthermore, at six months after discharge from the hospital, survivals in 33 °C and 36 °C group have similarly good quality of life and same level of cognitive function[89].
Clinical profit of TTM in patients with PCAS from other etiologies except OHCA with shockable rhythm remains not well-established[90]. Some small clinical trials report evidence of marginal outcomes benefit in OHCA subgroup with asystole/pulseless electrical activity, the so-called non-shockable rhythm, and also in IHCA subgroup[6,7,90]. For patients after OHCA with non-shockable rhythm, few observational studies show no difference in neurologic outcomes with TTM but possible reduction of mortality at six months[2,91,92]. A recent observational study included more than 90% of adult patients with non-shockable rhythm show improvement of neurologic outcomes and better survival to hospital discharge with TTM[73]. For patients with IHCA, few observational studies show marginal benefit of TTM in both neurologic outcomes and survival[73,93]. The most update recommendation declared by International Liaison Committee on Resuscitation, American Heart Association and European Resuscitation Council similarly state in 2015 that unconscious adult patients with ROSC after either OHCA or IHCA with either shockable or non-shockable rhythm should be treated with TTM at 32 °C to 36 °C for at least 24 h[8-10]. From the recent meta-analysis, TTM confers to better neurological outcomes than no temperature management in adult patients with PCAS, however, TTM in specific subgroup including initial non-shockable rhythm, IHCA and non-cardiac causes of arrest does not have sufficient data to make any conclusion[94]. The inclusion and exclusion criteria for TTM in adult patients with PCAS at Thammasat University Hospital are showed in Table 3.
| Inclusion criteria |
| Witnessed arrest |
| Any initial rhythm, However initial rhythm VF or pulseless VT is the first priority |
| Time to ACLS was less than 15 min and total of ACLS time less than 60 min |
| GCS of 8 or below |
| SBP of > 90 with or without vasopressors |
| Less than 8 h have elapsed since ROSC |
| Exclusion criteria |
| Pregnancy |
| Known functional dependence |
| Down time of > 30 min |
| ACLS preformed for > 60 min |
| Known terminal illness |
| Comatose state prior to cardiac arrest |
| Prolonged hypotension (i.e., MAP < 60 for > 30 min) |
| Evidence of hypoxemia for > 15 min following ROSC |
| Known coagulopathy that cannot be reversed |
Therapeutic Hypothermia after Paediatric Cardiac Arrest (THAPCA) Trial is a landmark study for TTM in all aspects of pediatric patients with PCAS[95]. The results from THAPCA trial demonstrated that TTM with target temperature of 33 °C in pediatric patients with PCAS due to either OHCA or IHCA did not show any outcomes benefit as compared with which of target temperature of 36.8 °C[96,97].
In non-human experimental models with on focal brain ischemia, TTM demonstrates a very capable neuroprotective outcomes[98]. However, application of TTM in patients with ischemic stroke still has a lot of limitations[99]. Invasive endovascular method is preferred to apply in patients with acute ischemic stroke due to its feasibility and safety as reported by most clinical studies[56,100]. Under endovascular method, shivering control is convenient with non-pharmacologic skin counter-warming technique[55]. For this reason, pharmacologic anti-shivering technique which usually associated with sedative effect can be avoided[54,100]. Endovascular method is apparently not associated with bleeding complications even in post-thrombolytic condition[100]. Unfortunately, the RCT of TTM with endovascular method at 33 °C following intravenous recombinant plasminogen activator (rtPA) in patients with ischemic stroke (ICTuS 2 Trial) is early stopped due to the approval of interventional thrombectomy and lack of funding[101]. The sample size of ICTus 2 Trial is too small to make any conclusion on efficacy or clinical outcomes of the treatment[101].
With its reperfusion protective action, TTM should be useful to decrease symptomatic intracerebral hemorrhage (sICH) after intravenous rtPA as well as after endovascular treatment[102,103]. The landmark RCT of TTM as neuroprotective treatment with target temperature at 34 °C to 35 °C in patients with acute ischemic stroke (EuroHYP-1) is still ongoing[104]. At this moment, routine application of TTM in patients with acute ischemic stroke is not recommended[105].
Fever control with TTM technique, to keep target temperature less than 37.5 °C, is helpful for patients with acute ischemic stroke[106]. Reduction of ICP with TTM in malignant brain infarct is demonstrated in both experimental and clinical studies[40,42,107]. TTM is helpful for ICP reduction in patients with large middle cerebral artery (MCA) infarct[40]. However, routine application of TTM as ICP reduction in any type of malignant brain infarct is controversial due to insufficient support clinical data of its benefit[3,106].
Pertaining to experimental animal models for TBI, TTM provides excellent mechanism of action in both Neuroprotection and ICP reduction[108-110]. Two clinical trials in patients with severe TBI from China demonstrated good effect of TTM on ICP control with favorable outcomes after six months to one year[111,112]. Unfortunately, the following meta-analysis, which includes small to medium scale RCTs before 2003, did not demonstrate any benefit to apply TTM as neuroprotective therapy in patients with TBI[113-115]. Finally, two landmark RCTs of TTM as neuroprotective therapy in either adults or children with TBI fail to demonstrate any beneficial outcomes[70,116,117]. Elevated ICP in patients with TBI is common and associated with poor outcomes[118,119]. The previous TTM trials begin rewarming when the peak of elevated ICP occurs at around 48 h after onset of TBI leading to clinical deterioration[120]. This rebound elevated ICP found during rewarming phase is assumed to be one of the key reasons of failure in previous landmark RCT[121]. Specific group of elevated ICP in patients with TBI may get clinical profit from ICP reduction with TTM[12]. Clinical trial of TTM according to high ICP in patients with TBI was proposed[122]. Unfortunately, large scale RCT of TTM in specific TBI patients with high ICP more than 20 mmHg (Eurotherm3235 Trial) does not demonstrate any clinical benefit[123]. Recent meta-analysis of TTM vs normothermia in adult patients with TBI does not demonstrate any clinical benefit of TTM but reveal risk of developing pneumonia and cardiovascular complications associated with TTM[124]. Large scale RCT of TTM in particular aspects of patients with TBI is still ongoing[125]. At this moment, ordinary application of TTM in patients with TBI without clinical study is not recommended[126].
Fever is commonly found in patients admitted in Neurological ICU, increases risk of complications, and is usually associated with unfavorable clinical outcomes[127,128]. For example, in patients with ischemic stroke, chance to develop poor outcomes increases 2.2 times in each one degree exceeding 37 °C when compared with patients who have normal temperature[129]. Most common cause of fever in Neurological ICU is infection[130]. Similar method of TTM can be applied for fever control in Neurological ICU[131,132]. The commonly use techniques such as surface and endovascular are convenient and save to employ for fever control[131,132]. Fever control in patients with septic shock with external TTM machine reduces early mortality[133]. However, overall benefit of antipyretic therapy with external TTM in patients with sepsis is still not approved[134,135]. Fever control in patients with acute ischemic stroke is recommended per standard guidelines[105].
TTM can apply as organ protective therapy from ischemic effect during cardiovascular surgery with circulatory arrest[136,137]. The landmark RCT of TTM for the period of operation in patients with benign grade SAH from ruptured intracranial aneurysm (World Federation of Neurological Surgeons scale between one and three) did not show any clinical benefit with more frequent associated infection[138]. TTM can reduce perilesional edema with favorable outcomes in animal models with intracerebral hemorrhage (ICH)[139]. The TTM after intracerebral hemorrhage (TTM-ICH) trial is ongoing[140]. At this moment, routine use of TTM in patients with ICH is not recommended[141]. The prospective protocol-selected trial demonstrated potential clinical benefit of local TTM in patients with neurologically complete spinal cord injury[142]. Experts recommend that TTM can be the option for ICP control in patients with fulminant hepatic encephalopathy particularly while waiting for liver transplantation[143].
Application of TTM in donors demonstrates organ protective effect on kidney in recipients[144]. This RCT is the first ever for clinical trial which demonstrates organ defensive action of TTM from hypoxic/ischemic cascade outside the brain. The process of TTM at 34 °C-35 °C in kidney donors in this study is convenient and the cost of treatment is economic[145]. TTM in kidney donors can be its second class I recommendation per standard guidelines following post-cardiac arrest in the near future.
Two main purposes of TTM in patients admitted in Neurological ICU are neuroprotective therapy and ICP control. TTM is the most potent neuroprotective treatment due to its numerous effects against ischemic/hypoxic injury. TTM provides reliable ICP reductive action. Two most popular methods of TTM using in clinical practice and clinical trials are invasive endovascular technique and non-invasive surface cooling. Fast induction, smooth maintenance and slow rewarming are the important steps to achieve ideal TTM. The strongest clinical benefit of TTM is the excellent outcomes with neuroprotective effect in patients with PCAS. TTM has been recommended as the essential treatment for OHCA with shockable rhythm for more than 10 years. Even with marginal benefit, TTM is still recommended for non-shockable rhythm and IHCA subgroup. TTM may give benefit in patients with acute ischemic stroke however its role needs to be proved with large scale RCT. TTM should be clinically useful for ICP reduction in patients with malignant MCA infarct. Routine use of TTM in patients with TBI as neuroprotective therapy or ICP control is still not recommended due to lacking of any benefit from many RCTs. TTM machine can be applied as fever control in patients with various conditions in Neurological ICU. Fever control should help to improve clinical outcomes in patients admitted in Neurological ICU.
The authors acknowledgement the National Research University Project of Thailand from Office of Higher Education Commission and Center of Excellence in Integrated Sciences for Holistic Stroke Research from Thammasat University.
Manuscript source: Invited manuscript
Specialty type: Medical Laboratory Technology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bugaj AM, Maric I, Ni Y, Sip M, Zhang Z S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3961] [Cited by in F6Publishing: 3688] [Article Influence: 167.6] [Reference Citation Analysis (0)] |
| 2. | Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4038] [Cited by in F6Publishing: 3712] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 3. | Nunnally ME, Jaeschke R, Bellingan GJ, Lacroix J, Mourvillier B, Rodriguez-Vega GM, Rubertsson S, Vassilakopoulos T, Weinert C, Zanotti-Cavazzoni S. Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Med. 2011;39:1113-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Mayer SA, Sessler DI. Therapeutic hypothermia. In: Mayer SA, Claassen J, editors. Prevention and Treatment of Fever in Neurocritical Care. New York 2005; 629. [Cited in This Article: ] |
| 5. | Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med. 2004;30:556-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, Böttiger BW, Morley PT, Nolan JP, Okada K. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 506] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 7. | Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768-S786. [PubMed] [Cited in This Article: ] |
| 8. | Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465-S482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 923] [Cited by in F6Publishing: 997] [Article Influence: 124.6] [Reference Citation Analysis (0)] |
| 9. | Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41:2039-2056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 10. | Hazinski MF, Nolan JP, Aickin R, Bhanji F, Billi JE, Callaway CW, Castren M, de Caen AR, Ferrer JM, Finn JC. Part 1: Executive Summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132:S2-S39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Kurtz P, Fitts V, Sumer Z, Jalon H, Cooke J, Kvetan V, Mayer SA. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care. 2011;15:477-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Sadaka F, Veremakis C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: a systematic review. Brain Inj. 2012;26:899-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Corry JJ. The use of targeted temperature management for elevated intracranial pressure. Curr Neurol Neurosci Rep. 2014;14:453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Muengtaweepongsa S. Methods and clinical applications of targeted temperature management. Neurology Asia. 2015;20:325-333. [Cited in This Article: ] |
| 15. | Gusev EISVI. Brain ischemia. New York: Kluwer Academic/Plenum Publishers 2003; . [Cited in This Article: ] |
| 16. | Zhao H, Kilgas S, Alam A, Eguchi S, Ma D. The Role of Extracellular Adenosine Triphosphate in Ischemic Organ Injury. Crit Care Med. 2016;44:1000-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7:378-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 18. | Stead SM, Daube JR. Basics of Neurophysiology. Clinical Neurophysiology. 2016;3. [Cited in This Article: ] |
| 19. | Hinkle JL, Bowman L. Neuroprotection for ischemic stroke. J Neurosci Nurs. 2003;35:114-118. [PubMed] [Cited in This Article: ] |
| 20. | Bennett MV, Pellegrini-Giampietro D, Gorter J, Aronica E, Connor J, Zukin RS. The GluR2 hypothesis: Ca -permeable AMPA receptors in delayed neurodegeneration. Proceedings of the Cold Spring Harbor symposia on quantitative biology. New York: Cold Spring Harbor Laboratory Press 1996; 373-384. [Cited in This Article: ] |
| 21. | Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004;1:46-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 290] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1568] [Cited by in F6Publishing: 1728] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 25. | Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371-383. [PubMed] [Cited in This Article: ] |
| 26. | Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, Abo-Ramadan U, Tatlisumak T. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Monro A. Observations on the structure and function of the nervous system. Edinburgh: Creech & Johnson 1783; . [Cited in This Article: ] |
| 28. | Kelly G. Appearances observed in the dissection of two individuals; death from cold and congestion of the brain. Trans Med Chir Sci Edinb. 1824;84-169. [Cited in This Article: ] |
| 29. | Neff S, Subramaniam RP. Monro-Kellie doctrine. J Neurosurg. 1996;85:1195. [PubMed] [Cited in This Article: ] |
| 30. | Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56:1746-1748. [PubMed] [Cited in This Article: ] |
| 31. | Kincaid MS, Lam AM. Monitoring and managing intracranial pressure. Continuum. 2006;12:16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Sheth K, McCullough M, Kazmi S, Lazaridis C, O’Phelan K, Shepherd SA, DeJesus-Alvelo I, Marshall SA, Willis AM, Durrant JC. Cerebral Herniation Syndromes and Intracranial. Hypertension: Rutgers University Press 2016; . [Cited in This Article: ] |
| 33. | Villa F, Citerio G. Intracranial pressure monitoring. Oxford: Oxford Textbook of Neurocritical Care 2016; 107. [DOI] [Cited in This Article: ] |
| 34. | Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186-S202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 753] [Cited by in F6Publishing: 763] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 35. | Talma N, Kok WF, de Veij Mestdagh CF, Shanbhag NC, Bouma HR, Henning RH. Neuroprotective hypothermia - Why keep your head cool during ischemia and reperfusion. Biochim Biophys Acta. 2016;1860:2521-2528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Madathil RJ, Hira RS, Stoeckl M, Sterz F, Elrod JB, Nichol G. Ischemia reperfusion injury as a modifiable therapeutic target for cardioprotection or neuroprotection in patients undergoing cardiopulmonary resuscitation. Resuscitation. 2016;105:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 291] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 38. | González-Ibarra FP, Varon J, López-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol. 2011;2:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Chi OZ, Liu X, Weiss HR. Effects of mild hypothermia on blood-brain barrier disruption during isoflurane or pentobarbital anesthesia. Anesthesiology. 2001;95:933-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 2001;32:2033-2035. [PubMed] [Cited in This Article: ] |
| 41. | Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery. 2009;64:86-93. [Cited in This Article: ] |
| 42. | Park CK, Jun SS, Kim MC, Kang JK. Effects of systemic hypothermia and selective brain cooling on ischemic brain damage and swelling. Acta Neurochir Suppl. 1998;71:225-228. [PubMed] [Cited in This Article: ] |
| 43. | Kawai N, Nakamura T, Okauchi M, Nagao S. Effects of hypothermia on intracranial pressure and brain edema formation: studies in a rat acute subdural hematoma model. J Neurotrauma. 2000;17:193-202. [PubMed] [Cited in This Article: ] |
| 44. | Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Suwannakin A, Muengtaweepongsa S. Initial experience of therapeutic hypothermia after cardiac arrest with surface cooling method in Thammasat Chalerm Prakiat Hospital: two cases report. J Med Assoc Thai. 2011;94 Suppl 7:S190-S193. [PubMed] [Cited in This Article: ] |
| 46. | Pinichjindasup A, Homvises B, Muengtaweepongsa S. Therapeutic hypothermia with extracorporeal membrane oxygenation (ECMO) and surface cooling in post-cardiac arrest patients: 4 case reports. J Med Assoc Thai. 2014;97 Suppl 8:S223-S227. [PubMed] [Cited in This Article: ] |
| 47. | Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality--Part 2: Practical aspects and side effects. Intensive Care Med. 2004;30:757-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 48. | Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med. 2005;33:2744-2751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 49. | Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9-13. [PubMed] [Cited in This Article: ] |
| 50. | Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, Rodde M, Burnham J, Wang D. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg. 2004;100:272-277. [PubMed] [Cited in This Article: ] |
| 51. | Oh SH, Oh JS, Kim YM, Park KN, Choi SP, Kim GW, Jeung KW, Jang TC, Park YS, Kyong YY. An observational study of surface versus endovascular cooling techniques in cardiac arrest patients: a propensity-matched analysis. Crit Care. 2015;19:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Glover GW, Thomas RM, Vamvakas G, Al-Subaie N, Cranshaw J, Walden A, Wise MP, Ostermann M, Thomas-Jones E, Cronberg T. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest - an analysis of the TTM trial data. Crit Care. 2016;20:381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Al-Senani FM, Graffagnino C, Grotta JC, Saiki R, Wood D, Chung W, Palmer G, Collins KA. A prospective, multicenter pilot study to evaluate the feasibility and safety of using the CoolGard System and Icy catheter following cardiac arrest. Resuscitation. 2004;62:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, Lutsep H, Dobak J, Matsubara BS, Zivin J. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 2005;14:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, Gilmore E, Malhotra R, Mayer SA, Lee K. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14:389-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312-317. [PubMed] [Cited in This Article: ] |
| 57. | Lyden PD, Hemmen TM, Grotta J, Rapp K, Raman R. Endovascular therapeutic hypothermia for acute ischemic stroke: ICTuS 2/3 protocol. Int J Stroke. 2014;9:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Wolfe MM, Boylan MO. Obesity and the gastrointestinal tract: you are what you eat. J Clin Gastroenterol. 2014;48:817-822. [PubMed] [Cited in This Article: ] |
| 59. | Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, Mayberg MR, Furlan AJ. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32:1847-1854. [PubMed] [Cited in This Article: ] |
| 60. | Fischer M, Lackner P, Beer R, Helbok R, Pfausler B, Schneider D, Schmutzhard E, Broessner G. Cooling Activity is Associated with Neurological Outcome in Patients with Severe Cerebrovascular Disease Undergoing Endovascular Temperature Control. Neurocrit Care. 2015;23:205-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Seder DB, Van der Kloot TE. Methods of cooling: practical aspects of therapeutic temperature management. Crit Care Med. 2009;37:S211-S222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Uray T, Sterz F, Janata A, Wandaller C, Holzer M, Laggner AN, Behringer W. Surface cooling with a new cooling-blanket for rapid induction of mild hypothermia in humans after cardiac arrest: A feasibility trial. Circulation. 2006;114:1190-1190. [Cited in This Article: ] |
| 63. | Dhaese HL, Martens PR, Müller NH, Casier IM, Mulier JP, Heytens L. The use of emergency medical cooling system pads in the treatment of malignant hyperthermia. Eur J Anaesthesiol. 2010;27:83-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Hegazy A, Lapierre D, Althenayan E. Targeted temperature management after cardiac arrest and fever control with an esophageal cooling device. Critical Care. 2015;19:P424. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | CritiCool Specification M (Rehovot, Israel). 510K for CritiCool system. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm?ID=DEN140018. [Cited in This Article: ] |
| 66. | Arrica M, Bissonnette B. Therapeutic hypothermia. Semin Cardiothorac Vasc Anesth. 2007;11:6-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Nair SU, Lundbye JB. The occurrence of shivering in cardiac arrest survivors undergoing therapeutic hypothermia is associated with a good neurologic outcome. Resuscitation. 2013;84:626-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Presciutti M, Bader MK, Hepburn M. Shivering management during therapeutic temperature modulation: nurses’ perspective. Crit Care Nurse. 2012;32:33-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Logan A, Sangkachand P, Funk M. Optimal management of shivering during therapeutic hypothermia after cardiac arrest. Crit Care Nurse. 2011;31:e18-e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Muizelaar JP, Wagner FC, Marion DW, Luerssen TG. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563. [PubMed] [Cited in This Article: ] |
| 71. | Thomsen JH, Hassager C, Bro-Jeppesen J, Søholm H, Nielsen N, Wanscher M, Køber L, Pehrson S, Kjaergaard J. Sinus bradycardia during hypothermia in comatose survivors of out-of-hospital cardiac arrest - a new early marker of favorable outcome? Resuscitation. 2015;89:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Muengtaweepongsa S, Jantanukul A, Suwanprasert K. Should the heart rate including the heart rate variability be important prognostigators in cardiac arrest? Resuscitation. 2016;98:e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Srivilaithon W, Muengtaweepongsa S. The Outcomes of Targeted Temperature Management After Cardiac Arrest at Emergency Department: A Real-World Experience in a Developing Country. Ther Hypothermia Temp Manag. 2017;7:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Varon J, Acosta P. Therapeutic hypothermia: past, present, and future. Chest. 2008;133:1267-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31:2041-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Lehot JJ, Piriz H, Villard J, Cohen R, Guidollet J. Glucose homeostasis. Comparison between hypothermic and normothermic cardiopulmonary bypass. Chest. 1992;102:106-111. [PubMed] [Cited in This Article: ] |
| 77. | Gagnon DJ, Nielsen N, Fraser GL, Riker RR, Dziodzio J, Sunde K, Hovdenes J, Stammet P, Friberg H, Rubertsson S. Prophylactic antibiotics are associated with a lower incidence of pneumonia in cardiac arrest survivors treated with targeted temperature management. Resuscitation. 2015;92:154-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 655] [Cited by in F6Publishing: 690] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 79. | Bro-Jeppesen J, Kjaergaard J, Stammet P, Wise MP, Hovdenes J, Åneman A, Horn J, Devaux Y, Erlinge D, Gasche Y. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation. 2016;98:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208-212. [PubMed] [Cited in This Article: ] |
| 81. | Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562-568. [PubMed] [Cited in This Article: ] |
| 82. | Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 700] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 83. | Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 84. | Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Müllner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33:414-418. [PubMed] [Cited in This Article: ] |
| 85. | Froehler MT, Geocadin RG. Hypothermia for neuroprotection after cardiac arrest: mechanisms, clinical trials and patient care. J Neurol Sci. 2007;261:118-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;CD004128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 87. | Nielsen N, Wetterslev J, al-Subaie N, Andersson B, Bro-Jeppesen J, Bishop G, Brunetti I, Cranshaw J, Cronberg T, Edqvist K. Target Temperature Management after out-of-hospital cardiac arrest--a randomized, parallel-group, assessor-blinded clinical trial--rationale and design. Am Heart J. 2012;163:541-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 88. | Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197-2206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1992] [Cited by in F6Publishing: 1950] [Article Influence: 177.3] [Reference Citation Analysis (0)] |
| 89. | Cronberg T, Lilja G, Horn J, Kjaergaard J, Wise MP, Pellis T, Hovdenes J, Gasche Y, Åneman A, Stammet P. Neurologic Function and Health-Related Quality of Life in Patients Following Targeted Temperature Management at 33°C vs 36°C After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA Neurol. 2015;72:634-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 90. | Dumas F, Grimaldi D, Zuber B, Fichet J, Charpentier J, Pène F, Vivien B, Varenne O, Carli P, Jouven X. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation. 2011;123:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 91. | Hessel EA. Therapeutic hypothermia after in-hospital cardiac arrest: a critique. J Cardiothorac Vasc Anesth. 2014;28:789-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Oddo M, Ribordy V, Feihl F, Rossetti AO, Schaller MD, Chioléro R, Liaudet L. Early predictors of outcome in comatose survivors of ventricular fibrillation and non-ventricular fibrillation cardiac arrest treated with hypothermia: a prospective study. Crit Care Med. 2008;36:2296-2301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Chan PS, Berg RA, Tang Y, Curtis LH, Spertus JA. Association Between Therapeutic Hypothermia and Survival After In-Hospital Cardiac Arrest. JAMA. 2016;316:1375-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 94. | Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2016;2:CD004128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 95. | Moler FW, Silverstein FS, Meert KL, Clark AE, Holubkov R, Browning B, Slomine BS, Christensen JR, Dean JM. Rationale, timeline, study design, and protocol overview of the therapeutic hypothermia after pediatric cardiac arrest trials. Pediatr Crit Care Med. 2013;14:e304-e315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 97. | Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Browning B, Pemberton VL, Page K. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med. 2017;376:318-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 98. | van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063-3074. [PubMed] [Cited in This Article: ] |
| 99. | van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab. 2010;30:1079-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 100. | Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, Wijman CA, Rapp KS, Grotta JC, Lyden PD. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 101. | Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, Parker S, Concha M, Hussain S, Agarwal S. Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke. 2016;47:2888-2895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 102. | Tang XN, Liu L, Koike MA, Yenari MA. Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke. Ther Hypothermia Temp Manag. 2013;3:74-83. [PubMed] [Cited in This Article: ] |
| 103. | Hong JM, Lee JS, Song HJ, Jeong HS, Choi HA, Lee K. Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke. 2014;45:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 104. | van der Worp HB, Macleod MR, Bath PM, Demotes J, Durand-Zaleski I, Gebhardt B, Gluud C, Kollmar R, Krieger DW, Lees KR. EuroHYP-1: European multicenter, randomized, phase III clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke. 2014;9:642-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 105. | Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947. [PubMed] [Cited in This Article: ] |
| 106. | Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ, Wintermark M. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1222-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 107. | Murtha LA, McLeod DD, Pepperall D, McCann SK, Beard DJ, Tomkins AJ, Holmes WM, McCabe C, Macrae IM, Spratt NJ. Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy. J Cereb Blood Flow Metab. 2015;35:592-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250-258. [PubMed] [Cited in This Article: ] |
| 109. | Jiang JY, Lyeth BG, Kapasi MZ, Jenkins LW, Povlishock JT. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 1992;84:495-500. [PubMed] [Cited in This Article: ] |
| 110. | Gu X, Wei ZZ, Espinera A, Lee JH, Ji X, Wei L, Dix TA, Yu SP. Pharmacologically induced hypothermia attenuates traumatic brain injury in neonatal rats. Exp Neurol. 2015;267:135-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 111. | Zhi D, Zhang S, Lin X. Study on therapeutic mechanism and clinical effect of mild hypothermia in patients with severe head injury. Surg Neurol. 2003;59:381-385. [PubMed] [Cited in This Article: ] |
| 112. | Jiang J, Yu M, Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93:546-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 113. | Henderson WR, Dhingra VK, Chittock DR, Fenwick JC, Ronco JJ. Hypothermia in the management of traumatic brain injury. A systematic review and meta-analysis. Intensive Care Med. 2003;29:1637-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 114. | Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 115. | McIntyre LA, Fergusson DA, Hébert PC, Moher D, Hutchison JS. Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA. 2003;289:2992-2999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 116. | Hutchison JS, Ward RE, Lacroix J, Hébert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447-2456. [PubMed] [Cited in This Article: ] |
| 117. | Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131-139. [PubMed] [Cited in This Article: ] |
| 119. | Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, Carli PA. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7:461-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 120. | Wijayatilake DS, Shepherd SJ, Sherren PB. Updates in the management of intracranial pressure in traumatic brain injury. Curr Opin Anaesthesiol. 2012;25:540-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Sinclair HL, Andrews PJ. Bench-to-bedside review: Hypothermia in traumatic brain injury. Crit Care. 2010;14:204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 122. | Lei J, Gao G, Mao Q, Feng J, Wang L, You W, Jiang J. Rationale, methodology, and implementation of a nationwide multicenter randomized controlled trial of long-term mild hypothermia for severe traumatic brain injury (the LTH-1 trial). Contemp Clin Trials. 2015;40:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, Murray GD. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N Engl J Med. 2015;373:2403-2412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 124. | Zhu Y, Yin H, Zhang R, Ye X, Wei J. Therapeutic hypothermia versus normothermia in adult patients with traumatic brain injury: a meta-analysis. Springerplus. 2016;5:801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Ahmad FU, Starke RM, Komotar RJ, Connolly ES. A Randomized Clinical Trial of Hypothermia as a Preferred Second-Line Treatment for Elevated Intracranial Pressure After Traumatic Brain Injury. Neurosurgery. 2016;78:N10-N11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Yokobori S, Yokota H. Targeted temperature management in traumatic brain injury. J Intensive Care. 2016;4:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 127. | Rehman T, Deboisblanc BP. Persistent fever in the ICU. Chest. 2014;145:158-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 128. | Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med. 2004;32:1489-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 129. | Olsen TS, Weber UJ, Kammersgaard LP. Therapeutic hypothermia for acute stroke. Lancet Neurol. 2003;2:410-416. [PubMed] [Cited in This Article: ] |
| 130. | Niven DJ, Laupland KB. Pyrexia: aetiology in the ICU. Crit Care. 2016;20:247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 131. | Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 2004;32:559-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 132. | Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, Yavagal DR, Du YE, Naidech AM, Janjua NA. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508-2515. [PubMed] [Cited in This Article: ] |
| 133. | Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, Dellamonica J, Bouadma L, Cook F, Beji O. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2012;185:1088-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 172] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 134. | Zhang Z. Antipyretic therapy in critically ill patients with established sepsis: a trial sequential analysis. PLoS One. 2015;10:e0117279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 135. | Zhang Z, Chen L, Ni H. Antipyretic therapy in critically ill patients with sepsis: an interaction with body temperature. PLoS One. 2015;10:e0121919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Bigelow WG, Callaghan JC, Hopps JA. General hypothermia for experimental intracardiac surgery; the use of electrophrenic respirations, an artificial pacemaker for cardiac standstill, and radio-frequency rewarming in general hypothermia. Trans Meet Am Surg Assoc Am Surg Assoc. 1950;68:211-219. [PubMed] [Cited in This Article: ] |
| 137. | Bigelow WC. Methods for inducing hypothermia and rewarming. Ann N Y Acad Sci. 1959;80:522-532. [PubMed] [Cited in This Article: ] |
| 138. | Todd MM, Hindman BJ, Clarke WR, Torner JC. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 139. | MacLellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2004;24:432-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 140. | Rincon F, Friedman DP, Bell R, Mayer SA, Bray PF. Targeted temperature management after intracerebral hemorrhage (TTM-ICH): methodology of a prospective randomized clinical trial. Int J Stroke. 2014;9:646-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 141. | Lopez GA. Temperature Management in the Neurointensive Care Unit. Curr Treat Options Neurol. 2016;18:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 142. | Hansebout RR, Hansebout CR. Local cooling for traumatic spinal cord injury: outcomes in 20 patients and review of the literature. J Neurosurg Spine. 2014;20:550-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Neuberger J. Management of the Patient with Fulminant Hepatic Failure Awaiting Liver Transplantation. Liver Transplantation: John Wiley & Sons, Ltd 2013; 93-100. [Cited in This Article: ] |
| 144. | Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, Broglio K, Hirose R, Roberts JP, Malinoski D. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med. 2015;373:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 145. | Jochmans I, Watson CJ. Taking the Heat Out of Organ Donation. N Engl J Med. 2015;373:477-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |