Published online May 6, 2015. doi: 10.5527/wjn.v4.i2.313
Peer-review started: June 12, 2014
First decision: August 14, 2014
Revised: February 3, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 6, 2015
AIM: To analyze the effects on the kidney of hypoxia-reoxygenation in an experimental model of normocapnic asphyxia.
METHODS: To this end, 40 newborn Landrace/Large-White piglets aged 1-4 d were studied in this work. Hypoxia was induced by decreasing the inspired fiO2 to 0.06-0.08. Animals were resuscitated with different fiO2 and subdivided into 4 groups: group 1, 2, 3 and 4 received 18%, 21%, 40% and 100% O2 respectively. Macroscopic examination was carried out to evidence possible pathological features. Tissue sample were obtained from both kidneys. Four or five micron paraffin sections were stained with H-E and PAS stain and examined under an optical microscope.
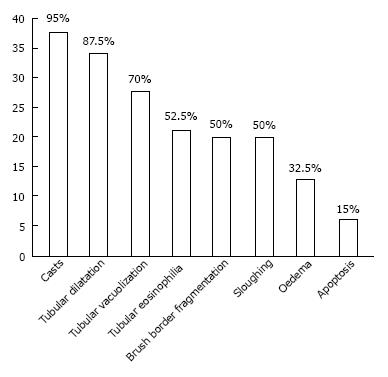
RESULTS: Pathological changes, mainly affecting tubular cells, were observed in the vast majority of kidneys of asphyxiated piglets. The most frequent tubular changes were: tubular casts (95%), tubular dilatation (87.5%), tubular vacuolization (70%), tubular eosinophilia (52.5%), sloughing (50%), fragmentation of the brush border (50%), oedema (32.5%), apoptosis (15%) and glomerular changes (meningeal cell proliferation, capsular adhesion between the flocculus and Bowman’s capsule, glomerulosclerosis and fibrous or cellular crescents associated with collapse of the glomerular tuft). Statistical analysis was carried out on changes observed when the animals were allocated in the 4 groups (χ2-test 0.05). The statistical analysis showed no evidence of differences regarding kidney lesions among the animals groups.
CONCLUSION: Our data show that renal pathology in newborn piglets is characterized by interindividual variability to hypoxia and is not associated with oxygen concentration.
Core tip: This work studied pathological renal changes following hypoxia-reoxygenation using an established experimental model of normocapnic asphyxia. Tubular dilatation, vacuolization, tubular eosinophilia, sloughing, fragmentation of the brush border and apoptosis were the most frequent changes detected in proximal tubules. Tubular dilatation, vacuolization and sloughing were the earliest lesions, interstitial oedema and apoptosis the late ones. In newborn piglets undergoing asphyxia, renal pathology was not associated with oxygen concentration used during resuscitation.
- Citation: Gerosa C, Iacovidou N, Argyri I, Fanni D, Papalois A, Aroni F, Faa G, Xanthos T, Fanos V. Histopathology of renal asphyxia in newborn piglets: Individual susceptibility to tubular changes. World J Nephrol 2015; 4(2): 313-318
- URL: https://www.wjgnet.com/2220-6124/full/v4/i2/313.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i2.313
Perinatal asphyxia is generally considered an important aetiological factor at the basis of neonatal mortality and morbidity in survivors, with involvement of all organs, including the kidney[1-3]. The kidney is particularly susceptible to hypoxia[4], since the renal tubular cells are characterized by a high metabolism rate, due to high demand and consumption of oxygen[5-7].
Neonates on the first or second day post-asphyxia, often present with symptoms of acute renal impairment or failure, including oliguria, anuria, oedema, and hyperkalemia[8]. Acute renal injury is a common condition encountered in neonates admitted to Neonatal Intensive Care Units[9,10], occurring in as many as 8% of them, and carrying a mortality rate of around 40%[11,12]. Acute kidney injury is generally considered to be related to severe pathological changes in proximal tubular cells, which are considered the typical diagnostic signs of the disease[13,14].
The most severe renal damage has been described to occur 2 h post-asphyxia. It is mainly attributed to the reperfusion injury of the tubular epithelial cells by free oxidant radicals accumulation and the influx of Ca2+[15]. This overload may activate the cysteine protease calpain in tubular cells, leading to hydrolysis of integrins and cytoskeleton components such as F-actin, talin, alpha-actin, and filamin, resulting in tubular cell membrane damage[16].
Acute renal failure has been reported to cause structural changes in tubular renal cells, including loss of brush border, vacuolization of tubular cells, apoptosis and cast formation of necrotic epithelial cells[6]. In experimental animal models of hypoxia, only minor histological changes, including mild brush border loss and vacuolization of tubular cells, are present in the kidney at 22 h post-peritonitis-induced septic shock in pigs[17]. Piglets subjected to mild hypothermic cardiopulmonary bypass exhibit tubular dilatation, vacuoles, leukocyte infiltration, epithelial destruction and interstitial oedema[18]. In cultured glomerular endothelial cells, hypoxia has been shown to induce apoptosis[19].
Resuscitation strategies modify the relationship between the inhibitors and the factors that promote angiogenesis. In particular, reoxygenation using 100% oxygen of neonatal piglets has been shown to decrease serum levels of angiostatin[20].
On clinical grounds, several questions remain unanswered at the moment: how does asphyxia cause acute renal tubular injury in neonates and what histological change corresponds to tubular dysfunction? What is the role of oxygen concentration used during resuscitation in determining kidney injury?
In the present study we investigated the renal injury caused at the histological level by hypoxia-reoxygenation in an experimental neonatal swine model previously described by our group[21].
Forty male Landrace/Large White newborn piglets, weighing 2.3-3.8 kg and aged 1-4 d were studied in this work. All experimental piglets came from the same breeding unit. Experimental procedures were previously approved by the General Directorate of Veterinary Services (Permit no. 404/21-04-09)[21].
Briefly, following sedation with 10 mg/kg ketamine and 0.5 mg/kg midazolam, anesthesia was induced with 1 mg/kg propofol and 10 mg/kg fentanyl, administered through a peripheral vein. Hypoxia was induced by decreasing the inspired fiO2 to 0.06-0.08; resuscitation efforts were carried out according to the Newborn Life Support (NLS) algorithm[1]. Animals were resuscitated with different fiO2 and subdivided into 4 groups: groups 1, 2, 3 and 4 received 18%-21%-40% and 100% O2 respectively (Table 1). Surviving animals were euthanatized by intravenous infusion of 30 mg/kg sodium thiopental (Pentothal, Hospira Enterprises BV, The Netherlands). A macroscopic examination was carried out to evidence possible pathological features. Tissue samples were obtained from both kidneys, fixed in 10% formalin, routinely processed, paraffin embedded. Four or five micron paraffin sections were stained with H-E and PAS stain and examined under an optical microscope. Depending on recovery time, the experimental animals were subdivided into 5 groups (Table 2): group A: fast recovery (< 15 min); group B: medium (15-45 min); group C: slow (45-90 min); group D: very slow recovery (> 90 min), and group E: dead animals.
| F. O2 | Deaths | Survivors | Mean time of resuscitation (min) | |
| Group 1 | 18% | 3 | 7 | 5-26 |
| Group 2 | 21% | 1 | 9 | 7-75 |
| Group 3 | 40% | 2 | 8 | 12-142 |
| Group 4 | 100% | 3 | 7 | 30-120 |
| Resuscitation | |||||
| Group AFast < 15’ (%) | Group BMiddle 16-45’ (%) | Group CSlow 45-90’ (%) | Group DVery slow > 90’ (%) | Group EDeath (%) | |
| Oedema | 12.5 | 50 | 28.50 | 0 | 44.40 |
| Eosinophilia | 50 | 50 | 42.80 | 100 | 44.40 |
| Tubular dilatation | 87.50 | 83.30 | 85.70 | 75 | 100 |
| Casts | 100 | 100 | 85.70 | 100 | 88.80 |
| Tubular vacuoles | 75 | 83.30 | 42.80 | 75 | 66.60 |
| Sloughing | 75 | 41.60 | 42.80 | 25 | 55.50 |
| Brush border fragmentation | 75 | 41.60 | 42.80 | 25 | 55.50 |
| Apoptosis | 25 | 8.30 | 0 | 25 | 22.20 |
Kidney samples from 4 male Landrace/large White newborn piglets not submitted to hypoxia served as subjects for the control group. Statistical analyses were based on the use of the χ2 test.
Kidneys from the control group preserved their architecture. In the subcapsular regions, active glomerulogenesis was detected, represented by the tubule-glomerular nodules recently reported by our group as the typical developing unit in piglets[21]. Moreover, in the deep cortex, some senescent glomeruli with different degrees of sclerosis were detected and were occasionally associated with cellular or fibrotic crescents. Few scattered PAS-positive tubular casts were also observed in all control kidneys.
Pathological changes, mainly affecting tubular cells, were observed in the vast majority of kidneys from asphyxiated piglets. The most frequent tubular changes are illustrated in Figure 1.
Tubular dilatation, mainly affecting distal tubules, was observed in 35/40 cases (87.5%); in 7 of the 35 positive cases, tubular dilatation was marked and diffuse. Dilatation was found to occur even in the subcapsular regions, in developing nephrons and, in particular, in renal vesicles and S-shaped bodies.
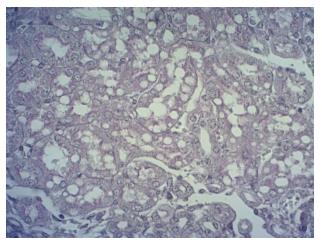
Tubular vacuolization was detected in 28/40 (70%) cases with an incidence similar to that of tubular dilatation. In the majority of cases, tubular vacuolization was focal, whereas in 7 cases it was diffuse. In kidneys with focal tubular vacuolization the lesions were mainly observed in the deep cortex at the cortico-medullary boundary. Only in rare cases did vacuolization affect tubular structures in the subcapsular regions. Vacuolization was mainly observed in proximal tubules. In the majority of affected kidneys, the size of vacuoles varied widely among cases: multiple microvacuoli were observed but, in few cases, larger vacuoles were found, sometimes occupying entirely the cytoplasm of affected tubular cells (Figure 2).
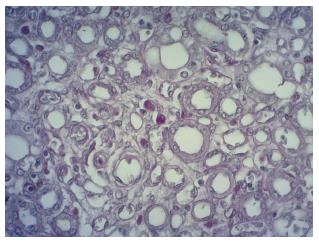
Apoptosis: this was diagnosed on the basis of morphological changes. It was detected only in 6/40 (15%) cases, was mainly focal and occurred in proximal tubular cells. Cells undergoing apoptosis showed cell fragmentation with the formation of multiple eosinophilic globules, some of which contained nuclear remnants (Figure 3). Apoptotic bodies often appeared strongly PAS-positive.
Tubular eosinophilia was observed in 21/40 (52.5%) cases. Eosinophilic changes were mainly focal, localized in tubular structures of single nephrons with their cytoplasm intensely stained by eosin. Eosinophilia was detected in the superficial and deep cortical regions: subcapsular areas with active nephrogenesis were frequently affected. At high power, tubules affected by eosinophilia often showed associated degenerative changes, including vacuolization.
Tubular casts were observed in 38/40 cases (95%), as well as in the kidneys from the control group. Casts were mainly hyaline, detected in areas affected by interstitial oedema and tubular cell vacuolization. Occasionally, granular casts were found in areas with tubular cells apoptosis.
Oedema was observed in the interstitial space of the cortical region in 13/40 cases (32.5%): in 10 cases it was focal and mainly localized in the subcortical regions; in 3 cases it was diffuse in the whole cortex, including the deep areas.
Sloughing. Detachment of tubular cells from the basal membrane was observed in 20/40 cases (50%) mainly in proximal tubuli. Inside the affected tubules, sloughing was focal, with scattered cells detaching from the basal lamina of tubuli. Sloughing was unevenly distributed throughout the entire cortex, with no preference for superficial or deep cortical zones.
Fragmentation of the brush border. In 20 out of 40 cases (50%), proximal tubules at high power revealed a previously underestimated lesion: the brush border had lost its integrity and appeared fragmented. Brush border fragmentation was often associated with sloughing and with other tubular changes, including dilatation and cytoplasmic vacuolization.
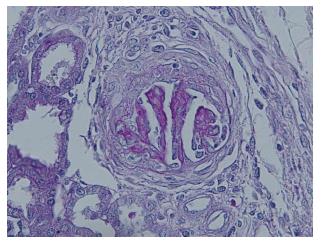
Glomerular changes were found in all samples examined, most frequently in the deep cortex and at the cortico-medullary junction. Mesangial cell proliferation, capsular adhesion between the flocculus and Bowman’s capsule, glomerulosclerosis and fibrous or cellular crescents associated with collapse of the glomerular tuft (Figure 4) were detected. In some cases, these glomerular lesions were also detected in the subcapsular regions, affecting glomeruli during the initial phases of their development. Focal glomerulosclerosis and hyalinization were observed even in controls, but always restricted to the deep cortex.
As for the glomerular changes, these lesions will not be discussed, given their presence in all the control kidneys examined. They might represent physiological senescence of “not well developed” glomeruli.
Statistical analysis was carried out on changes observed when the animals were allocated in the 4 groups depending on the concentration of oxygen used during resuscitation and when they were allocated in 5 groups on the basis of the time of resuscitation. Statistical analysis showed no evidence of differences regarding kidney lesions among animals groups.
Despite improvement in knowledge over the last decade[5] regarding the molecular mechanisms by which perinatal asphyxia causes tissue damage, the insult leading to acute kidney injury is partly unknown and still under discussion. Some authors claim that the predominant role in tubular cell death is played by hypoxia per se, while others attribute this to dysoxia 4 or reoxygenation[20].
This study presents data on histological changes occurring in kidneys of newborn piglets exposed to acute hypoxia-reoxygenation. The degenerative and necrotic changes were predominantly localized in the proximal tubules, followed by distal tubules, in absence of significant pathological changes in glomerular cells and vascular structures. These data confirm that kidney proximal tubular cells are the main target of hypoxia, due to their high physiological metabolic rate[4] and high rate of aerobic glycolysis[5]. Distal tubular cells, despite their lower metabolism rate, were however not devoid of pathological changes in this study, with tubular dilatation being the main one. Tubular vacuolization was detected both in proximal and distal tubules.
As for the role of oxygen concentration used for resuscitation in acute kidney injury, the present data suggest that it did not play a significant role in acute kidney injury following hypoxia-reoxygenation in our experimental model. No significant difference in the incidence of renal pathological changes among animals of groups 1-4 were detected (Table 1). However, we cannot exclude long-term effects of different oxygen concentrations in the kidney.
Interesting data on the changes of renal cells were obtained when piglets were subdivided into groups A-E depending on the time required for resuscitation (Table 2). Interstitial oedema appeared to be a “late” lesion, detected in only 12.5% of animals in group A. On the contrary, eosinophilia of tubular cells should be considered an “early” lesion as it was found in 50% of piglets in group A, and a similar percentage was found in deceased animals. Tubular dilatation appeared frequently in group A (87.5%) thus suggesting that it is a “very early” response of kidney structures to hypoxia. The observation of dilated renal tubules in 100% of deceased piglets reinforces the hypothesis of a major role of this early pathological lesion in the pathogenesis of acute kidney injury following hypoxia. Tubular casts were observed in the majority of animals, ranging from 85.7% up to 100% of cases (Figure 1). This association with their detection in control kidneys suggests a minor, if any, role of casts in acute kidney injury.
As for the vacuolization occurring in the cytoplasm of proximal and distal tubular cells, it appears to be an “early” lesion, observed in 75% of piglets in group A. Contrary to other lesions, the incidence of this pathological change had not significantly increased in animals with a longer time of resuscitation and was found in 66.6% of deceased animals, thus suggesting that some of them probably did not survive long enough after asphyxia for cytoplasmic vacuoles to appear. Sloughing and brush border changes appeared to be “early” lesions observed in 75% of animals in group A and decreased in piglets with late and very late (> 90 min) resuscitation, finally to be observed in about 50% of deceased animals (Table 2).
The rare presence of apoptotic globules with nuclear condensation and fragmentation in animals of group A may be attributed to the short time to complete the whole process of apoptotic cell death. The same explanation can be applied to deceased animals: as apoptosis is an active process, we can speculate that the majority of deceased piglets did not survive long enough to allow apoptosis to occur. On the contrary, the incidence of apoptotic globules in kidneys of animals characterized by a very late resuscitation time (> 90 min) suggests an important role of apoptosis in asphyxia-related acute kidney injury. We may also speculate that sloughing should be considered the morphological sign of tubular cell apoptosis: the detachment of tubular cells from each other and from the basal lamina and their removal by the urinary flow may be a limit for the development of the complete sequence of the apoptotic process, thus limiting their finding to few kidney samples.
Our data clearly show that the Landrace/Large White piglet model provides useful data for the study of hypoxia-induced kidney injury. Tubular dilatation, tubular cell vacuolization, sloughing and brush border changes are the main, earliest and most severe lesions in the post-asphyxia kidney. A strong interindividual variability in the severity of renal changes to asphyxia was observed. The extent of kidney lesions was not associated with the concentration of oxygen used during resuscitation, thus suggesting a previously unreported individual susceptibility to hypoxia. Further studies on pathological kidney changes from human and non-human models are needed to verify their role in the short- and long-term kidney damage following hypoxia-reoxygenation.
The study focuses on the histological renal change following asphyxia in a experimental model of neonatal asphyxia. On the basis of recent data suggesting a role of reoxygenation in the development of renal lesions, the authors evaluated the presence and degree of renal lesions in piglets submitted to different oxygen percentages following asphyxia.
The study is mainly related to the research field of tissue damage following asphyxia and reoxygenation in the perinatal period.
The most important data of the study regard the usefulness of the Landrace/Large White piglet model for the study of hypoxia-induced kidney injury. The strong interindividual variability in the severity of renal changes to asphyxia described in the study represents a new finding that may lead the neonatologist towards an individualized sartorial approach in each newborn affected by perinatal asphyxia.
In the study, tubular dilatation, tubular cell vacuolization, sloughing and brush border changes are the main, earliest and most severe lesions observed in the post-asphyxia kidney. These data may help pathologists involved in the study of asphyxia-induced renal pathology.
The study is interesting and well-conducted.
P- Reviewer: Menezes RG, Revuelta KL, Wagner KD S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147-1152. [PubMed] [Cited in This Article: ] |
| 2. | Huang CC. Perinatal hypoxic/ischemic encephalopathy: clinical challenge and experimental implications. Zhonghua Minguoxiaoerke Yixuehui Zazhi. 1998;39:157-165. [PubMed] [Cited in This Article: ] |
| 3. | Martín-Ancel A, García-Alix A, Gayá F, Cabañas F, Burgueros M, Quero J. Multiple organ involvement in perinatal asphyxia. J Pediatr. 1995;127:786-793. [PubMed] [Cited in This Article: ] |
| 4. | Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 5. | Fanos V, Dessì A, D’Aloja E, Finco G, Iacovidou N, Faa G. The kidney in post asphytic syndrome: state of the art. Development nephrology: from embryology to metabolomics. Quartu S. Elena: Hygeia Press 2011; 159-179. [Cited in This Article: ] |
| 6. | Baricos WH. Protease mediated tubular injury: a new direction in acute renal failure? Kidney Int. 2002;61:1174-1175. [PubMed] [Cited in This Article: ] |
| 7. | Rosen S, Epstein FH, Brezis M. Determinants of intrarenal oxygenation: factors in acute renal failure. Ren Fail. 1992;14:321-325. [PubMed] [Cited in This Article: ] |
| 8. | Subramanian PT. Acute renal failure in the neonate. Saudi J Kidney Dis Transpl. 1998;9:320. [PubMed] [Cited in This Article: ] |
| 9. | Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24:253-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Nouri S, Mahdhaoui N, Beizig S, Zakhama R, Salem N, Ben Dhafer S, Methlouthi J, Seboui H. [Acute renal failure in full term neonates with perinatal asphyxia. Prospective study of 87 cases]. Arch Pediatr. 2008;15:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Putensen C. [Acute renal failure. Still a cause of increased mortality]. Anaesthesist. 2007;56:1103-1104. [PubMed] [Cited in This Article: ] |
| 12. | Stapleton FB, Jones DP, Green RS. Acute renal failure in neonates: incidence, etiology and outcome. Pediatr Nephrol. 1987;1:314-320. [PubMed] [Cited in This Article: ] |
| 13. | Hoste EA, Kellum JA. Acute kidney injury: epidemiology and diagnostic criteria. Curr Opin Crit Care. 2006;12:531-537. [PubMed] [Cited in This Article: ] |
| 14. | Rosen S, Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int. 2001;60:1220-1224. [PubMed] [Cited in This Article: ] |
| 15. | Yu B, Li S, Yao Y, Lin Z. Changes in beta(1) integrin in renal tubular epithelial cells after intrauterine asphyxia of rabbit pups. J Perinat Med. 2009;37:59-65. [PubMed] [Cited in This Article: ] |
| 16. | Elliget KA, Phelps PC, Trump BF. Cytosolic Ca2+ elevation and calpain inhibitors in HgCl2 injury to rat kidney proximal tubule epithelial cells. Pathobiology. 1994;62:298-310. [PubMed] [Cited in This Article: ] |
| 17. | Chvojka J, Sykora R, Krouzecky A, Radej J, Varnerova V, Karvunidis T, Hes O, Novak I, Radermacher P, Matejovic M. Renal haemodynamic, microcirculatory, metabolic and histopathological responses to peritonitis-induced septic shock in pigs. Crit Care. 2008;12:R164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Tirilomis T, Tempes T, Waldmann-Beushausen R, Ballat C, Bensch M, Schoendube FA. Histological changes in neonatal kidneys after cardiopulmonary bypass and deep hypothermic circulatory arrest. Thorac Cardiovasc Surg. 2009;57:7-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Tanaka T, Miyata T, Inagi R, Kurokawa K, Adler S, Fujita T, Nangaku M. Hypoxia-induced apoptosis in cultured glomerular endothelial cells: involvement of mitochondrial pathways. Kidney Int. 2003;64:2020-2032. [PubMed] [Cited in This Article: ] |
| 20. | Emara M, Obaid L, Johnson S, Bigam DL, Cheung PY. Angiostatins decrease in the kidney of newborn piglets after hypoxia-reoxygenation. Eur J Pharmacol. 2010;644:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Aroni F, Xanthos T, Varsami M, Argyri I, Alexaki A, Stroumpoulis K, Lelovas P, Papalois A, Faa G, Fanos V. An experimental model of neonatal normocapnic hypoxia and resuscitation in Landrace/Large White piglets. J Matern Fetal Neonatal Med. 2012;25:1750-1754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |