Published online May 6, 2015. doi: 10.5527/wjn.v4.i2.307
Peer-review started: November 27, 2014
First decision: December 18, 2014
Revised: December 24, 2014
Accepted: January 18, 2015
Article in press: January 20, 2015
Published online: May 6, 2015
Renal proximal tubules (PTs) play important roles in the regulation of acid/base, plasma volume and blood pressure. Recent studies suggest that there are substantial species differences in the regulation of PT transport. For example, thiazolidinediones (TZDs) are widely used for the treatment of type 2 diabetes mellitus, but the use of TZDs is associated with fluid overload. In addition to the transcriptional enhancement of sodium transport in distal nephrons, TZDs rapidly stimulate PT sodium transport via a non-genomic mechanism depending on peroxisome proliferator activated receptor γ/Src/epidermal growth factor receptor (EGFR)/MEK/ERK. In mouse PTs, however, TZDs fail to stimulate PT transport probably due to constitutive activation of Src/EGFR/ERK pathway. This unique activation of Src/ERK may also affect the effect of high concentrations of insulin on mouse PT transport. On the other hand, the effect of angiotensin II (Ang II) on PT transport is known to be biphasic in rabbits, rats, and mice. However, Ang II induces a concentration-dependent, monophasic transport stimulation in human PTs. The contrasting responses to nitric oxide/guanosine 3’,5’-cyclic monophosphate pathway may largely explain these different effects of Ang II on PT transport. In this review, we focus on the recent findings on the species differences in the regulation of PT transport, which may help understand the species-specific mechanisms underlying edema formation and/or hypertension occurrence.
Core tip: Renal proximal tubule (PT) transport is essential for the regulation of plasma volume and blood pressure. Several species differences are found as to the stimulatory effects of thiazolidinediones, insulin, and angiotensin II on PT sodium transport. This review focuses on this topic, which may be relevant to species-specific mechanisms underlying edema formation and/or hypertension occurrence.
- Citation: Seki G, Nakamura M, Suzuki M, Satoh N, Horita S. Species differences in regulation of renal proximal tubule transport by certain molecules. World J Nephrol 2015; 4(2): 307-312
- URL: https://www.wjgnet.com/2220-6124/full/v4/i2/307.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i2.307
The kidney plays an essential role in the homeostatic regulation of electrolytes, acid-base and plasma volume in the body. Some species differences are known to exist in the distribution patterns of solute transporters as well as the hormonal actions in distal nephron[1,2]. In proximal tubules (PTs), on the other hand, there are no major species differences in the distribution patterns and functions of main sodium transporters such as the apical Na+/H+ exchanger type 3 NHE3 and the basolateral electrogenic Na+-HCO3- cotransporter NBCe1[3,4]. While the transport stoichiometry of NBCe1 was reported to be 1Na+ to 3HCO3- in rat PTs in vivo[5], it was found to be 1Na+ to 2HCO3- in rabbit PTs in vitro[6]. However, this difference was turned out to be due to the differences in experimental conditions[7,8].
Nevertheless, recent studies identified substantial species differences in the regulation of PT transport, which might be important for uncovering the species-specific causes for edema and/or hypertension. Species differences in renal physiologic and/or metabolic responses may be also potentially important for understanding mechanisms for the different effects of several agents against diabetic nephropathy between animal models and human patients. For example, inhibition of advanced glycosylation end products by aminoguanidine or pyridoxamine was reported to be protective in rodent models of diabetic nephropathy[9-12]. However, these agents were not found effective in initial human trials in diabetic patients[13,14]. Effectiveness of a selective PKC-inhibitor Ruboxistaurin found in rodent models of diabetic nephropathy[15,16] was also not confirmed in human type 2 diabetic patients[17]. Indeed, rodent models of diabetic nephropathy exhibit proteinuria and pathological glomerular changes, but do not exhibit progressive renal failure[18]. Dogs, pigs and other non-human primates have been used for toxicology testing. However, ideal animal models have not been established for revealing drug side effects[19]. We focus on species differences in the regulation of PT transport in this review.
Thiazolidinediones (TZDs) activate a nuclear receptor peroxisome proliferator activated receptor γ (PPARγ), thereby improving insulin resistance through the transcriptional modulation of the relevant genes[20]. TZDs have been widely used for the treatment of type 2 diabetes. However, fluid retention is a serious clinical problem, which may exert an adverse effect on the cardiovascular system[21].
Initially, PPARγ-mediated transcriptional enhancement of the epithelial Na channel ENaCγ subunit in collecting ducts was proposed to be a main mechanism for TZDs-induced volume expansion[22,23]. Subsequent analysis in renal principal cell culture models, however, did not support the central role of ENaC in TZDs-induced volume expansion[24]. Moreover, TZDs-induced volume expansion was preserved in collecting ducts specific ENaC deficient mice[25]. These results indicate that mechanism(s) other than the activation of ENaC in collecting ducts may be also involved in TZDs-induced volume expansion. Interestingly, Muto and colleagues reported the rapid stimulation of NBCe1 activity by troglitazone in isolated rabbit PTs[26]. Because TDZs were reported to stimulate PT transport also in humans[27], we performed the detailed analysis on the mechanism of TZDs-induced stimulation of PT sodium transport.
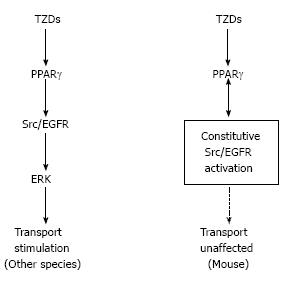
We found that TZDs rapidly induced transport stimulation within minutes in isolated PTs from rabbits, rats, and humans. Our subsequent analysis revealed that TZDs-induced PT transport stimulation was dependent on the non-genomic signaling cascade consisting of PPARγ/Src/epidermal growth factor receptor (EGFR)/MEK/ERK[28]. Notably, however, TZDs failed to induce transport stimulation in isolated mouse PTs, despite the definite expression of PPARγ in these tubules. We speculated that some factor(s) specific for mouse PTs might interfere with TZDs-induced rapid signaling. Consistent with this view, Kiley et al[29] reported the constitutive activation of Src/EGFR pathway that was found only in mouse PTs. This unique constitutive activation of Src/EGFR, which could potentially explain the different effects of exogenous EGF on unilateral ureteral obstruction in rats and mice[30], was confined to mouse PTs and not found in other mouse tissues[29]. Because TZDs rapidly activated the Src/ERK pathway in kidney cortex of rabbits and rats but not mice, we concluded that the constitutive activation of Src/EGFR prevented TZDs-induced transport stimulation in mouse PTs[28]. Consistent with this conclusion, the constitutive activation of Src was also reported to interfere with the non-genomic signaling of another nuclear receptor for estrogen[31].
Most likely, TZDs-induced volume expansion is multifactorial, depending on both genomic and non-genomic actions of PPARγ on tubular sodium transport. One of potential targets of non-genomic actions of PPARγ may be distal nephron, where WNK kinases regulate the balance between renal NaCl absorption and K+ secretion[32,33]. WNK4 is also known to affect Cl- transport in extrarenal epithelia[34]. It remains to be determined whether PPARγ regulates the WNK kinase system.
Insulin is thought to enhance renal sodium retention by activating sodium transport in several nephron segments[35]. In PTs, insulin stimulates the major sodium transporters NHE3, NBCe1, and the Na+/K+-ATPase[36-38]. In isolated rabbit PTs, Baum found that insulin, at the concentrations between 10-10 mol/L and 10-8 mol/L, dose-dependently stimulates volume and bicarbonate absorption[39]. Recently, we also found the similar dose-dependent stimulation of NBCe1 by up to 10-8 mol/L insulin in isolated PTs from rats and humans[40]. This stimulatory effect of insulin on PT sodium transport, which is completely preserved in insulin resistant rats and humans, may play an important role in the pathogenesis of hypertension associated with insulin resistance[40].
Insulin actions are initiated by the activation of tyrosine kinase in the cell membrane receptor, which induces a series of phosphorylation in multiple insulin receptor substrates (IRSs). The two major IRS proteins, IRS1 and IRS2 often mediate distinct insulin actions, and defects at the levels of IRS1 or IRS2 may be responsible for the occurrence of selective insulin resistance in several tissues[41]. We have shown that IRS2 but not IRS1 mediates the stimulatory effect of insulin on PT transport in both mice and rats. Moreover, we have confirmed that insulin activates Akt in kidney cortex of both mice and rats[40,42]. We found that up to 10-9 mol/L insulin stimulated mouse PT sodium transport. Unlike in rats and humans, however, the higher concentrations of insulin (i.e., more than 10-8 mol/L) failed to stimulate PT transport in mice[42]. In isolated mouse collecting ducts, by contrast, insulin at the high concentrations up to 10-7 mol/L was reported to activate ENaC[43]. Importantly, insulin is known to activate both Akt and ERK pathways, and these two pathways are interconnected with each other[44,45]. It is therefore possible that the constitutive activation of Src/ERK may somehow interfere with the effects of high concentrations of insulin in mouse PTs.
The stimulation of PT sodium transport by Angiotensin II (Ang II) may be essential for Ang II-induced hypertension[46,47]. Actually, however, Ang II is known to regulate PT transport in a biphasic way: transport is stimulated by low (picomolar to nanomolar) concentrations of Ang II, but inhibited by high (nanomolar to micromolar) concentrations of Ang II. This biphasic regulation of PT transport by Ang II has been confirmed in rats, mice, and rabbits[48-51]. Regarding the receptor subtype(s) responsible for the biphasic effects of Ang II, controversial results had been reported[52-54]. However, the analyses on isolated PTs from type 1A Ang II receptor (AT1A) deficient mice have clearly shown that AT1A mediates both the stimulatory and inhibitory effects of Ang II[49,51].
The stimulatory effect of Ang II is dependent on the activation of protein kinase C and/or the decrease in the intracellular cAMP concentration, which may result in the activation of ERK[55,56]. On the other hand, the inhibitory effect of Ang II is dependent on the activation of phospholipase A2/arachidonic acid/5,6-epoxyeicosatrienoic acid (EET) pathway and/or nitric oxide (NO)/guanosine 3’,5’-cyclic monophosphate (cGMP) pathway[53,55,57]. Because little had been known about the direct effects of Ang II on human PT transport, we tried to clarify this issue using isolated, intact human PTs obtained from nephrectomy surgery.
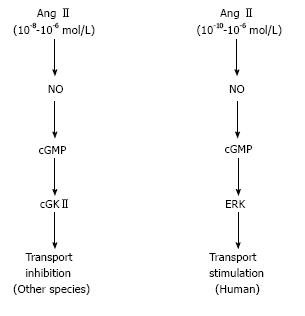
Surprisingly, we found that the inhibitory effect of Ang II is lost in human PTs. Actually, up to 10-5 mol/L Ang II dose-dependently stimulated human PT transport. In view of high intrarenal concentrations of Ang II, these data suggest that Ang II may play an even more important role in the regulation of plasma volume and blood pressure in humans than in other species[58].
The detailed analysis revealed that the contrasting responses to NO/cGMP could explain the different modes of PT transport regulation in humans and other species. While the NO/cGMP/ERK pathway mediates the dose-dependent stimulatory effect of Ang II in humans, the NO/cGMP/cGMP-dependent kinase II (cGKII) pathway mediates the inhibitory effect of high concentrations of Ang II in mice[58]. In cGKII-deficient mice, the inhibitory effect of Ang II was lost, but the NO/cGMP pathway failed to stimulate PT transport. These results indicate that the loss of cGKII alone in mice cannot reproduce the NO/cGMP/ERK-dependent stimulatory effect of Ang II in humans.
Currently, the reason why the NO/cGMP pathway exerts different effects on PT transport in humans and other species remains unknown. Nevertheless, several previous reports supported the existence of such species differences. For example, renal NO production was enhanced by salt loading in rodents, which might facilitate sodium diuresis and prevent blood pressure elevation[59,60]. Thus, renal NO is thought to induce a natriuretic response to salt loading in rodents[61]. However, renal NO production was not significantly enhanced by salt loading in humans[62,63], and an adaptive role of renal NO to salt loading is less clear in humans[64,65]. In any case, the stimulatory effect of NO/cGMP pathway on PT transport may represent a human-specific target for hypertension.
The NO/cGMP pathway is thought to be inhibitory on sodium transport in thick ascending limb and collecting ducts[61]. Therefore, it will be interesting to examine whether the similar species differences may also exist in the effects of NO/cGMP pathway on these nephron segments. Such knowledge may help understand the mechanism of fluid overload by selective endothelin receptor antagonism[66].
In summary, non-genomic stimulation of PT transport by TZDs is uniquely absent in mice probably because of the constitutive activation of Src/EGFR as shown in Figure 1. As shown in Figure 2, on the other hand, the inhibitory effect of Ang II is lost in human PTs, where the NO/cGMP pathway is stimulatory unlike in other species. These species differences may be at least partially responsible for the species-specific mechanisms underlying edema formation and/or hypertension occurrence.
P- Reviewer: Duan SB, Trimarchi H S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. Human cortical distal nephron: distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 2002;13:836-847. [PubMed] [Cited in This Article: ] |
| 2. | Morel F. Sites of hormone action in the mammalian nephron. Am J Physiol. 1981;240:F159-F164. [PubMed] [Cited in This Article: ] |
| 3. | Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev. 1990;70:79-114. [PubMed] [Cited in This Article: ] |
| 4. | Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol. 2006;17:2368-2382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Yoshitomi K, Burckhardt BC, Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985;405:360-366. [PubMed] [Cited in This Article: ] |
| 6. | Seki G, Coppola S, Frömter E. The Na(+)-HCO3- cotransporter operates with a coupling ratio of 2 HCO3- to 1 Na+ in isolated rabbit renal proximal tubule. Pflugers Arch. 1993;425:409-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Müller-Berger S, Nesterov VV, Frömter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. II. Change of Na-HCO3 cotransport stoichiometry and of response to acetazolamide. Pflugers Arch. 1997;434:383-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Seki G, Coppola S, Yoshitomi K, Burckhardt BC, Samarzija I, Müller-Berger S, Frömter E. On the mechanism of bicarbonate exit from renal proximal tubular cells. Kidney Int. 1996;49:1671-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Alderson NL, Chachich ME, Youssef NN, Beattie RJ, Nachtigal M, Thorpe SR, Baynes JW. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003;63:2123-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Bucala R, Vlassara H. Advanced glycosylation end products in diabetic renal and vascular disease. Am J Kidney Dis. 1995;26:875-888. [PubMed] [Cited in This Article: ] |
| 11. | Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Friedman EA, Distant DA, Fleishhacker JF, Boyd TA, Cartwright K. Aminoguanidine prolongs survival in azotemic-induced diabetic rats. Am J Kidney Dis. 1997;30:253-259. [PubMed] [Cited in This Article: ] |
| 13. | Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728-731. [PubMed] [Cited in This Article: ] |
| 16. | Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439-447. [PubMed] [Cited in This Article: ] |
| 17. | Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28:2686-2690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Breyer MD, Böttinger E, Brosius FC, Coffman TM, Fogo A, Harris RC, Heilig CW, Sharma K. Diabetic nephropathy: of mice and men. Adv Chronic Kidney Dis. 2005;12:128-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Helke KL, Swindle MM. Animal models of toxicology testing: the role of pigs. Expert Opin Drug Metab Toxicol. 2013;9:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1492] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 21. | Mudaliar S, Chang AR, Henry RR. Thiazolidinediones, peripheral edema, and type 2 diabetes: incidence, pathophysiology, and clinical implications. Endocr Pract. 2003;9:406-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 468] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA. 2005;102:9406-9411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Nofziger C, Chen L, Shane MA, Smith CD, Brown KK, Blazer-Yost BL. PPARgamma agonists do not directly enhance basal or insulin-stimulated Na(+) transport via the epithelial Na(+) channel. Pflugers Arch. 2005;451:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol. 2009;20:721-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Muto S, Miyata Y, Imai M, Asano Y. Troglitazone stimulates basolateral rheogenic Na+/HCO3- cotransport activity in rabbit proximal straight tubules. Exp Nephrol. 2001;9:191-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Zanchi A, Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Effects of the peroxisomal proliferator-activated receptor-gamma agonist pioglitazone on renal and hormonal responses to salt in healthy men. J Clin Endocrinol Metab. 2004;89:1140-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Endo Y, Suzuki M, Yamada H, Horita S, Kunimi M, Yamazaki O, Shirai A, Nakamura M, Iso-O N, Li Y. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARγ-dependent nongenomic signaling. Cell Metab. 2011;13:550-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Kiley SC, Chevalier RL. Species differences in renal Src activity direct EGF receptor regulation in life or death response to EGF. Am J Physiol Renal Physiol. 2007;293:F895-F903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kiley SC, Thornhill BA, Belyea BC, Neale K, Forbes MS, Luetteke NC, Lee DC, Chevalier RL. Epidermal growth factor potentiates renal cell death in hydronephrotic neonatal mice, but cell survival in rats. Kidney Int. 2005;68:504-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, Sanjay A, Collinge M, Baron R, Sessa WC, Bender JR. Variant estrogen receptor-c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proc Natl Acad Sci USA. 2007;104:16468-16473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Kahle KT, Wilson FH, Leng Q, Lalioti MD, O’Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 361] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl- flux in extrarenal epithelia. Proc Natl Acad Sci USA. 2004;101:2064-2069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int J Hypertens. 2011;2011:391762. [PubMed] [Cited in This Article: ] |
| 36. | Féraille E, Carranza ML, Rousselot M, Favre H. Insulin enhances sodium sensitivity of Na-K-ATPase in isolated rat proximal convoluted tubule. Am J Physiol. 1994;267:F55-F62. [PubMed] [Cited in This Article: ] |
| 37. | Gesek FA, Schoolwerth AC. Insulin increases Na(+)-H+ exchange activity in proximal tubules from normotensive and hypertensive rats. Am J Physiol. 1991;260:F695-F703. [PubMed] [Cited in This Article: ] |
| 38. | Ruiz OS, Qiu YY, Cardoso LR, Arruda JA. Regulation of the renal Na-HCO3 cotransporter: IX. Modulation by insulin, epidermal growth factor and carbachol. Regul Pept. 1998;77:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104-1109. [PubMed] [Cited in This Article: ] |
| 40. | Nakamura M, Yamazaki O, Shirai A, Horita S, Satoh N, Suzuki M, Hamasaki Y, Noiri E, Kume H, Enomoto Y. Preserved Na/HCO3 cotransporter sensitivity to insulin may promote hypertension in metabolic syndrome. Kidney Int. 2014;87:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006;17:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Zheng Y, Yamada H, Sakamoto K, Horita S, Kunimi M, Endo Y, Li Y, Tobe K, Terauchi Y, Kadowaki T. Roles of insulin receptor substrates in insulin-induced stimulation of renal proximal bicarbonate absorption. J Am Soc Nephrol. 2005;16:2288-2295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Pavlov TS, Ilatovskaya DV, Levchenko V, Li L, Ecelbarger CM, Staruschenko A. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J. 2013;27:2723-2732. [PubMed] [Cited in This Article: ] |
| 44. | Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1943] [Cited by in F6Publishing: 1923] [Article Influence: 106.8] [Reference Citation Analysis (3)] |
| 45. | Vigneri R, Squatrito S, Sciacca L. Insulin and its analogs: actions via insulin and IGF receptors. Acta Diabetol. 2010;47:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985-17990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 516] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 47. | Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 48. | Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch. 1977;367:295-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 285] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Horita S, Zheng Y, Hara C, Yamada H, Kunimi M, Taniguchi S, Uwatoko S, Sugaya T, Goto A, Fujita T. Biphasic regulation of Na -HCO3- cotransporter by angiotensin II type 1A receptor. Hypertension. 2002;40:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest. 1984;73:507-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 266] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Zheng Y, Horita S, Hara C, Kunimi M, Yamada H, Sugaya T, Goto A, Fujita T, Seki G. Biphasic regulation of renal proximal bicarbonate absorption by luminal AT(1A) receptor. J Am Soc Nephrol. 2003;14:1116-1122. [PubMed] [Cited in This Article: ] |
| 52. | Haithcock D, Jiao H, Cui XL, Hopfer U, Douglas JG. Renal proximal tubular AT2 receptor: signaling and transport. J Am Soc Nephrol. 1999;10 Suppl 11:S69-S74. [PubMed] [Cited in This Article: ] |
| 53. | Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M. Signaling pathways in the biphasic effect of angiotensin II on apical Na/H antiport activity in proximal tubule. Kidney Int. 1996;50:1496-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Poggioli J, Lazar G, Houillier P, Gardin JP, Achard JM, Paillard M. Effects of angiotensin II and nonpeptide receptor antagonists on transduction pathways in rat proximal tubule. Am J Physiol. 1992;263:C750-C758. [PubMed] [Cited in This Article: ] |
| 55. | Li Y, Yamada H, Kita Y, Kunimi M, Horita S, Suzuki M, Endo Y, Shimizu T, Seki G, Fujita T. Roles of ERK and cPLA2 in the angiotensin II-mediated biphasic regulation of Na+-HCO3(-) transport. J Am Soc Nephrol. 2008;19:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Liu FY, Cogan MG. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest. 1989;84:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 155] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Zhang C, Mayeux PR. NO/cGMP signaling modulates regulation of Na+-K+-ATPase activity by angiotensin II in rat proximal tubules. Am J Physiol Renal Physiol. 2001;280:F474-F479. [PubMed] [Cited in This Article: ] |
| 58. | Shirai A, Yamazaki O, Horita S, Nakamura M, Satoh N, Yamada H, Suzuki M, Kudo A, Kawakami H, Hofmann F. Angiotensin II dose-dependently stimulates human renal proximal tubule transport by the nitric oxide/guanosine 3’,5’-cyclic monophosphate pathway. J Am Soc Nephrol. 2014;25:1523-1532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 192] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol. 2002;282:F777-F784. [PubMed] [Cited in This Article: ] |
| 62. | Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension. 1999;33:1008-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Schmidt RJ, Beierwaltes WH, Baylis C. Effects of aging and alterations in dietary sodium intake on total nitric oxide production. Am J Kidney Dis. 2001;37:900-908. [PubMed] [Cited in This Article: ] |
| 64. | Parker JD, Farrell B, Fenton T, Cohanim M, Parker JO. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991;84:2336-2345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 122] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 65. | White WB, Halley SE. Comparative renal effects of intravenous administration of fenoldopam mesylate and sodium nitroprusside in patients with severe hypertension. Arch Intern Med. 1989;149:870-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Sarafidis PA, Lasaridis AN. Diabetic nephropathy: Endothelin antagonism for diabetic nephropathy. Nat Rev Nephrol. 2010;6:447-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |