Published online Sep 26, 2015. doi: 10.5662/wjm.v5.i3.127
Peer-review started: March 2, 2015
First decision: March 20, 2015
Revised: May 18, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: September 26, 2015
Lesions of the ampulla of Vater represent an uncommon group of gastrointestinal malignancies. The majority of lesions of the ampulla of Vater are either adenomas or adenocarcinomas. Ampullary lesions are often incidental findings. Accurate preoperative diagnosis and staging of ampullary tumors is imperative for predicting prognosis and determining the most appropriate therapeutic approach. Endoscopic ampullectomy is a safe and efficacious therapeutic procedure that can obviate the need for potentially major surgical intervention. This review will provide the framework for the diagnosis and management of ampullary lesions from the perspective of the practicing gastroenterologist. Strategies for safe and successful endoscopic ampullectomy with a focus on accurate preoperative diagnosis and staging, resection technique, and management of complications are presented.
Core tip: Adenomatous ampullary lesions are rare. Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound (EUS) have changed the management of patients with these lesions. Endoscopic ampullectomy is a technique that has revolutionized the treatment of these lesions avoiding potential complications of surgery. We herein discuss the epidemiology, the role of EUS in the local staging and the role of endoscopy in the treatment of the adenomatous ampullary neoplasms.
- Citation: Espinel J, Pinedo E, Ojeda V, Rio MGD. Endoscopic management of adenomatous ampullary lesions. World J Methodol 2015; 5(3): 127-135
- URL: https://www.wjgnet.com/2222-0682/full/v5/i3/127.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i3.127
The anatomy of the ampulla of Vater is complex. Ampullary adenomas (AA) are an uncommon group of gastrointestinal malignances. With the advances in esophagogastroduodenoscopy and ultrasonography, detection of ampullary neoplasms has increased. Most periampullary lesions are malignant tumors appearing from the ampulla, duodenum, or pancreas. Benign neoplasms entail in this region only < 10% of neoplasms[1-3]. Advances in endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) have changed the clinical management of these patients. Endoscopic ampullectomy may be considered in patients with smaller lesions that do not contain invasive carcinoma, and in patients who are poor surgical candidates[4-6]. Many series have reported low morbidity and mortality with endoscopic therapy[4,7-19]. Detailed preoperative assessment and staging is needed in other to decide on the best therapeutic option. We review the epidemiology, the role of EUS, ERCP and endoscopy in the approach of ampullary neoplasms.
Ampullary neoplasms comprise several lesions: adenoma, adenocarcinoma, adenoendocrine carcinoma, small cell carcinoma, adenosquamous carcinoma, and undifferentiated carcinoma[20]. Adenomas or adenocarcinomas representing > 95% of these lesions[21,22]. AA are benign lesions but, can potencially develop into ampullary carcinomas in a comparable progression to that of colorectal cancer[2,3,23-29]. AA can be sporadic or in the context of a familial polyposis syndromes [e.g., familial adenomatous polyposis (FAP)]. FAP is a risk factor; 80% of affected patients develop duodenal adenomas, which are often multiple[30]. In this polyposis syndrome, the lifetime incidence of peri-AA is 50%-100%. The prevalence of AA has increased in the last years with the extensive availability of endoscopy.
Ampullary lesions are often found incidentally on cross-sectional imaging or by endoscopic examination. Presenting symptoms are usually non-specific, reflecting biliary or pancreatic obstruction. The most common presentation is with painless jaundice, which is present in 50%-75% of patients[21,31-34]. Cholangitis or acute pancreatitis are rare manifestations[35-38]. Nausea, vomiting, biliary colic, and weight loss may also occur[21,33].
Accurate preoperative diagnosis and staging is critical to decide on the best treatment option and establish a prognosis.
The best endoscopic examination of the papilla of Vater is performed with a side-viewing endoscope[20]. This endoscope allows an adequate assessment of the morphological features of the lesion. Thus the following features are suggestive of benign disease: (1) a regular margin; (2) absence of ulceration or spontaneous bleeding; and (3) a soft consistency[39]. Furthermore, the side-viewing endoscope enables an easy acquisition of tissue by biopsy, at the time of procedure. However, on this respect, we know that sensitivity with forceps biopsies for demonstrating the presence of adenoma is > 90%; this is lower for adenocarcinoma, and there is up to 30% of miss diagnosis[11,40-42]. Thus, a negative histological diagnosis of carcinoma on endoscopic biopsy of an ampullary adenoma does not exclude a possible focus of adenocarcinoma[42-47]. The accuracy of endoscopic biopsies can be enhanced when additional techniques are employed. Thus, taking biopsies several days after sphincterotomy[48], and taking at least six biopsies, minimizes the chance of false negative results[49] . Despite its gaps, endoscopic forceps biopsy is the mainstay of pre-excisional histological assessment in lesions of the ampulla. However, we ought to remember that resection of all AA might be the best approach for excluding the presence of carcinoma.
ERCP has a central role in the staging and management of obstructive jaundice in AA. Adenoma ingrowth into the pancreatic or biliary ducts does not always indicate malignancy, but may hinder endoscopic excision and considerably decreases the chance of complete endoscopic resection. ERCP at the time of endoscopic papillectomy permits: (1) evaluate the intraductal extension; (2) the placement of a pancreatic stent in order to reduce the risk of pancreatitis; and (3) deploy, if required, a biliary duct stent for the palliation of obstructive jaundice.
EUS, in conjunction with ERCP, allows to assess for infiltration of the periampullary wall layers and pancreatobiliary ducts but, it does not have to be universally incorpored into the diagnostic evaluation of an ampullary adenoma[45,50-57]. The use of EUS in the assessment of AA is undefined. There is no consensus on the requirement or not for EUS prior to consideration of treatment on all patients with AA. It has been suggested by some experts that EUS is not required if the lesion is less than 1 cm in diameter or there are no endoscopic signs to suggest malignancy[58]. Others claim that, if accessible, EUS testing ought to be taken into consideration prior to endoscopic or surgical resection[59]. EUS has been reported to be of help in recognizing non-invasive lesions amenable to local resection, but as yet there are no preoperative test which are as accurate as clinical judgment and intraoperative pathological diagnosis[45,60]. A recent retrospective review concluded that EUS is useful in predicting the depth of mucosal invasion in the preoperative evaluation of suspected peri-ampullary and duodenal adenomas (specificity: 88%; negative predictive value: 90%)[53]. However, EUS is an invasive technique, operator dependent, with different rates of over-diagnosis and under-diagnosis. In this context, peritumoral inflammatory changes can lead to over-staging and likewise focal pancreatic infiltration to under-staging[61,62]. A recent meta-analisis of 14 studies and a systematic review, concluded that the results obtained by EUS were comparable to the histological results with moderate strength of agreement in the following: preoperative staging of papillary neoplasm, predicting lymph node involvement and tumor invasion[63]. The modest EUS sensitivity (77%) and specificity (78%) in predicting T1 neoplasms makes it not optimal in choosing the right patients for endoscopic papillectomy. EUS sensitivity and specificity for detecting nodal invasion was 70% and 74%, respectively. We believe, as other authors that if the clinical suspicion for invasive carcinoma is low (e.g., absence of jaundice, endoscopic features of noncancerous lesion), and the lesion appears amenable to endoscopic resection, then EUS may not impact the endoscopist’s decision to stage the lesion via ampullectomy. Few studies have been reported comparing efficacy of EUS and intraductal ultrasound (IDUS) for ampullary neoplasms[54,60,64]. IDUS was superior to EUS in terms of tumor visualization and staging (staging accuracy: 78%-93%). Therefore, IDUS can be particularly appropriate in deciding which patients should undergo endoscopic ampullectomy. However, the availability of this technique is limited and therefore the number of patients undergoing IDUS is small.
Patients diagnosed with an ampullary adenoma have three treatment options: pancreaticoduodenectomy (Whipple procedure), surgical local excision (surgical ampullectomy), or endoscopic ampullectomy. There are no clear guidelines about the surgical or endoscopic management of AA and, if they should undergo postprocedure surveillance[66]. Surgical excision is typically recommended for patients with larger lesions, lesions that contain carcinoma, lesions with lymph node involvement on preprocedure imaging, or for patients who do not have access to an experienced endoscopist in ampullectomy. Pancreaticoduodenectomy is more likely to achieve complete excision compared with local excision, but it is associated with higher operative morbidity and mortality rates (25%-65% and 0%-10%, respectively)[67,68]. Perioperative mortality rates were lowest (< 4%) in centers with a high procedure volume. Surgical ampullectomy has lower morbidity and mortality, but has the disadvantage of having more recurrence rate. Randomized trials comparing surgical ampullectomy with pancreaticoduodenectomy have not been performed. Endoscopic ampullectomy was first described in 1983 by Suzuki et al[59] and ten years later Binmoeller et al[4] described a considerable case series. More recently, many other series have reported low morbidity and mortality with endoscopic therapy[7-19]. However, the role of endoscopic ampullectomy is still debatable and it is largely performed only in reference hospitals with skill in therapeutic endoscopy. Endoscopic ampullectomy may be considered in smaller lesions (< 30 mm) that do not contain carcinoma and in patients with severe diseases. Lesions with endoscopic characteristics suggestive of posible malignancy (e.g., nonlifting, firmness, ulceration, friability) should be offered surgical resection[6].
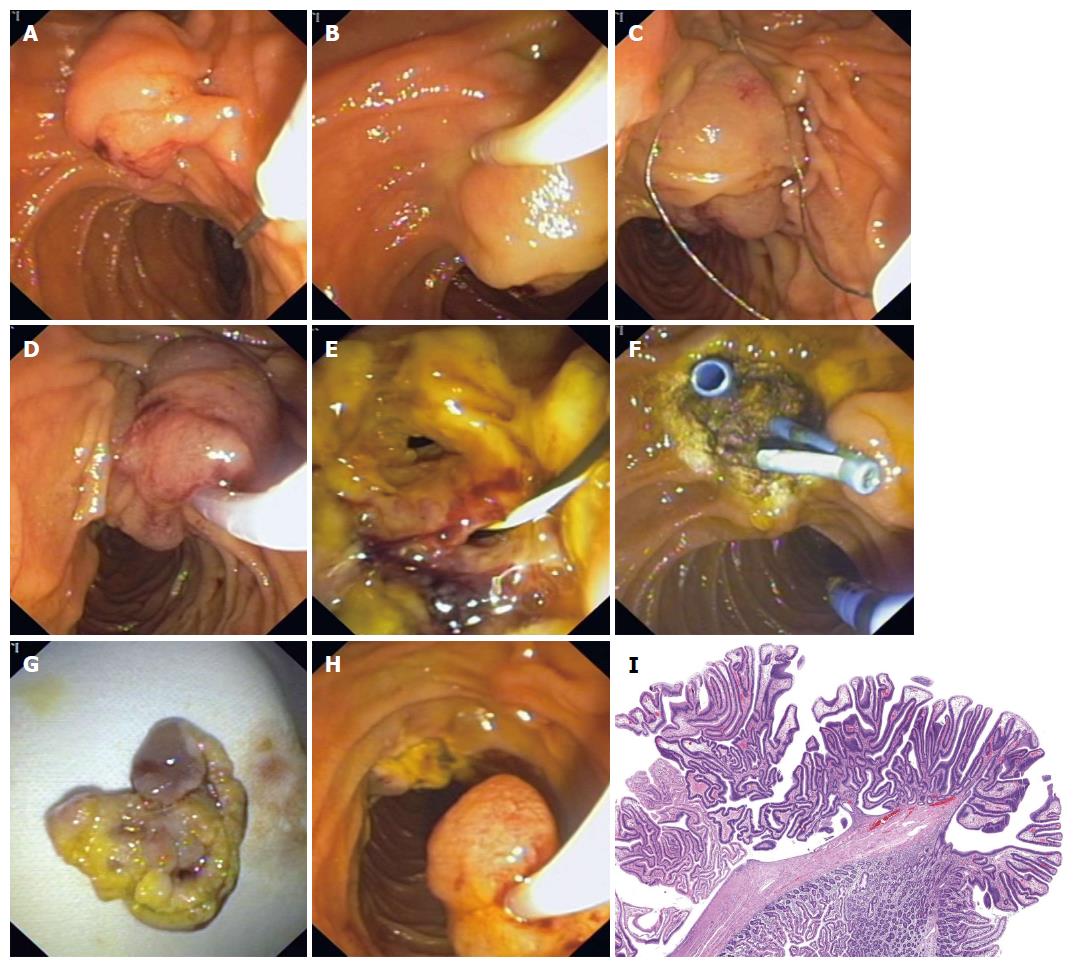
Endoscopic ampullectomy is a therapeutic modality which must be undertaken by an endoscopist with enough training and expertise. The goal with AA is for total en-bloc removal of the neoplasm. Initially, the endoscopist must determine whether resection of the entire lesion in one piece (“en bloc”) is feasible and locate the margins of the lesion. This method has several advantages: (1) it increases the likelihood of complete removal; (2) it provides clear margins for histopathologic evaluation; and (3) it reduces the procedure time. However, en bloc excision may not be technically feasible if the adenoma is of a large size, and/or there is a limited endoscopic accessibility. Piecemeal excision is usually reserved for these cases, frequently with adjuvant ablative therapy[69]. It has been postulated that this technique can reduce recurrence rates, bleeding and perforation. However, comparative trials are lacking[13] (Figure 1).
The role of submucosal injection of saline, which may be combined with epinephrine or methylene blue before ampullectomy, is controversial[6,62,66]. Epinephrine and methylene blue may help minimize bleeding and enhance endoscopic visualization of the lesions margins, respectively[13]. Local saline injection may increase technical success and decrease complications similar to mucosectomy[13,70]. However, this technique is not recommended by other authors because submucosal saline injection may involve certain disadvantages: (1) the ampullary lesion may not lift due to tethering by the biliary and pancreatic ducts; (2) The dome effect created by submucosal injection may cause difficulty in the placement of the snare for effective en bloc resection[13,70-72]; and (3) increased risk of postresection pancreatitis has been reported. Currently, the evidence to support submucosal injection before ampullectomy is not significant. A possible indication may be adenomas with lateral extraampullary spread[72].
There is no specific type of snare for endoscopic ampullectomy. For the majority of usual adenomas both hexagonal or oval snares of 3 cm are recommended. Standard braided polypectomy snares are typically used. The use of a thin wire snare is advised by some authors, limiting dispersion of the energy and risk of injury to the pancreatic orifice[72]. Occasionally, a peripheral circumferential incision to the adenoma with a needle knife device may make easier the snare capture[6]. To resect the lesion, the tip of the snare is placed on the top of adenoma; then, the snare is closed maximally and, after previously checking for papilla mobility, the lesion is sectioned by continuous application of current.
There is no general recommendation regarding the optimal current and power output for endoscopic ampulectomy. Some investigators recommend pure-cutting current for this purpose[4,15,73] to preclude the edema originated by the coagulation mode, although, a pure cutting current has been related to bleeding. Others, using a blended electrosurgical current[4,6,9] or alternating cut/coagulation modes[6,62,74]. Power output oscillates between 30 W and 150 W[6,9,13,73,75]. Most experts, advocate a blended current[76]. We prefer to use Erbe electrosurgical generators (Endocut, effect 2)[77].
Retrieval of the specimen is essential for total evaluation and detection of small malignant foci. An anti-peristaltic agent administration (e.g., glucagon or hyoscine butylbromide) to avoid intestinal migration is recommended. Retrieval should be performed immediately after excision since there is a tendency for the excised specimen to migrate distally into the jejunum. For this purpose, the snare that was used for the excision or a retrieval net is ideal. Endoscopic suction can also prevent the tissue migration. However, the specimen should not be aspirated through the accessory channel of the duodenoscope into a trap because this could lead to fragmentation of the specimen. Once retrieved, the specimen can be pinned to a polystyrene block to aid orientation and facilitate margin analysis.
After specimen retrieval, the duodenoscope is reintroduced to examine the resection site for: (1) active bleeding or bleeding stigmata; (2) residual tissue ablation. Usually, ablation therapy is used as adjunctive therapy to treat residual adenomatous tissue remaining after, en bloc or piecemeal, snare resection. With piecemeal excision, the tissue near the duct holes may be hard to excise completely. However, the benefits of this adjunctive therapy remain controversial. The overall success rate was comparable in patients with and without adjuvant thermal ablation (81% vs 78%, respectively)[9]. Ablation can be performed with monopolar or bipolar coagulation[49,70], and others devices[11,13,70,78]. We often use argon plasma coagulation (APC) (setting of 40 to 50 watts) to ablate residual tissue. We carry out a biliary sphincterotomy prior to fulguration, and we place a pancreatic stent before thermally coagulating around the pancreatic orifice.
The aim with a pancreatic or biliar sphincterotomy and stent placement is to enhance the technical success and decrease the complications of endoscopic ampullectomy[4,13,70,79-81]. However, a preresection sphincterotomy has some drawbacks. First, en bloc resection can be more difficult and will hinder total histologic evaluation of the resected specimen as result of thermal injury. Secondly, it may increase risks of bleeding, perforation and tumor seeding[82].
Usually, a meticulous inspection of the ampullectomy site allows identification of focal biliary and pancreatic orifices within the duodenal wall. Otherwise, secretin administration can produce juice flow to identify the pancreatic orifice. A 5 French pancreatic stent placement is advised to decrease the incidence and severity of pancreatitis[6,9,81,83,84]. Therefore, pancreatic duct stenting after endoscopic ampullectomy appears recomendable[74]. If ERCP or prior MRCP have demonstrated a pancreas divisum, pancreatic duct stenting is usually not necessary. Acute cholangitis after papillectomy is uncommon[76], and prophylactic biliary stent placement is generally unnecessary. However, we often perform either a biliary sphincterotomy or a prophylactic biliary stent is placed to minimize this probability. Biliary stenting may ensure the correct bile drainage if major bleeding occurs. The pancreatic and biliary stents are generally removed two or three weeks later, at which time any suspicious-appearing residual polypoid tissue can be removed to ensure complete excision.
Complications after endoscopic ampullectomy include bleeding (0%-25%), pancreatitis (0%-25%), perforation (0%-4%), papillary stenosis (0%-8%) and cholangitis (0%-2%)[4,6,9,11,13,62,85-87]. Pancreatitis, perforation and delayed bleeding are the most severe complications[62]. The overall complication rate is around 15%[4,11,49,70,80]. Ampullectomy-related mortality is exceptional, occurring in 0.3%[76].
The duodenal wall has a high vascularization. Bleeding can habitually stopped by hemostatic procedures (e.g., adrenaline injection, APC, clipping)[88]. If substantial bleeding is expected then, biliary stent placement is useful to avoid cholagitis. If massive bleeding occurs, urgent arteriography is probably the best diagnostic and treatment option. In patients with a high risk of cardiovascular incidents aspirin may be continued; however, anti-coagulants agents should be discontinued.
Perforation is usually retroperitoneal. Therefore, if perforation is suspected (endoscopic features, ongoing pain) a CT is more sensitive than simple radiology. Not all cases of perforation need surgical treatment, selected patients can be treated conservatively (intravenous antibiotics, gut rest)[6,14]. In anycase, a multi-disciplinary management is imperative to reach the best result.
The success rates for endoscopic resection of AA is high (range: 45%-92%), with recurrence rates of 0%-33%[9,89]. Intraductal adenoma growth had less favorable outcomes compared with adenomas without intraductal growth[15]. Predictors of success include: (1) lack of a genetic predisposition to adenoma formation (e.g., FAP); (2) age > 48 years; (3) male sex; and (4) lesion size < 2.4 cm[70].
After ampullectomy patients should remain fasting for 4-12 h. Then, they are discharged home on a liquid diet and later continue with a normal diet. To reduce the risk of ductal lesion, the pancreatic stent should be removed in 2 wk.
Adenoma recurrence can occur in up to 25% of cases despite of complete removal during the index procedure[6,9,76]. In the absence of symptoms, surveillance endoscopy can be accomplished using a side-viewing duodendoscope without ERCP. Intervals change based on the histology and margin status of the resected lesion, history of FAP, patient age and comorbidities.
Recommended intervals (Table 1): (1) If there was no residual polyp after the primary resection: endoscopy 3 mo later; (2) If the result is negative for residual adenoma: surveillance 1 year later; (3) Beyond this, the yield of long-term surveillance in sporadic AA is unknown. We usually perform surveillance every 3-5 years; and (4) Given the risk for metachronous duodenal lesions, patients with FAP should undergo routine surveillance every 3 years.
| Surveillance | |
| No residual polyp after the primary resection | 3 mo later |
| If negative result for residual adenoma | 1 yr later |
| Beyond this | every 3-5 yr |
| Patients with FAP | every 3 yr |
Advances in endoscopy, EUS and ERCP have influenced the management to patients with ampullary lesions. Endoscopic ampullectomy has replaced surgical interventions for the treatment of AA without ductal extension. Endoscopic ampullectomy has lower morbidity and mortality rates than surgical approaches. Disadvantages include: difficult technique, few experienced endoscopists, several procedures to achieve total resection, moderate recurrence rates (30%), and, as with surgical ampullectomy, the need for postprocedure endoscopic surveillance. The best technique for endoscopic ampullectomy is subject to the adenoma size. En bloc resection is recomended for lesions confined to the papilla. Endoscopic ampullectomy is an effective and safe treatment for AA in experienced endoscopist but, the endoscopist must be alert to potential complications. Long-term follow-up information is required to clarify the appropiate surveillance interval for patients with sporadic AA.
P- Reviewer: Kobayashi N, Meister T, Midorikawa Y, Tepes B, Vezakis A S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, Hochwald SN. Contemporary results with ampullectomy for 29 “benign” neoplasms of the ampulla. J Am Coll Surg. 2008;206:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Sato T, Konishi K, Kimura H, Maeda K, Yabushita K, Tsuji M, Miwa A. Adenoma and tiny carcinoma in adenoma of the papilla of Vater--p53 and PCNA. Hepatogastroenterology. 1999;46:1959-1962. [PubMed] [Cited in This Article: ] |
| 3. | Baker HL, Caldwell DW. Lesions of the ampulla of Vater. Surgery. 1947;21:523-531. [PubMed] [Cited in This Article: ] |
| 4. | Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 244] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Beger HG, Staib L, Schoenberg MH. Ampullectomy for adenoma of the papilla and ampulla of Vater. Langenbecks Arch Surg. 1998;383:190-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Matsumoto T, Iida M, Nakamura S, Hizawa K, Yao T, Tsuneyoshi M, Fujishima M. Natural history of ampullary adenoma in familial adenomatous polyposis: reconfirmation of benign nature during extended surveillance. Am J Gastroenterol. 2000;95:1557-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Demetriades H, Zacharakis E, Kirou I, Pramateftakis MG, Sapidis N, Kanellos I, Betsis D. Local excision as a treatment for tumors of ampulla of Vater. World J Surg Oncol. 2006;4:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Jung MK, Cho CM, Park SY, Jeon SW, Tak WY, Kweon YO, Kim SK, Choi YH. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568-2574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Ponchon T, Berger F, Chavaillon A, Bory R, Lambert R. Contribution of endoscopy to diagnosis and treatment of tumors of the ampulla of Vater. Cancer. 1989;64:161-167. [PubMed] [Cited in This Article: ] |
| 12. | Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Bohnacker S, Seitz U, Nguyen D, Thonke F, Seewald S, deWeerth A, Ponnudurai R, Omar S, Soehendra N. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Katsinelos P, Paroutoglou G, Kountouras J, Beltsis A, Papaziogas B, Mimidis K, Zavos C, Dimiropoulos S. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc. 2006;20:608-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Jeanniard-Malet O, Caillol F, Pesenti C, Bories E, Monges G, Giovannini M. Short-term results of 42 endoscopic ampullectomies: a single-center experience. Scand J Gastroenterol. 2011;46:1014-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Ceppa EP, Burbridge RA, Rialon KL, Omotosho PA, Emick D, Jowell PS, Branch MS, Pappas TN. Endoscopic versus surgical ampullectomy: an algorithm to treat disease of the ampulla of Vater. Ann Surg. 2013;257:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Ito K, Fujita N, Noda Y. Endoscopic diagnosis and treatment of ampullary neoplasm (with video). Dig Endosc. 2011;23:113-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Treitschke F, Beger HG. Local resection of benign periampullary tumors. Ann Oncol. 1999;10 Suppl 4:212-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Allgaier HP, Schwacha H, Kleinschmidt M, Thimme R, Schöffel U, Blum HE. Ampullary hamartoma: A rare cause of biliary obstruction. Digestion. 1999;60:497-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Park SH, Kim YI, Park YH, Kim SW, Kim KW, Kim YT, Kim WH. Clinicopathologic correlation of p53 protein overexpression in adenoma and carcinoma of the ampulla of Vater. World J Surg. 2000;24:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Heiskanen I, Kellokumpu I, Järvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, Hamilton SR. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980-1982. [PubMed] [Cited in This Article: ] |
| 26. | Vasen HF, Bülow S, Myrhøj T, Mathus-Vliegen L, Griffioen G, Buskens E, Taal BG, Nagengast F, Slors JF, de Ruiter P. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut. 1997;40:716-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg. 1988;207:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 105] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Shapiro PF, Lifvendahl RA. Tumors of the Extrahepatic Bile-ducts. Ann Surg. 1931;94:61-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 446] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Ashkar K, Deeb LS, Bikhazi K, Arnaout MS. Unusual manifestation of an ampullary tumor presenting with severe upper gastrointestinal bleeding. Digestion. 1999;60:583-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Sharp KW, Brandes JL. Local resection of tumors of the ampulla of Vater. Am Surg. 1990;56:214-217. [PubMed] [Cited in This Article: ] |
| 33. | Taxier M, Sivak MV, Cooperman A. Villous adenoma of the ampulla of Vater. Gastrointest Endosc. 1979;25:155-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Greco S, Cassinotti A, Massari A, Bossi I, Trabucchi E, Bianchi Porro G. Isolated ampullary adenoma causing biliary obstruction. J Gastrointestin Liver Dis. 2008;17:329-332. [PubMed] [Cited in This Article: ] |
| 35. | Sand JA, Nordback IH. Transduodenal excision of benign adenoma of the papilla of Vater. Eur J Surg. 1995;161:269-272. [PubMed] [Cited in This Article: ] |
| 36. | Sato T, Konishi K, Kimura H, Maeda K, Yabushita K, Tsuji M, Miwa A. Necrotizing acute pancreatitis caused by tiny carcinoma in adenoma in Vater’s papilla. Gastrointest Endosc. 1999;50:672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Akatsu T, Aiura K, Takahashi S, Kameyama K, Kitajima M, Kitagawa Y. Recurrent pancreatitis caused by ampullary carcinoma and minor papilla adenoma in familial polyposis: report of a case. Surg Today. 2008;38:440-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Murakami Y, Uemura K, Hayashidani Y, Sudo T, Sueda T. Relapsing acute pancreatitis due to ampullary adenoma in a patient with familial adenomatous polyposis. J Gastroenterol. 2006;41:798-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Martin JA, Haber GB, Kortan PP, Raijman I, Abedi M, DuVall GA, Dorais JA, Silva S. Endoscopic snare ampullectomy for resection of benign ampullary neoplasms. Gastrointest Endosc. 1997;45:AB139. [DOI] [Cited in This Article: ] |
| 40. | Sauvanet A, Chapuis O, Hammel P, Fléjou JF, Ponsot P, Bernades P, Belghiti J. Are endoscopic procedures able to predict the benignity of ampullary tumors? Am J Surg. 1997;174:355-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Cahen DL, Fockens P, de Wit LT, Offerhaus GJ, Obertop H, Gouma DJ. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990;36:588-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Yamaguchi K, Enjoji M. Adenoma of the ampulla of Vater: putative precancerous lesion. Gut. 1991;32:1558-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Yamaguchi K, Enjoji M. Carcinoma of the ampulla of vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer. 1987;59:506-515. [PubMed] [Cited in This Article: ] |
| 45. | Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery. 2000;128:694-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Clary BM, Tyler DS, Dematos P, Gottfried M, Pappas TN. Local ampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery. 2000;127:628-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Lee SY, Jang KT, Lee KT, Lee JK, Choi SH, Heo JS, Paik SW, Rhee JC. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Bourgeois N, Dunham F, Verhest A, Cremer M. Endoscopic biopsies of the papilla of Vater at the time of endoscopic sphincterotomy: difficulties in interpretation. Gastrointest Endosc. 1984;30:163-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Shemesh E, Nass S, Czerniak A. Endoscopic sphincterotomy and endoscopic fulguration in the management of adenoma of the papilla of Vater. Surg Gynecol Obstet. 1989;169:445-448. [PubMed] [Cited in This Article: ] |
| 50. | Rattner DW, Fernandez-del Castillo C, Brugge WR, Warshaw AL. Defining the criteria for local resection of ampullary neoplasms. Arch Surg. 1996;131:366-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Tio TL, Sie LH, Verbeek PC, Dé Wit LT, Tytgat GN. Endosonography in diagnosing and staging duodenal villous adenoma. Gut. 1992;33:567-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Azih LC, Broussard BL, Phadnis MA, Heslin MJ, Eloubeidi MA, Varadarajulu S, Arnoletti JP. Endoscopic ultrasound evaluation in the surgical treatment of duodenal and peri-ampullary adenomas. World J Gastroenterol. 2013;19:511-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Lim GJ, Devereaux BM. EUS in the assessment of ampullary lesions prior to endoscopic resection. Tech Gastroint Endosc. 2010;12:49–52. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Itoh A, Goto H, Naitoh Y, Hirooka Y, Furukawa T, Hayakawa T. Intraductal ultrasonography in diagnosing tumor extension of cancer of the papilla of Vater. Gastrointest Endosc. 1997;45:251-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, Stotland B, Rosato EF, Morris JB, Eckhauser F. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK. Reappraisal of endosonography of ampullary tumors: correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Manta R, Conigliaro R, Castellani D, Messerotti A, Bertani H, Sabatino G, Vetruccio E, Losi L, Villanacci V, Bassotti G. Linear endoscopic ultrasonography vs magnetic resonance imaging in ampullary tumors. World J Gastroenterol. 2010;16:5592-5597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Baillie J. Endoscopic ampullectomy. Am J Gastroenterol. 2005;100:2379-2381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Suzuki K, Kantou U, Murakami Y. Two cases with ampullary cancer who underwent endoscopic excision. Prog Dig Endosc. 1983;23:236-239. [Cited in This Article: ] |
| 60. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Okano N, Igarashi Y, Miura T. The study of early complications of endoscopic papillectomy of the tumor with duodenal major papilla. Tando (Journal of Japan Biliary Association). 2007;21:623–629. [Cited in This Article: ] |
| 62. | Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Trikudanathan G, Njei B, Attam R, Arain M, Shaukat A. Staging accuracy of ampullary tumors by endoscopic ultrasound: meta-analysis and systematic review. Dig Endosc. 2014;26:617-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Menzel J, Hoepffner N, Sulkowski U, Reimer P, Heinecke A, Poremba C, Domschke W. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT--a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49:349-357. [PubMed] [Cited in This Article: ] |
| 65. | Artifon EL, Couto D, Sakai P, da Silveira EB. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Standards of Practice Committee, Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T, Baron TH. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849-854. [PubMed] [Cited in This Article: ] |
| 67. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 68. | de Castro SM, van Heek NT, Kuhlmann KF, Busch OR, Offerhaus GJ, van Gulik TM, Obertop H, Gouma DJ. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Hopper AD, Bourke MJ, Williams SJ, Swan MP. Giant laterally spreading tumors of the papilla: endoscopic features, resection technique, and outcome (with videos). Gastrointest Endosc. 2010;71:967-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649-669. [PubMed] [Cited in This Article: ] |
| 71. | Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 56] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Bassan M, Bourke M. Endoscopic ampullectomy: a practical guide. J Interv Gastroenterol. 2012;2:23-30. [PubMed] [Cited in This Article: ] |
| 73. | Aiura K, Imaeda H, Kitajima M, Kumai K. Balloon-catheter-assisted endoscopic snare papillectomy for benign tumors of the major duodenal papilla. Gastrointest Endosc. 2003;57:743-747. [PubMed] [Cited in This Article: ] |
| 74. | Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, Koshita S, Kanno Y, Ogawa T, Kato Y. Impact of technical modification of endoscopic papillectomy for ampullary neoplasm on the occurrence of complications. Dig Endosc. 2012;24:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Menees SB, Schoenfeld P, Kim HM, Elta GH. A survey of ampullectomy practices. World J Gastroenterol. 2009;15:3486-3492. [PubMed] [Cited in This Article: ] |
| 76. | El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Espinel J, Pinedo E, Vaquero LM, Alvarez-Cuenllas B. Ampulectomia endoscópica en el diagnóstico y tratamiento de tumores ampulares. Endoscopy. 2013;45:A84. [DOI] [Cited in This Article: ] |
| 78. | Lambert R, Ponchon T, Chavaillon A, Berger F. Laser treatment of tumors of the papilla of Vater. Endoscopy. 1988;20 Suppl 1:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Bertoni G, Sassatelli R, Nigrisoli E, Bedogni G. Endoscopic snare papillectomy in patients with familial adenomatous polyposis and ampullary adenoma. Endoscopy. 1997;29:685-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Norton ID, Geller A, Petersen BT, Sorbi D, Gostout CJ. Endoscopic surveillance and ablative therapy for periampullary adenomas. Am J Gastroenterol. 2001;96:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, Irie J. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Lee SK, Kim MH, Seo DW, Lee SS, Park JS. Endoscopic sphincterotomy and pancreatic duct stent placement before endoscopic papillectomy: are they necessary and safe procedures? Gastrointest Endosc. 2002;55:302-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 84. | Singh P, Das A, Isenberg G, Wong RC, Sivak MV, Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 85. | Vogt M, Jakobs R, Benz C, Arnold JC, Adamek HE, Riemann JF. Endoscopic therapy of adenomas of the papilla of Vater. A retrospective analysis with long-term follow-up. Dig Liver Dis. 2000;32:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Patel R, Davitte J, Varadarajulu S, Wilcox CM. Endoscopic resection of ampullary adenomas: complications and outcomes. Dig Dis Sci. 2011;56:3235-3240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Nguyen N, Shah JN, Binmoeller KF. Outcomes of endoscopic papillectomy in elderly patients with ampullary adenoma or early carcinoma. Endoscopy. 2010;42:975-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Espinel J, Pinedo E, Bailador C. Clipping of a post-sphincterotomy bleeding. Rev Esp Enferm Dig. 2010;102:385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006;63:292-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |