Published online Sep 20, 2022. doi: 10.5662/wjm.v12.i5.365
Peer-review started: February 28, 2022
First decision: June 16, 2022
Revised: June 30, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: September 20, 2022
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has affected the entire world, causing the coronavirus disease 2019 (COVID-19) pandemic since it was first discovered in Wuhan, China in December 2019. Among the clinical presentation of the disease, in addition to fever, fatigue, cough, dyspnea, diarrhea, nausea, vomiting, and abdominal pain, infected patients may also experience neurological and psychiatric repercussions during the course of the disease and as a post-COVID-19 sequelae. Thus, headache, dizziness, olfactory and gustatory dysfunction, cerebrovascular disorders, neuromuscular abnor
Core Tip: Severe acute respiratory syndrome coronavirus 2 infection may also involve neurological and psychiatric manifestations, both by the viral action itself and by social distancing and quarantine. Headache, dizziness, cerebrovascular disorders, olfactory and gustatory dysfunction, neuromuscular abnormalities, anxiety, depression, and post-traumatic stress disorder may occur in this setting. Supporting these repercussions, this virus is able to reach the central nervous system by the interaction between the angiotensin-converting enzyme 2 and the transmembrane protease serine 2 expressed in the host nerve cells, and the viral spike glycoprotein. Finally, the management of these patients is complex and we review current evidence on the subject.
- Citation: da Silva Júnior RT, Santos Apolonio J, Cuzzuol BR, da Costa BT, Silva CS, Araújo GRL, Silva Luz M, Marques HS, Santos LKS, Pinheiro SLR, Lima de Souza Gonçalves V, Calmon MS, Freire de Melo F. COVID-19 neuropsychiatric repercussions: Current evidence on the subject. World J Methodol 2022; 12(5): 365-380
- URL: https://www.wjgnet.com/2222-0682/full/v12/i5/365.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i5.365
The coronavirus disease 2019 (COVID-19) outbreak has affected the whole world, causing fear and concern due to its transmissibility and severe life-threatening conditions. This pandemic infectious disease was first discovered in Wuhan, China, in December 2019[1]. Since then, the number of cases has increased, spreading rapidly globally and becoming a major pandemic disease[2]. By February 1, 2022, the World Health Organization confirmed more than 370 million cases worldwide, leading to 5658702 deaths[3].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a β-coronavirus with positive spherical single-stranded RNA and spike proteins that project on the surface of the virion, which is characterized by its crown-shaped morphology[4]. Common symptoms are fever, fatigue, cough, dyspnea, diarrhea, chest tightness, nausea, vomiting, sputum production, anorexia, pharyngalgia, hemoptysis, and abdominal pain[5]. Frequently, SARS-CoV-2 contamination is associated with the nasopharyngeal and pulmonary tracts. However, important findings show that manifestations of this virus can be found in the central nervous system (CNS).
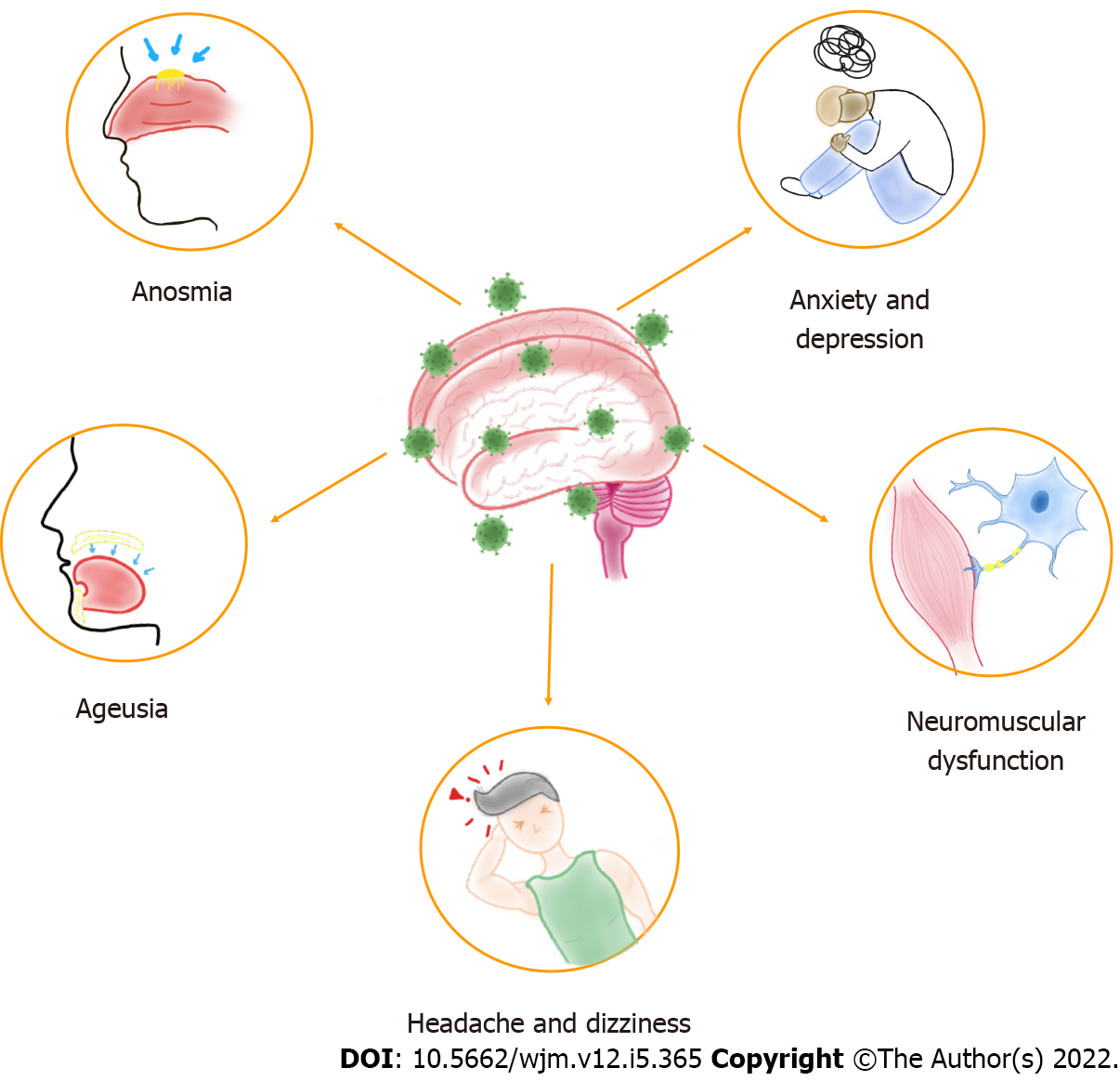
Neurological alterations have been described in patients with COVID-19, which vary from mild to fatal effects and can occur in severe or asymptomatic infection[6]. On the other hand, the global effects of SARS-CoV-2 infection result in various viral-related physical and mental health problems[7]. Thus, physical and social isolation, financial stress, and fear of contagion contribute to this scenario[8]. Therefore, this infection can present neuropsychiatric repercussions, such as headache, dizziness, anosmia, ageusia, neuromuscular dysfunction, anxiety, and depression[9], shown in Figure 1, in addition to other symptoms related to physiological and psychiatric changes, such as post-traumatic stress disorder (PTSD) and neuropsychiatric syndromes[8]. These manifestations seem to be caused as much by the infection itself as by social distancing and quarantine, which means that specific therapy should be used according to each case, seeking the most efficient healing process.
Therefore, this review describes the reported CNS manifestations associated with COVID-19, in order to help professionals who treat these patients, review the manner in which the virus reaches the CNS, and the intervention possibilities available to date in the literature.
For this minireview, the authors surveyed relevant and current articles published in the United States National Library of Medicine (PubMed). The descriptors used were COVID-19; SARS-CoV-2, coronavirus; angiotensin-converting enzyme 2 (ACE2), central nervous system, neuroimmune, cytokine storm, pathophysiology, neuroinvasion, neurological symptoms, neurological manifestations, olfactory dysfunction, gustatory dysfunction, ischemic stroke, hemorrhagic stroke, Guillain-Barré syndrome, neuropsychiatric symptoms, mental health, mental suffering, psychiatric disorder, and quarantine. The eligibility criteria were based on the discussion of aspects related to the neuropsychiatric repercussions of SARS-CoV-2 infection, dealing with everything from viral neuroinvasive mechanisms to neurological and psychiatric manifestations, due to the infection itself or to the need for full isolation in the read full-text. Thus, 26035 articles were found in the database, of which 109 complied with the inclusion criteria. The exclusion criteria were articles that did not address the topics in the title and/or abstract, or were written in languages other than English. The search was complemented by a manual search of the references of the included articles to identify additional references, 13 of which were added later, totaling 122 articles included in this review.
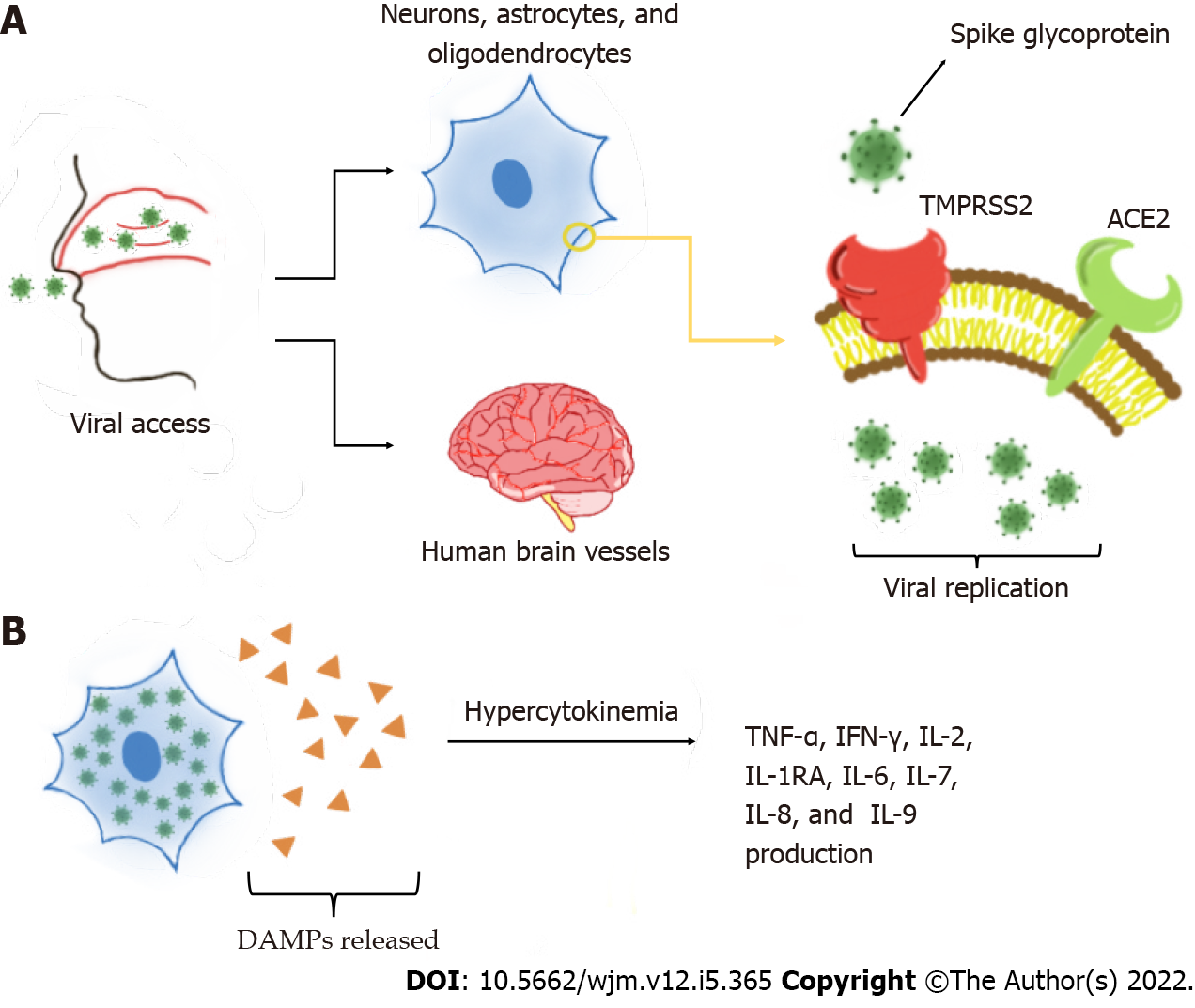
One of the main mechanisms of neurological invasion of SARS-CoV-2 is through ACE2 receptors on host cells[6,10]. Several studies have shown the presence of ACE2 protein in human brain vessels, mainly in dopaminergic neurons, astrocytes, oligodendrocytes, and neurons[11-13]. ACE2 has also been observed in the substantia nigra, ventricles, middle temporal gyrus, posterior cingulate cortex, and olfactory bulb[11,13]. One study reported that ACE2 is more highly expressed in neuronal cell bodies than in axons and dendrites[14]. During infection, transmembrane protease serine 2 (TMPRSS2) activates the spike (S) glycoprotein on the SARS-CoV-2 envelope, which allows the virion to bind to ACE2 receptors[12,15]. TMPRSS2 is also found in oligodendrocytes and astrocytes located in the substantia nigra, cortex, and endothelial cells of cerebral capillaries[6,14], and is fundamental in the priming and activation of S proteins, which leads to membrane fusion. This interaction is responsible for SARS-CoV-2 entry on the CNS[14,16]. Later, the virus may affect the nervous system by disturbing the renin angiotensin system[11]. This process is exacerbated by slower circulation in the brain capillaries, which intensifies the interaction between the viral S glycoprotein with the ACE2 on brain cells[9,16]. Thus, CoV interacts with ACE2 expressed in the capillary endothelium, causing neuronal death and neurodegeneration[9,18].
Compared with SARS-CoV, SARS-CoV-2 has higher affinity to ACE2[15]. This enzyme is also known as a cardiocerebral vascular protection factor, and influences blood pressure regulation and anti-atherosclerosis mechanism, in view of its vasoconstrictor function and pro-inflammatory effects[9,19]. When the virus binds to the enzyme, it may cause elevated blood pressure and increase the risk of arterial wall rupture, cerebral hemorrhage, and ischemic stroke[13,17]. On other hand, ACE2 and TMPRSS2 have been detected in the nasal mucosa, one of the main mechanisms of entry into the brain[15,20]. Once the infection of the olfactory system occurs, the virus may be internalized in the nerve by endocytosis via the olfactory bulb and be transported retrogradely and disseminated to the brain via the cribriform plate[6,12,20].
SARS-CoV-2 may also affect the CNS indirectly, as the virus provokes alveolar and lung tissue damage[17,21]. This inflammation and edema caused by lung invasion disturbs oxygen exchange and results in hypoxemia. Thus, this scenario may lead to increased anaerobic metabolism in brain cells, brain hypoxia with vasodilation, hyperemia, ischemia, and brain edema and injury[17,20,21].
As previously mentioned, the virus can access the CNS through the olfactory nerve and also through the hematoretinal route. However, there is another potential pathway that allows CNS infection, which is via the blood-brain barrier (BBB), which occurs through hematogenous dissemination[22]. The BBB is one of the body's protections against disturbances in the nervous system. It is composed of endothelial cells, astrocytes, microglia and neurons, which act together. Under normal circumstances, these cells accurately regulate what enters and leaves the nervous system. However, in pro-inflammatory situations, such as that caused by SARS-CoV-2, this homeostasis is disturbed, which may be the genesis of virus entry into the CNS[23-25].
One factors that has been extensively studied currently is what causes this damage to the BBB, enabling infection in the CNS. The most likely one is the hyperinflammatory situation caused by the cytokine storm[1]. When viral replication occurs, damage-associated molecular patterns, which induce inflammatory states in neighboring cells through Toll-like receptors, are released. These receptors promote several cytokine production pathways. However, in COVID-19, there is hypercytokinemia. The main cytokines involved in this exacerbated process are tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin 2 (IL-2), IL-1RA, IL-2, IL-6, IL-7, IL-8, IL-9, and the granulocyte-macrophage colony-stimulating factor. These cytokines ultimately potentiate the activation of immune system cells, creating a cycle of increasing and perpetuating inflammation[26,27]. These processes are represented in Figure 2. Severe patients can also present with elevated levels of IL-17 compared to non-severe patients[28]. A comparative study showed a particularly strong inflammatory response triggered with the activation of this cytokine[29].
This cytokine storm damages the vascular endothelial cells of the CNS, affecting the integrity of tight junction proteins in the BBB, allowing the virus to enter. In addition to increased cytokines, the virus itself has cytopathic power, which can lead to pathogenic inflammation with cellular damage in the CNS including edema, ischemia, bleeding, and neurodegenerative disorders[1,30]. In pathological situations, the migration of cells from the immune system to the CNS is increased and in severe SARS-CoV-2 infection, it is even greater. This is supplanted in histopathological examinations of the brain parenchyma where large numbers of macrophages and lymphocytes were found[27].
Another factor that corroborates this increase in cell migration is that in the presence of damage to endothelial cells, the number of intercellular adhesion molecules increases. This favors the entry of the virus into the CNS, which is transported by cells of the immune system through a mechanism known as the “trojan horse” in which the virus enters the nervous system inside the host cell[31,32].
SARS-CoV-2 infection can reach the CNS through the olfactory tract and access the cortex, basal ganglia and midbrain, which may be affected during propagation[33], supporting the existence of neurological symptoms as headache, anosmia, dysgeusia, dizziness, and impaired consciousness[34,35].
Although this symptom is very nonspecific, several studies have reported the prevalence of headache in patients infected with SARS-CoV-2. The symptom may be present at the beginning of the disease, or even be the initial presentation of the clinical picture, as it may also be present after resolution of the infection[9]. In addition, this symptom may be related to other diseases present in the patient, and therefore, the prevalence varies greatly according to the work[36]. One study[28] indicated a combined prevalence of headache in about 8% of patients, whereas others reported higher numbers such as 20%[37] and 25%[38], as well as variation from 0.6% to 70.3%[39,40]. Accordingly, among the neurological effects reported in COVID-19 infection, especially headache, may be the result of complications of the viral infection, the host immune response, the presence of severe installed disease or even the drug therapy used[41].
The prevalence of dizziness is estimated to be about 8%[42] to 9%[5], which indicates a combined overall prevalence of 8.77% in a systematic review that analyzed neurological symptoms in patients infected with SARS-CoV-2[43]. As with headache, studies vary in determining the period in which dizziness appears in the clinical picture. However, a retrospective and observational case series reports dizziness as the main neurological symptom[44]. This is not a surprise, after all, since dizziness is historically reported in patients with viral infections. This symptom has been proposed to result from the neuroinvasive potential of the virus, such as direct injury by binding to the ACE2, or even by hypoxia and coagulation disorders[45]. Because it is nonspecific, it is important that the healthcare team perform a thorough investigation to determine its cause, given that it can be due to acute labyrinthitis, acute otitis media, vestibular neuritis, or even stroke due to COVID-19[46,47].
In retrospective studies that analyzed the incidence of neurological symptoms in patients with COVID-19, a range of 3.3% to 19.6% was reported for disturbances of consciousness/delusion[48,49]. The cause of this involvement is still poorly understood, and may even be related to the post-inflammation inflammatory state, meningoencephalitis and encephalopathy, or may just be a sequela after a traumatic event[48]. Thus, a long-term follow-up is necessary for these patients, so that the real cause of this condition can be investigated with a detailed clinical history and serial imaging examinations.
Viral neuroinvasion and subsequent central neuronal injury have been proposed to contribute to the pathogenesis of the disease. The interaction of SARS-CoV-2 with ACE2 receptors may be related to the episodes of intracerebral hemorrhage found in some cases, resulting in receptor inactivation and consequent dysfunction in blood pressure regulation[50]. In patients who already have pre-existing vascular risk factors, ischemic stroke is related to late complications in the severity of COVID-19 infection. The elderly with alterations in vascular hemodynamics resulting from age or associated pathology, once again, are groups that deserve special attention for the involvement of these injuries[51-54].
Studies have observed that patients presented neurological symptoms on average 3 to 4 d after the onset of respiratory symptoms, with hemifacial paresis, dysarthria, hemiparesis, loss of level of consciousness, hemiparesthesia and ataxia being symptoms found less frequently[51,52,55,56]. One study reported that the sex-based distribution of patients affected by COVID-19 shows that female patients report more central nervous system-related symptoms than males. This sex-based difference may be attributed to humoral and innate immune responses to viral infections that are more pronounced in women than in men[54-57]. Yet, autopsy results from COVID-19 patients showed that brain tissue was hyperemic and swollen and that some neurons degenerate[55,58].
Although they are now among the most well-known symptoms during SARS-CoV-2 infection, olfactory dysfunctions (OD), mainly hyposmia and anosmia, were initially seen as less relevant conditions in the pandemic context. Thus, OD throughout the pandemic became part of the symptoms that carry a warning sign of a possible ongoing infection, even when they appear in isolation[59].
Hyposmia, reduced sense of smell, and anosmia, the complete loss of smell, are common in patients with COVID-19. The OD can be evidenced subjectively, when the patient reports any degree of alteration, or objectively, when specific tests are applied in order to evidence the conditions of each individual. These parameters have guided researchers worldwide to carry out studies in which OD was evaluated in patients with COVID-19. In this context, a meta-analysis involving 3563 patients found that 47% of individuals had a self-reported loss of smell. In addition, researchers show that OD is more frequent in women and young patients. However, despite the relationship with the infectious condition, studies suggest that OD is not related to the severity of the condition[59,60].
The mechanisms that can cause OD have not yet been fully defined. In this context, studies point to several possibilities that can lead to olfactory impairment, such as conductive loss due to edema in the olfactory cleft, injury to the respiratory epithelium - due to the local inflammatory response with an increase in pro-inflammatory cytokines and chemokines such as IL-6 and IFN-γ, or lesion in the olfactory bulb, with neuronal damage. Symptoms begin on average within the 1st week of infection, vary in duration, and can last for weeks or months[61,62].
The impairment of smell has a direct impact on the quality of life of the individual, since it can make it difficult to recognize odors in food that indicate its disposal and odors associated with risks, such as flammable or toxic substances, for example. In addition, it can cause impairment in social interactions, and several studies associate olfactory dysfunction with a higher risk of developing depressive disorders[63].
Gustatory dysfunction (GD), mainly hypogeusia and ageusia, are prevalent symptoms in infected individuals. They are mostly associated with OD, but they may manifest in isolation in some cases, as pointed out by a systematic review that revealed a combined prevalence of GD of 43.93% in SARS-CoV-2-positive individuals[64]. Like OD, GD is more prevalent in young and female patients[65]. Fur
The gustatory function is able to identify sweet, salty, sour, bitter, and umami flavors. Among patients infected by SARS-CoV-2 with DG, sweet and sour tastes had the most altered sensitivity[65]. Among the DG, hypogeusia, mild to moderate, is more common than ageusia. For example, an Italian study with 72 participants found that hypogeusia occurred in 47.1% of cases, while ageusia occurred in only 1.4% of patients[67]. Like OD, GD is a common manifestation mainly in the 1st week of symptoms and has a resolution in a variable period with most patients completely regressing in approximately 10 d[68].
Guillain-Barré syndrome (GBS) is considered a post-infectious and immune-mediated syndrome characterized mainly by manifestations such as rapid, progressive, and symmetrical limb weakness, impairment of tendon reflexes, which may be reduced or absent and to sensory impairment. Their subtypes are acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy, and acute motor and sensory axonal neuropathy, in addition to Miller Fisher syndrome, a variant. It is usually associated with respiratory or gastrointestinal infections caused by Campylobacter jejuni, the most common agent, Mycoplasma pneumoniae, Haemophilus influenzae, Epstein-Barr virus, influenza A, and Zika virus, for example[69-71].
However, during the COVID-19 pandemic, an increase in the number of GBS cases associated with individuals infected with SARS-CoV-2 was observed. In this context, a study conducted suggested an up to 2.6-fold increase in the baseline GBS case rate, from 0.93/100000/year to 2.43/100000/year. Furthermore, the authors noted that GBS associated with COVID-19 is more severe than those not associated with this virus[69]. In this sense, among patients positive for SARS-CoV-2, GBS is more prevalent in males and those aged over 60 years and, among the GBS subtypes, the most prevalent is AIDP[71-73].
As with other conditions arising from SARS-CoV-2 infection, the onset of GBS-related symptoms is variable, but studies suggest that the onset of symptoms occurs approximately during the 2nd week of infection, reinforcing the hypotheses of a post-infectious etiology of GBS. The causal mechanisms are still uncertain. However, there are hypotheses that relate the development of GBS to the cytokine storm that occurs during the second phase of infection, which usually occurs in the 2nd week of infection, related to the elevation of cytokines such as TNF-α, IL-1β, -6, -17 and IFN-γ, which are capable of causing tissue damage. In addition, there are hypotheses that relate GBS to autoimmune mechanisms by cross-reaction against ganglioside components of peripheral nerves, causing impairment of nerve structures and, consequently, the development of the syndrome[72,74,75].
A summary of the hypotheses and mechanisms related to development of the neurological manifestations of the SARS-CoV-2 infection, as well as prevalence/incidence of these repercussions, are shown in Table 1.
| Neurological repercussion | Hypotheses/mechanisms related to their development | Prevalence/incidence of manifestation |
| Headache | Complications of the viral infection, the host immune response, the presence of severe installed disease or even the drug therapy used[41,45] | The headache prevalence varies from 8%[56] to 25%[38] and 70.3%[40], according to the study |
| Dizziness | The direct injury by binding to the ACE2, or even by hypoxia and coagulation disorders may be related[45] | For dizziness, the prevalence is estimated to be around 8%[42] to 9%[5] |
| OD | Conductive loss due to edema in the olfactory cleft, injury to the respiratory epithelium or lesion in the olfactory bulb[61,62] | About 47% of individuals had a self-reported loss of smell. More frequent in women and young patients[59,60] |
| GD | Local inflammatory reactions and the relationship with cranial nerves VII, IX, and X[66] | About 43.93% of the patients[64]. More prevalent in young and female patients[65] |
| Disturbances of consciousness | Post-inflammatory state, meningoencephalitis, or may just be a sequela after a traumatic event[48] | A range of 3.3% to 19.6% in COVID-19 patients[48,49] |
| Acute cerebrovascular disease | Intracerebral hemorrhage can be caused by viral interaction with ACE2 receptors and ischemic stroke is related to late complications in the disease severity[50] | The incidence of ischemic stroke in patients with COVID-19 was reported to be between 0.9%[76] and 4.6%[77] |
| GBS | There are hypotheses that relate the development of GBS to the cytokine storm and autoimmune mechanisms by cross-reaction[74,75] | A study suggested an up to 2.6-fold increase in the baseline GBS case rate, from 0.93/100000/yr to 2.43/100000/yr |
Neurological complications following COVID-19 infection are still poorly understood due to the recent onset of the pandemic and the question remains whether neurological symptoms are a definite sequelae or just a late effect of the disease. As the virus attacks and grows in lung tissue, alveolar gas exchange is disrupted due to systemic inflammation and edema, which can cause hypoxia and acid accumulation. A study describing the autopsy of 8 confirmed cases with SARS-CoV-2 infection reported brain swelling and severe neuronal damage[78], as did another study, with 18 brain autopsies of patients testing positive for the viral infection, indicated alteration by hypoxia in the cerebellum and brain, with loss of neurons in the cerebral cortex and hippocampus[79]. In addition, recent studies have shown that the infection caused by SARS-CoV-2 affects the central nervous system and the peripheral nervous system, and also directly or indirectly damages neurons, which causes long-term neurological sequelae[80].
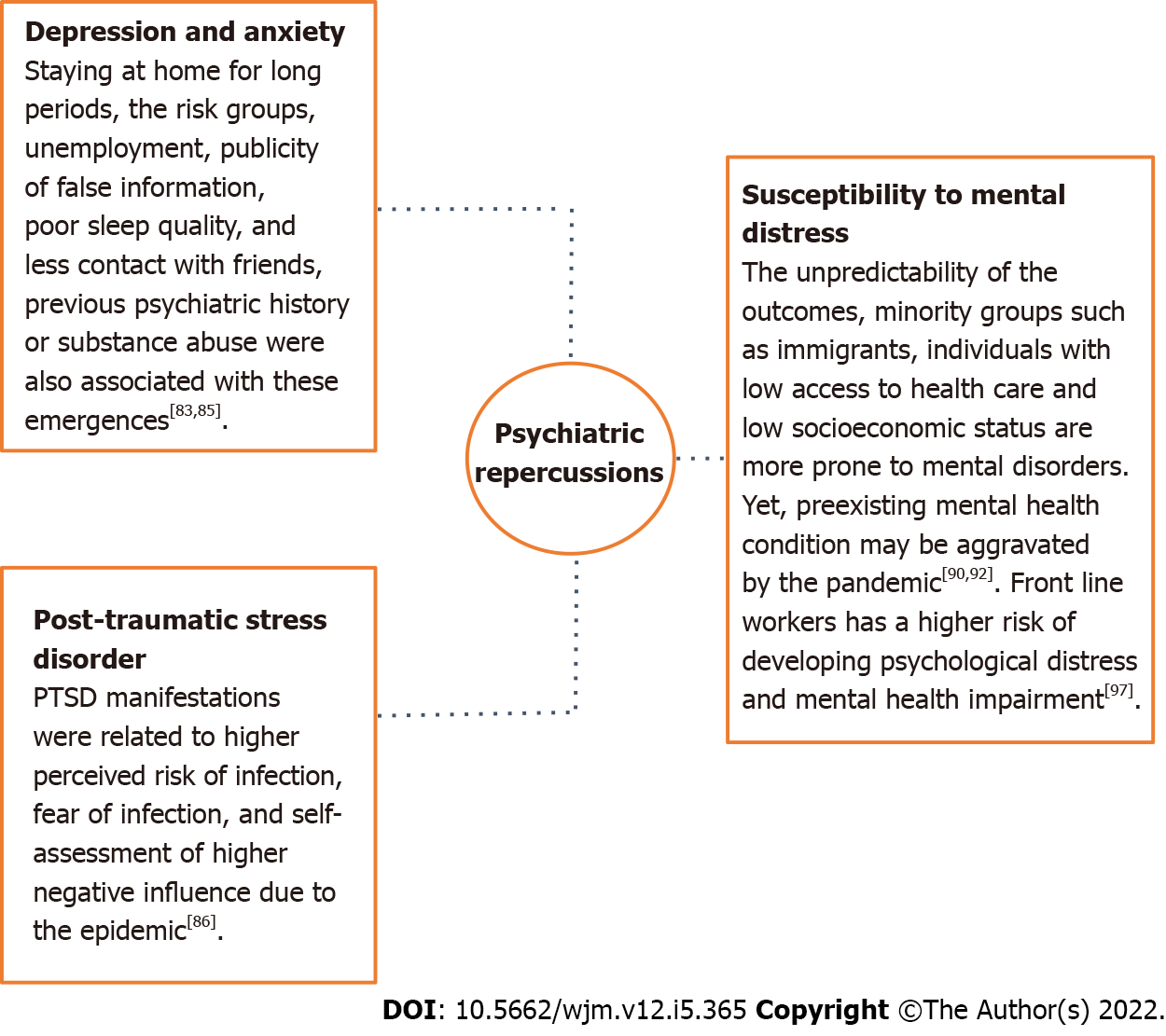
Along with the physical damages caused by SARS-CoV-2 infection, quarantine and social distancing significantly impacted the mental health of the population. In this sense, studies have demonstrated that during this period there was an increase in the rates of psychiatric manifestations such as irritability, stress, insomnia and depression[81], which can influence the individual's daily life even after the epidemic has ended.
The subjectivity of life demonstrates an impact in reference to the development of mental disorders which contributes to different mental suffering rates for each group of people[82]. The necessity of staying at home for long periods[83], the vulnerability of the risk groups, increase in unemployment rates and publicity of false information contribute to both the illness of the population and increased number of suicides cases[84]. Other factors such as living alone, having children, being a student or a health worker, poor sleep quality, family support lack, less contact with friends and previous psychiatric history or substance abuse were also associated with the emergence of depression and anxiety[85].
A study conducted with the Chinese population showed that of the 1.210 respondents, about 30% had severe to moderate anxiety symptoms and 17% had severe to moderate depression[83]. Similarly, an online cross-sectional study conducted in China included 1.456 participants and assessed factors that influenced the mental health of adults during the pandemic. Its results showed that loneliness, depression and anxiety were associated with more somatic symptoms and lower self-efficacy. In addition, depression was associated with fear of infection, excessive alcohol consumption, and longer screen time. Loneliness was associated with single, divorced or widowed marital status, low education, medication use and frequent going out[86]. Furthermore, a survey of the Belgian population aged 18 years to 65 years reported that in just 2 wk of isolation the stress of individuals increased by about 25%[87].
Literature reports also describe an increase in rates of PTSD in children, parents and even health care professionals after contact with infection, which demonstrates that subjective experience is also related to mental illness[88]. PTSD manifestations were related to higher perceived risk of infection, fear of infection, and self-assessment of higher negative influence due to the epidemic[86]. Corroborating, an electronic records cohort of 69 million individuals, of which 62.354 tested positive for infection, found that patients with COVID-19 had a higher incidence of unprecedented psychiatric diagnoses between 14-90 d after infection compared to other illnesses such as respiratory tract infections, skin infection, large bone fracture, urolithiasis, and cholelithiasis. Interestingly, previous psychiatric diagnoses also appear to be an independent risk factor for COVID-19[89].
The unpredictability of the outcomes of a possible infection can also increase susceptibility to mental distress, especially in people considered to be in the risk groups, which shows that subjective experience is an important predictor of the onset of psychological problems[90]. Studies performed during the pandemic reported a higher prevalence of mental disorders, such as anxiety in people with comorbidities or depression in individuals diagnosed with type 2 diabetes mellitus, when compared to the general population[91]. Similarly, minority groups such as immigrants, individuals with low access to health care and low socioeconomic status are more prone to mental disorders, since not only does the pandemic contribute to increasing marginalization and discrimination of these groups, but they are also considered more susceptible to infection and deaths caused by COVID-19[92]. In addition, women were also more affected than men by major depressive disorders and anxiety[93]. Violation of women's human rights with increasing rates of domestic violence and restrictions on access to prenatal health care services also contribute to greater mental illness in this group[94].
On the other hand, a preexisting mental health condition may be aggravated by the pandemic, as people diagnosed with mental disorders are considered more vulnerable to changes in their health status due to varying risk factors[95]. Interestingly, Pan et al[96] noted that people who did not have a psychiatric diagnosis of anxiety, depression, or obsessive-compulsive disorder prior to the pandemic reported an increase in symptoms related to these comorbidities. However, those individuals who already had a diagnosis of one of these disorders did not experience greater worsening of symptoms post-pandemic.
Healthcare professionals who worked on the front lines in the pandemic are a particularly affected population at higher risk of developing psychological distress and mental health impairment[97]. In this context, a cross-sectional study of 3852 healthcare professionals assessed the mental health of professionals who worked in the COVID-19 pandemic and SARS outbreak. The authors reported that health care workers achieved moderate and severe scores for symptoms of PTSD, anxiety, and depression[98]. A summary of highlights related to development of the psychiatric disorders in COVID-19 are shown in Figure 3.
The management of SARS-CoV-2 infection seems more complex when it involves the CNS. The dense parenchyma and impermeability of brain tissue, despite protecting the brain from the infectious process, may also hinder virus elimination. Still, the treatment of patients with neurological complications from this infection requires caution, because some drugs used in non-COVID-19 situations can lead to worsening of the disease-related acute respiratory syndrome, such as corticosteroids and immunosuppressant[99,100]. Moreover, viral damage can affect renal, immunological, hematological, hepatic, pulmonary and cardiac organ systems, as well as lead to pharmacokinetic changes that influence the absorption, distribution, metabolism and/or excretion of medications, such as psychotropic drugs. Susceptibility to side effects may be increased and adjustments in treatment regimens should potentially be considered[101], in addition to the pro-inflammatory, pro-thrombotic and arrhythmogenic implications of this infection[102]. Therefore, the approach to COVID-19-positive patients with neuropsychiatric repercussions still needs further long-term studies for evaluation and establishment of guidelines.
One study reports the requirement for appropriate neuroimaging protocols in coronavirus infections to detect encephalitis, leptomeningeal and vascular changes such as stroke, microhemorrhages and cerebral infarction[103]. It is important that appropriate therapies are applied at the correct time, and that assessment of adjacent comorbidities, as well as damage to other organs and general condition is done using the sequential organ failure assessment score, which influences the COVID-19 prognosis[104,105]. Severe individuals can present right levels of the inflammatory markers, as C-reactive protein and D-dimer, and administration of tissue plasminogen activator in these patients with ischemic stroke predicted worse prognostic[44,106]. Thrombectomy can be used, evaluating the risks and benefits of therapy, and antiplatelet and anticoagulant agents remain uncertain. Thus, due to the lack of definitive studies, it is recommended to follow the existing guidelines[104].
Patients with demyelinating conditions and mild infection may be acceptable to continue treatment and the interruption may be considered in the use of potent immunosuppressant with risk factors for severe disease, returning after 4 wk or complete remission of symptoms[107]. The remission time of olfactory and gustatory dysfunction is controversial in literature, with studies reporting spontaneous resolution in 1 to 3 wk[108]. On the other hand, the smell performance of SARS-CoV-2-positive patients with mild or no symptoms can also not recover completely after 4 mo or more of acute infection[109]. Therefore, this treatment is still uncertain, but studies point to benefits of practicing olfactory training for those with persistent symptoms. In addition, some studies evaluate the role of local corticosteroids in recovery, but there is no consensus[110]. A study evaluating the efficacy of locally applied steroids in the form of fluticasone nasal sprays for olfaction disorders and triamcinolone paste for taste disorders reported that olfaction and taste function improved significantly in patients with COVID-19 within 1 wk[111]. Treatment for GDs is rarely addressed in the currently published literature, so more studies are needed to understand the best therapeutic options, especially in cases in which symptom regression does not occur as expected. Yet, there are different treatments that can be used against GBS, depending on the health structure and clinical context of each individual. Thus, among treatment possibilities, the use of intravenous immunoglobulins, plasmapheresis, or corticosteroids, alone or in combination, may be necessary[73,112].
The management of headache during SARS-CoV-2 infection can be accomplished by administering previously established therapeutic regimens for the treatment of acute crisis[113]. Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, are already widely used drugs in the treatment of headache crises and, although they are demonstrated during infection, their use is questioned. Reports in the literature note that NSAIDs could be related to increased ACE2 levels[114], which would contribute to ease the entry of the virus into the host cell as more viral receptors would be expressed, and negatively impact the immune system through cyclooxygenase inhibition, decreasing neutrophil chemotaxis and leading to an inefficient response against the virus, and reducing the expression of lipoxins and resolving that contribute to the resolution of inflammation. However, there are not enough studies to prove or rule out these theories[115]. On other hand, the administration of triptans like sumatriptan has been shown to be quite effective in the treatment of migraine, but despite this, for the drug choice, it is also important to consider both the patient's pre-existing comorbidities and the severity of the infection at the time[116]. Other therapies such as the administration of paracetamol, which is considered a safe and effective drug in these cases[114], neuroleptics such as chlorpromazine and neuromodulation devices can also be considered for treatment[113,117]. Lastly, the use of oral corticosteroids has also been related to improving migraine cases and managing the transition to cluster headache; however, studies have reported that these drugs could also contribute to perpetuating the replication of the virus[117].
With regard to psychiatric manifestations secondary to SARS-CoV-2 infection, delirium is mainly related to the hyperactive/mixed variety associated with elevated anxiety, and isolation itself is considered a factor that can both trigger and/or increase delirium symptoms[118,119]. This makes management difficult and lower potency antipsychotics such as olanzapine and quetiapine are preferred[118] and haloperidol is the most considered for agitation control in delusional patients[101]. Immune modulation therapies for depression secondary to infection-initiated hyperinflammation are being investigated, such as IL-6 inhibitors and melatonin[120], but more studies are required. On the other hand, technologies with online psychotherapies can support the pediatric population in this situation[121], cognitive behavioral therapy and mindfulness-based cognitive therapy can also assist in stress reduction[122].
Although low dosages of benzodiazepines are indicated in anxiety, these drugs have the potential for respiratory depression and the risk and benefits in patients with respiratory symptoms should be considered. Thus, according to the situation, gabapentin, hydroxyzine or lower doses of selective serotonin reuptake inhibitors (SSRIs) can be used, as well as non-pharmacological interventions, such as psychotherapy[101]. Treatment of PTSD typically involves SSRIs and serotonin-norepinephrine reuptake inhibitors, and the potential risks should be analyzed on a case-by-case basis. Paroxetine is not recommended due to the short half-life, anticholinergic side effect profile, and increased risk of drug interactions[102].
Thus, in this review we described the SARS-CoV-2 ability to infect the CNS and to cause manifestations related to neurology and psychiatry. Some nonspecific symptoms, such as headache, may be part of the initial clinical presentation as also be present after the resolution of the infection. Viral interaction with ACE2 receptors may be related to the onset or worsening of episodes of cerebrovascular disorders and demyelinating conditions, and to the development of olfactory and taste dysfunction by migration through the olfactory tract, one of the virus pathways. Yet, mental illnesses such as depression, anxiety, and PTSD may be caused by the social distancing and quarantine in both patients and health care workers who worked on the front lines, and these disorders may remain even after the pandemic has ended. Our work contributes to the elucidation of the disease pathogenesis, as well as the under
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tiejun W, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Achar A, Ghosh C. COVID-19-Associated Neurological Disorders: The Potential Route of CNS Invasion and Blood-Brain Relevance. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2317] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Geneva: 2022. Weekly operational update on COVID-19. [cited 1 February 2022]. Available from: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---1-february-2022. [Cited in This Article: ] |
| 4. | Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 337] [Article Influence: 84.3] [Reference Citation Analysis (1)] |
| 5. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2289] [Cited by in F6Publishing: 2428] [Article Influence: 607.0] [Reference Citation Analysis (2)] |
| 6. | Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the Nervous System. Cell. 2020;183:16-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 7. | Mukaetova-Ladinska EB, Kronenberg G, Raha-Chowdhury R. COVID-19 and neurocognitive disorders. Curr Opin Psychiatry. 2021;34:149-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Saeed SA, Pastis IS, Santos MG. COVID-19 and its impact on the brain and Mind- A conceptual model and supporting evidence. Psychiatr Q. 2022;93:271-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological Involvement in COVID-19 and Potential Mechanisms: A Review. Neurocrit Care. 2021;34:1062-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 174] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 10. | Abdel Hafez SMN. Can Covid-19 attack our nervous system? J Chem Neuroanat. 2021;117:102006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Divani AA, Andalib S, Biller J, Di Napoli M, Moghimi N, Rubinos CA, Nobleza CO, Sylaja PN, Toledano M, Lattanzi S, McCullough LD, Cruz-Flores S, Torbey M, Azarpazhooh MR. Central Nervous System Manifestations Associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SAJ, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 565] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 13. | Alomari SO, Abou-Mrad Z, Bydon A. COVID-19 and the central nervous system. Clin Neurol Neurosurg. 2020;198:106116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Satarker S, Nampoothiri M. Involvement of the nervous system in COVID-19: The bell should toll in the brain. Life Sci. 2020;262:118568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Payus AO, Liew Sat Lin C, Mohd Noh M, Jeffree MS, Ali RA. SARS-CoV-2 infection of the nervous system: A review of the literature on neurological involvement in novel coronavirus disease-(COVID-19). Bosn J Basic Med Sci. 2020;20:283-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 663] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 17. | Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. COVID-19 and SARS-Cov-2 Infection: Pathophysiology and Clinical Effects on the Nervous System. World Neurosurg.. 2020;140:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 18. | Yesilkaya UH, Balcioglu YH. Neuroimmune correlates of the nervous system involvement of COVID-19: A commentary. J Clin Neurosci. 2020;78:449-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Mahalakshmi AM, Ray B, Tuladhar S, Bhat A, Paneyala S, Patteswari D, Sakharkar MK, Hamdan H, Ojcius DM, Bolla SR, Essa MM, Chidambaram SB, Qoronfleh MW. Does COVID-19 contribute to development of neurological disease? Immun Inflamm Dis. 2021;9:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Gusev EI, Martynov MY, Boyko AN, Voznyuk IA, Latsh NY, Sivertseva SA, Spirin NN, Shamalov NA. [Novel coronavirus infection (COVID-19) and nervous system involvement: pathogenesis, clinical manifestations, organization of neurological care]. Zh Nevrol Psikhiatr Im S S Korsakova. 2020;120:7-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Abdennour L, Zeghal C, Dème M, Puybasset L. Interaction cerveau-poumon [Interaction brain-lungs]. Ann Fr Anesth Reanim. 2012;31:e101-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sharma S, Jagadeesh H, Saxena A, Chakravarthy H, Devanathan V. Central nervous system as a target of novel coronavirus infections: Potential routes of entry and pathogenic mechanisms. J Biosci. 2021;46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41:2657-2669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 205] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 24. | Alquisiras-Burgos I, Peralta-Arrieta I, Alonso-Palomares LA, Zacapala-Gómez AE, Salmerón-Bárcenas EG, Aguilera P. Neurological Complications Associated with the Blood-Brain Barrier Damage Induced by the Inflammatory Response During SARS-CoV-2 Infection. Mol Neurobiol. 2021;58:520-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Chakravarty N, Senthilnathan T, Paiola S, Gyani P, Castillo Cario S, Urena E, Jeysankar A, Jeysankar P, Ignatius Irudayam J, Natesan Subramanian S, Lavretsky H, Joshi S, Garcia G Jr, Ramaiah A, Arumugaswami V. Neurological pathophysiology of SARS-CoV-2 and pandemic potential RNA viruses: a comparative analysis. FEBS Lett. 2021;595:2854-2871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Reynolds JL, Mahajan SD. SARS-COV2 Alters Blood Brain Barrier Integrity Contributing to Neuro-Inflammation. J Neuroimmune Pharmacol. 2021;16:4-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Maiese A, Manetti AC, Bosetti C, Del Duca F, La Russa R, Frati P, Di Paolo M, Turillazzi E, Fineschi V. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021;31:e13013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 28. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28479] [Article Influence: 7119.8] [Reference Citation Analysis (3)] |
| 29. | Hasan MZ, Islam S, Matsumoto K, Kawai T. SARS-CoV-2 infection initiates interleukin-17-enriched transcriptional response in different cells from multiple organs. Sci Rep. 2021;11:16814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Welcome MO, Mastorakis NE. Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology. 2021;29:939-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Almutairi MM, Sivandzade F, Albekairi TH, Alqahtani F, Cucullo L. Neuroinflammation and Its Impact on the Pathogenesis of COVID-19. Front Med (Lausanne). 2021;8:745789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Alipoor SD, Mortaz E, Varahram M, Garssen J, Adcock IM. The Immunopathogenesis of Neuroinvasive Lesions of SARS-CoV-2 Infection in COVID-19 Patients. Front Neurol. 2021;12:697079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5228] [Cited by in F6Publishing: 5526] [Article Influence: 1381.5] [Reference Citation Analysis (2)] |
| 34. | Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32:667-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 35. | Das G, Mukherjee N, Ghosh S. Neurological Insights of COVID-19 Pandemic. ACS Chem Neurosci. 2020;11:1206-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 36. | Favas TT, Dev P, Chaurasia RN, Chakravarty K, Mishra R, Joshi D, Mishra VN, Kumar A, Singh VK, Pandey M, Pathak A. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol Sci. 2020;41:3437-3470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 37. | Choi WS, Kang CI, Kim Y, Choi JP, Joh JS, Shin HS, Kim G, Peck KR, Chung DR, Kim HO, Song SH, Kim YR, Sohn KM, Jung Y, Bang JH, Kim NJ, Lee KS, Jeong HW, Rhee JY, Kim ES, Woo H, Oh WS, Huh K, Lee YH, Song JY, Lee J, Lee CS, Kim BN, Choi YH, Jeong SJ, Lee JS, Yoon JH, Wi YM, Joung MK, Park SY, Lee SH, Jung SI, Kim SW, Lee JH, Lee H, Ki HK, Kim YS; Korean Society of Infectious Diseases. Clinical Presentation and Outcomes of Middle East Respiratory Syndrome in the Republic of Korea. Infect Chemother. 2016;48:118-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, Oh DH, Kim JH, Koh B, Kim SE, Yun NR, Lee JH, Kim JY, Kim Y, Bang JH, Song KH, Kim HB, Chung KH, Oh MD; Korea National Committee for Clinical Management of COVID-19. Clinical Course and Outcomes of Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection: a Preliminary Report of the First 28 Patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35:e142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 39. | Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, Bentvelsen RG, van den Bijllaardt W, van Oudheusden AJG, van Rijen MML, Verweij JJ, Koopmans MPG, Kluytmans JAJW. Prevalence and Clinical Presentation of Health Care Workers With Symptoms of Coronavirus Disease 2019 in 2 Dutch Hospitals During an Early Phase of the Pandemic. JAMA Netw Open. 2020;3:e209673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 40. | Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, Huet K, Plzak J, Horoi M, Hans S, Rosaria Barillari M, Cammaroto G, Fakhry N, Martiny D, Ayad T, Jouffe L, Hopkins C, Saussez S; COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 499] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 41. | Wan D, Du T, Hong W, Chen L, Que H, Lu S, Peng X. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduct Target Ther. 2021;6:406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 42. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12365] [Article Influence: 3091.3] [Reference Citation Analysis (1)] |
| 43. | Pinzon RT, Wijaya VO, Buana RB, Al Jody A, Nunsio PN. Neurologic Characteristics in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 44. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4761] [Cited by in F6Publishing: 4395] [Article Influence: 1098.8] [Reference Citation Analysis (0)] |
| 45. | Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11:995-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1435] [Cited by in F6Publishing: 1340] [Article Influence: 335.0] [Reference Citation Analysis (0)] |
| 46. | Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1219] [Cited by in F6Publishing: 1175] [Article Influence: 293.8] [Reference Citation Analysis (0)] |
| 47. | Saniasiaya J, Kulasegarah J. Dizziness and COVID-19. Ear Nose Throat J. 2021;100:29-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, González E, Redondo-Peñas I, Perona-Moratalla AB, Del Valle-Pérez JA, Gracia-Gil J, Rojas-Bartolomé L, Feria-Vilar I, Monteagudo M, Palao M, Palazón-García E, Alcahut-Rodríguez C, Sopelana-Garay D, Moreno Y, Ahmad J, Segura T. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060-e1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 506] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 49. | Zhao XY, Xu XX, Yin HS, Hu QM, Xiong T, Tang YY, Yang AY, Yu BP, Huang ZP. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 50. | Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1284] [Cited by in F6Publishing: 1461] [Article Influence: 365.3] [Reference Citation Analysis (0)] |
| 51. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 33:1007-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 629] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 52. | Kantonen J, Mahzabin S, Mäyränpää MI, Tynninen O, Paetau A, Andersson N, Sajantila A, Vapalahti O, Carpén O, Kekäläinen E, Kantele A, Myllykangas L. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30:1012-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 53. | Toljan K. Letter to the Editor Regarding the Viewpoint "Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanism". ACS Chem Neurosci. 2020;11:1192-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 54. | Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26:499-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 55. | Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, Chang SC, Chang YC. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669-1673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 56. | Liguori C, Pierantozzi M, Spanetta M, Sarmati L, Cesta N, Iannetta M, Ora J, Mina GG, Puxeddu E, Balbi O, Pezzuto G, Magrini A, Rogliani P, Andreoni M, Mercuri NB. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020;88:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 57. | Pallanti S. Importance of SARs-Cov-2 anosmia: From phenomenology to neurobiology. Compr Psychiatry. 2020;100:152184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Conklin J, Frosch MP, Mukerji S, Rapalino O, Maher M, Schaefer PW, Lev MH, Gonzalez RG, Das S, Champion SN, Magdamo C, Sen P, Harrold GK, Alabsi H, Normandin E, Shaw B, Lemieux J, Sabeti P, Branda JA, Brown EN, Westover MB, Huang SY, Edlow BL. Cerebral Microvascular Injury in Severe COVID-19. J Neurol Sci. 2021;421:117308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Karamali K, Elliott M, Hopkins C. COVID-19 related olfactory dysfunction. Curr Opin Otolaryngol Head Neck Surg. 2022;30:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 60. | Sedaghat AR, Gengler I, Speth MM. Olfactory Dysfunction: A Highly Prevalent Symptom of COVID-19 With Public Health Significance. Otolaryngol Head Neck Surg. 2020;163:12-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 61. | Las Casas Lima MH, Cavalcante ALB, Leão SC. Pathophysiological relationship between COVID-19 and olfactory dysfunction: A systematic review. Braz J Otorhinolaryngol. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Desai M, Oppenheimer J. The Importance of Considering Olfactory Dysfunction During the COVID-19 Pandemic and in Clinical Practice. J Allergy Clin Immunol Pract. 2021;9:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Izquierdo-Dominguez A, Rojas-Lechuga MJ, Mullol J, Alobid I. Olfactory Dysfunction in the COVID-19 Outbreak. J Investig Allergol Clin Immunol. 2020;30:317-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 64. | Wu D, Wang VY, Chen YH, Ku CH, Wang PC. The prevalence of olfactory and gustatory dysfunction in covid-19 - A systematic review. Auris Nasus Larynx. 2022;49:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 65. | Harikrishnan P. Gustatory Dysfunction as an Early Symptom in COVID-19 Screening. J Craniofac Surg. 2020;31:e656-e658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Pang KW, Tham SL, Ng LS. Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review. Life (Basel). 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, Ferrari M, Gagliardini L, Pipolo C, Deiana G, Fiore V, De Vito A, Turra N, Canu S, Maglio A, Serra A, Bussu F, Madeddu G, Babudieri S, Giuseppe Fois A, Pirina P, Salzano FA, De Riu P, Biglioli F, De Riu G. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42:1560-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 68. | Vaira LA, Lechien JR, Salzano G, Salzano FA, Maglitto F, Saussez S, De Riu G. Gustatory Dysfunction: A Highly Specific and Smell-Independent Symptom of COVID-19. Indian J Otolaryngol Head Neck Surg. 2020;1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Sansone P, Giaccari LG, Aurilio C, Coppolino F, Esposito V, Fiore M, Paladini A, Passavanti MB, Pota V, Pace MC. Post-Infectious Guillain-Barré Syndrome Related to SARS-CoV-2 Infection: A Systematic Review. Life (Basel). 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 70. | Makhluf H, Madany H. SARS-CoV-2 Infection and Guillain-Barré Syndrome. Pathogens. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Shoraka S, Ferreira MLB, Mohebbi SR, Ghaemi A. SARS-CoV-2 Infection and Guillain-Barré Syndrome: A Review on Potential Pathogenic Mechanisms. Front Immunol. 2021;12:674922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 72. | Palaiodimou L, Stefanou MI, Katsanos AH, Fragkou PC, Papadopoulou M, Moschovos C, Michopoulos I, Kokotis P, Bakirtzis C, Naska A, Vassilakopoulos TI, Chroni E, Tsiodras S, Tsivgoulis G. Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: A systematic review and meta-analysis. Eur J Neurol. 2021;28:3517-3529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 73. | Elzouki AN, Osman MAM, Ahmed MAE, Al-Abdulmalek A, Altermanini M, Al-Ani HA, Naeem M, Habas E. COVID-19 infection presented as Guillain-Barre Syndrome: Report of two new cases and review of 116 reported cases and case series. Travel Med Infect Dis. 2021;44:102169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J Peripher Nerv Syst. 2020;25:204-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 75. | Agosti E, Giorgianni A, D'Amore F, Vinacci G, Balbi S, Locatelli D. Is Guillain-Barrè syndrome triggered by SARS-CoV-2? Neurol Sci. 2021;42:607-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 76. | Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LLL, Chan BPL, Tan BYQ. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50:587-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 77. | Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 533] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 78. | Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF Jr, Sabeti P. Neuropathological Features of Covid-19. N Engl J Med. 2020;383:989-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 553] [Cited by in F6Publishing: 560] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 79. | Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 80. | Wang F, Kream RM, Stefano GB. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med Sci Monit. 2020;26:e928996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 149] [Article Influence: 37.3] [Reference Citation Analysis (1)] |
| 81. | Rossi R, Socci V, Talevi D, Mensi S, Niolu C, Pacitti F, Di Marco A, Rossi A, Siracusano A, Di Lorenzo G. COVID-19 Pandemic and Lockdown Measures Impact on Mental Health Among the General Population in Italy. Front Psychiatry. 2020;11:790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 709] [Cited by in F6Publishing: 714] [Article Influence: 178.5] [Reference Citation Analysis (0)] |
| 82. | Pierce M, Hope H, Ford T, Hatch S, Hotopf M, John A, Kontopantelis E, Webb R, Wessely S, McManus S, Abel KM. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7:883-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1777] [Cited by in F6Publishing: 1496] [Article Influence: 374.0] [Reference Citation Analysis (0)] |
| 83. | Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, Ho RC. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4551] [Cited by in F6Publishing: 4849] [Article Influence: 1212.3] [Reference Citation Analysis (1)] |
| 84. | Xiong J, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, Chen-Li D, Iacobucci M, Ho R, Majeed A, McIntyre RS. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J Affect Disord. 2020;277:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2424] [Cited by in F6Publishing: 2596] [Article Influence: 649.0] [Reference Citation Analysis (0)] |
| 85. | Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2096] [Cited by in F6Publishing: 1696] [Article Influence: 424.0] [Reference Citation Analysis (0)] |
| 86. | Xu Z, Zhang D, Xu D, Li X, Xie YJ, Sun W, Lee EK, Yip BH, Xiao S, Wong SY. Loneliness, depression, anxiety, and post-traumatic stress disorder among Chinese adults during COVID-19: A cross-sectional online survey. PLoS One. 2021;16:e0259012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Fofana NK, Latif F, Sarfraz S, Bilal, Bashir MF, Komal B. Fear and agony of the pandemic leading to stress and mental illness: An emerging crisis in the novel coronavirus (COVID-19) outbreak. Psychiatry Res. 2020;291:113230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 88. | Hossain MM, Sultana A, Purohit N. Mental health outcomes of quarantine and isolation for infection prevention: a systematic umbrella review of the global evidence. Epidemiol Health. 2020;42:e2020038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 289] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 89. | Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 736] [Cited by in F6Publishing: 805] [Article Influence: 268.3] [Reference Citation Analysis (0)] |
| 90. | Cowden RG, Davis EB, Counted V, Chen Y, Rueger SY, VanderWeele TJ, Lemke AW, Glowiak KJ, Worthington EL Jr. Suffering, Mental Health, and Psychological Well-being During the COVID-19 Pandemic: A Longitudinal Study of U.S. Adults With Chronic Health Conditions. Wellbeing Space Soc. 2021;2:100048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Wu T, Jia X, Shi H, Niu J, Yin X, Xie J, Wang X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J Affect Disord. 2021;281:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 611] [Article Influence: 203.7] [Reference Citation Analysis (0)] |
| 92. | Garcini LM, Rosenfeld J, Kneese G, Bondurant RG, Kanzler KE. Dealing with distress from the COVID-19 pandemic: Mental health stressors and coping strategies in vulnerable latinx communities. Health Soc Care Community. 2022;30:284-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 93. | COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2140] [Cited by in F6Publishing: 1661] [Article Influence: 553.7] [Reference Citation Analysis (0)] |
| 94. | Rahman M, Ahmed R, Moitra M, Damschroder L, Brownson R, Chorpita B, Idele P, Gohar F, Huang KY, Saxena S, Lai J, Peterson SS, Harper G, McKay M, Amugune B, Esho T, Ronen K, Othieno C, Kumar M. Mental Distress and Human Rights Violations During COVID-19: A Rapid Review of the Evidence Informing Rights, Mental Health Needs, and Public Policy Around Vulnerable Populations. Front Psychiatry. 2020;11:603875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 95. | Neelam K, Duddu V, Anyim N, Neelam J, Lewis S. Pandemics and pre-existing mental illness: A systematic review and meta-analysis. Brain Behav Immun Health. 2021;10:100177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 96. | Pan KY, Kok AAL, Eikelenboom M, Horsfall M, Jörg F, Luteijn RA, Rhebergen D, Oppen PV, Giltay EJ, Penninx BWJH. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: a longitudinal study of three Dutch case-control cohorts. Lancet Psychiatry. 2021;8:121-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 332] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 97. | Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, Wu J, Du H, Chen T, Li R, Tan H, Kang L, Yao L, Huang M, Wang H, Wang G, Liu Z, Hu S. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw Open. 2020;3:e203976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4130] [Cited by in F6Publishing: 4031] [Article Influence: 1007.8] [Reference Citation Analysis (0)] |
| 98. | Styra R, Hawryluck L, Mc Geer A, Dimas M, Sheen J, Giacobbe P, Dattani N, Lorello G, Rac VE, Francis T, Wu PE, Luk WS, Ng E, Nadarajah J, Wingrove K, Gold WL. Surviving SARS and living through COVID-19: Healthcare worker mental health outcomes and insights for coping. PLoS One. 2021;16:e0258893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 99. | de Sousa Moreira JL, Barbosa SMB, Vieira JG, Chaves NCB, Felix EBG, Feitosa PWG, da Cruz IS, da Silva CGL, Neto MLR. The psychiatric and neuropsychiatric repercussions associated with severe infections of COVID-19 and other coronaviruses. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 100. | Jasti M, Nalleballe K, Dandu V, Onteddu S. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J Neurol. 2021;268:2007-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 101. | Bilbul M, Paparone P, Kim AM, Mutalik S, Ernst CL. Psychopharmacology of COVID-19. Psychosomatics. 2020;61:411-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 102. | Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL. Neuropsychiatric Complications of COVID-19. Curr Psychiatry Rep. 2021;23:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 103. | Almqvist J, Granberg T, Tzortzakakis A, Klironomos S, Kollia E, Öhberg C, Martin R, Piehl F, Ouellette R, Ineichen BV. Neurological manifestations of coronavirus infections - a systematic review. Ann Clin Transl Neurol. 2020;7:2057-2071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 104. | Roy D, Ghosh R, Dubey S, Dubey MJ, Benito-León J, Kanti Ray B. Neurological and Neuropsychiatric Impacts of COVID-19 Pandemic. Can J Neurol Sci. 2021;48:9-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 117] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 105. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13771] [Cited by in F6Publishing: 13723] [Article Influence: 1715.4] [Reference Citation Analysis (2)] |
| 106. | Hsu PJ, Chen CH, Yeh SJ, Tsai LK, Tang SC, Jeng JS. High Plasma D-Dimer Indicates Unfavorable Outcome of Acute Ischemic Stroke Patients Receiving Intravenous Thrombolysis. Cerebrovasc Dis. 2016;42:117-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94:949-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 108. | Lee Y, Min P, Lee S, Kim SW. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 2020;35:e174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 109. | Rebholz H, Pfaffeneder-Mantai F, Knoll W, Hassel AW, Frank W, Kleber C. Olfactory dysfunction in SARS-CoV-2 infection: Focus on odorant specificity and chronic persistence. Am J Otolaryngol. 2021;42:103014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Whitcroft KL, Hummel T. Olfactory Dysfunction in COVID-19: Diagnosis and Management. JAMA. 2020;323:2512-2514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 111. | Singh CV, Jain S, Parveen S. The outcome of fluticasone nasal spray on anosmia and triamcinolone oral paste in dysgeusia in COVID-19 patients. Am J Otolaryngol. 2021;42:102892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 112. | Patnaik UJ. Review article on COVID-19 and Guillain-Barré syndrome. Front Biosci (Schol Ed). 2021;13:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Chowdhury D, Datta D. Managing Migraine in the Times of COVID-19 Pandemic. Ann Indian Acad Neurol. 2020;23:S33-S39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | MaassenVanDenBrink A, de Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 115. | Arca KN, Smith JH, Chiang CC, Starling AJ, Robertson CE, Halker Singh RB, Schwedt TJ, Kissoon NR, Garza I, Rozen TD, Boes CJ, Whealy MA, VanderPluym JH. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020;60:1558-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Caronna E, Pozo-Rosich P. Headache as a Symptom of COVID-19: Narrative Review of 1-Year Research. Curr Pain Headache Rep. 2021;25:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Bobker SM, Robbins MS. COVID-19 and Headache: A Primer for Trainees. Headache. 2020;60:1806-1811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 118. | Beach SR, Praschan NC, Hogan C, Dotson S, Merideth F, Kontos N, Fricchione GL, Smith FA. Delirium in COVID-19: A case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 119. | Moretti P, Brufani F, Pierotti V, Pomili G, Di Buò A, Giulietti C, Masini F, Tanku V, Bachetti MC, Menculini G, Tortorella A. Neurotropism and Neuropsychiatric Symptomsi in Patients with COVID-19. Psychiatr Danub. 2021;33:10-13. [PubMed] [Cited in This Article: ] |
| 120. | Ferrando SJ, Klepacz L, Lynch S, Tavakkoli M, Dornbush R, Baharani R, Smolin Y, Bartell A. COVID-19 Psychosis: A Potential New Neuropsychiatric Condition Triggered by Novel Coronavirus Infection and the Inflammatory Response? Psychosomatics. 2020;61:551-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 121. | Rajkumar RP. COVID-19 and mental health: A review of the existing literature. Asian J Psychiatr. 2020;52:102066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1943] [Cited by in F6Publishing: 1731] [Article Influence: 432.8] [Reference Citation Analysis (0)] |
| 122. | Ho CS, Chee CY, Ho RC. Mental Health Strategies to Combat the Psychological Impact of Coronavirus Disease 2019 (COVID-19) Beyond Paranoia and Panic. Ann Acad Med Singap. 2020;49:155-160. [PubMed] [Cited in This Article: ] |