Published online Nov 6, 2014. doi: 10.5527/wjn.v3.i4.156
Revised: September 30, 2014
Accepted: October 14, 2014
Published online: November 6, 2014
Chronic kidney disease (CKD) is recognised as a health concern globally and leads to high rates of morbidity, mortality and healthcare expenditure. CKD is itself an independent risk factor for unfavorable health outcomes that include cardiovascular disease (CVD). Coronary artery disease is the primary type of CVD in CKD patients and a significant cause of death among renal transplant patients. Traditional and non-traditional risk factors for CVD exist in patients with CKD. Traditional factors include smoking, hypertension, dyslipidemia and diabetes which are highly prevalent in CKD patients. Non-traditional risk factors of CKD are mainly uraemia-specific and increase in prevalence as kidney function declines. Some examples of uraemia-specific risk factors that have been well documented include low levels of haemoglobin, albuminuria, and abnormal bone and mineral metabolism. Therapeutic interventions targeted at more traditional risk factors which contribute to CVD, have not had the desired effect on lowering CVD events and mortality in those suffering with CKD. Future research is warranted to delineate clear evidence to the benefit of modifying non-traditional risk factors.
Core tip: Chronic kidney disease (CKD) has been recognised as a health concern globally and leads to high morbidity, mortality and healthcare expenditure. CKD is an independent risk factor for several different unfavourable outcomes including cardiovascular disease (CVD). Traditional and non-traditional risk factors for CVD exist in patients with CKD. Non-traditional risk factors of CKD are mainly uraemia-specific and include release of large levels of inflammatory and prothrombotic factors, low levels of haemoglobin, albuminuria, and abnormal bone and mineral metabolism. Future research is warranted to delineate clear evidence to the benefit of modifying non-traditional risk factors
- Citation: Alani H, Tamimi A, Tamimi N. Cardiovascular co-morbidity in chronic kidney disease: Current knowledge and future research needs. World J Nephrol 2014; 3(4): 156-168
- URL: https://www.wjgnet.com/2220-6124/full/v3/i4/156.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i4.156
CKD has become recognised as a key independent risk factor for several adverse health outcomes including cardiovascular disease (CVD). It is now increasingly apparent that individuals are more likely to die from cardiovascular disease than to develop end stage renal disease (ESRD)[1-3]. Initial evidence indicating a relationship between renal dysfunction and adverse cardiovascular events became apparent in those on dialysis, where the number of CVD deaths was found to be raised. Almost 50% of those suffering from established ESRD are unlikely to survive a CVD event[2-5]. Compared to the age adjusted CVD mortality in the general populations this is approximately 15 to 30 times higher[4,6]. Although true across all ages it is particularly more profound in the 25-34 year age group, where a 500-fold increase in CVD mortality rate is found when comparing it to their counterparts in the general population[1].
According to the Kidney Disease Improving Global Outcomes (KDIGO), CKD can be defined as either damage to kidneys or a glomerular filtration rate (GFR) of < 60 mL/min per 1.73 m2 for a period of ≥ 3 mo, with implications for health. Kidney damage can be defined by structural (detected by imaging) or functional abnormalities of the kidneys with or without a decrease in GFR. These may be apparent as either pathological irregularities or as indicators of kidney damage which include albuminuria > 30 mg/d, urine sediment abnormalities and electrolyte and other abnormalities secondary to tubular disorders.
Individuals with CKD are usually staged according to their GFR levels (Stage 1-5) and albuminuria category, with a higher stage representing lower GFR levels[7,8].
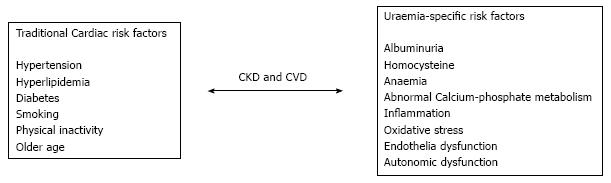
Traditional risk factors for atherosclerotic CVD are not enough to justify the significant upsurge in cardiovascular mortality seen amongst CKD patients and in particular ESRD. This has led to the suggestion that in patients with CKD two groups of CVD risk factors can be defined; traditional and non-traditional. Traditional factors are those described in the Framingham study[9] including hypertension, smoking, dyslipidemia and diabetes that are well known to contribute to the acceleration of the atherosclerotic process, and are highly prevalent in CKD patients[10]. Non-traditional risk factors of CKD increase in prevalence as kidney function declines. Some examples of such risk factors have been and includes, of large levels of inflammatory and prothrombotic factors, low levels of haemoglobin, albuminuria, and abnormal bone and mineral metabolism[4,11]. Some of these non-traditional risk factors will be discussed in more detail below (Figure 1).
Echocardiographic studies report a high prevalence of left ventricular hypertrophy (LVH), systolic/diastolic dysfunction and ventricular dilatation (dilated cardiomyopathy) in patients with ESRD[12-15]. They are more strongly associated with an unfavourable prognosis than more established cardiovascular risk factors[14,16]. These structural and functional abnormalities can lead to sudden, presumed arrhythmic death and account for 50% of cardiovascular deaths in patients with ESRD[17-19].
LVH exists in 70% of patients starting dialysis and is an independent risk factor for cardiac death[12,13,20]. In a cross-sectional study by Stewart and colleagues, 296 non-diabetic renal disease patients underwent echocardiographic monitoring. The results showed that left ventricular mass was increased from even the earliest stages of renal disease (near-normal renal function). Eccentric LVH was found to be the prevalent pattern. The increase in LVH was progressive and 80% of those on renal replacement therapy were found to have LVH, with the concentric pattern being more dominant[19].
In an early study by Levin et al[21], 175 pre-dialysis patients underwent echocardiographic monitoring and had their left ventricular mass index (LVMI) assessed. The study demonstrated that the presence of LVH increases with progressive renal decline, reaching 45.2% in patients having severe renal impairment (CrCl < 25 mL/min)[21].
Paoletti et al[22] studied 244 non-diabetic pre-dialysis patients and found a greater prevalence, with LVH being associated with 51% in CKD stage 1 and 2 patients and 78% in CKD stages 3 to 5. In all studies - age, haemoglobin, systolic blood pressure and CrCl were found to be significantly different between those who either did or did not have LVH.
Coronary artery disease (CAD) is one of the primary types of CVD in patients with CKD and is a major cause of death among renal transplant patients[23-27]. The prevalence varies from 24% in young patients without diabetes, to 85% in elderly haemodialysis patients with diabetes[28,29].
In earlier studies it was found that approximately 25%-40% of asymptomatic patients undergoing coronary angiography before their renal transplant exhibited evidence of significant stenosis (50%-70%) in one or more coronary arteries[30-32]. Furthermore, Liu and colleagues, showed that patients with CKD were found to have a 2.5 times higher chance of having 3-vessel disease compared with patients without CKD[33].
Kiyosue et al[27] studied the association found amongst renal dysfunction and the severity of CAD. It looked at 572 patients and graded severity according to how many stenotic coronary arteries were there and the estimated GFR (eGFR). More stenotic coronary arteries were present in the CKD group compared to other groups. Multivessel stenosis was also greater in the CKD group[27].
Whether or not CAD should be screened for and interventions offered for patients with advanced kidney disease remains a contentious issue. Coronary angiography remains the modality of choice for CAD investigation in CKD patients, however its invasiveness and associated risks in a group at risk of contrast-induced nephropathy, makes it unfavourable[34,35]. While other imaging modalities exist which are effective (CT coronary angiography, cardiovascular MRI), there are several factors including significant costs, adverse affects as well as technical difficulties that must be considered[36,37].
Anaemia is an anticipated consequence as renal function declines, and generally begins to develop before ESRD. The severity of anaemia however increases with declining kidney function[38,39]. There is a strong association between anaemia and cardiovascular complications. Specifically, anaemia is linked to LVH development, found in up to 74% of patients at the commencement of renal replacement therapy and is an independent predictor of consequent cardiac morbidity and mortality among patients with ESRD[12,13,21,40]. In an Observational study it was demonstrated that each 10 g/L drop in haemoglobin, leads to a 20%-40% increased risk of developing heart failure, LVH or mortality in those patients on long-term dialysis[41]. Interestingly sustained anaemia is often associated with eccentric hypertrophy, whereas hypertension is associated with concentric hypertrophy[21].
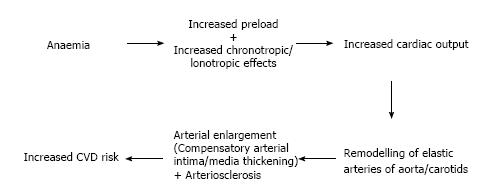
Physiologically, chronic anaemia leads to an increased cardiac output (CO) as a result of decreased afterload, an increase in preload and an increase in chronotropic/inotropic effects. This will eventually lead to ventricular dilation and LVH[42-44]. This chronic rise in CO eventually causes remodeling of the central elastic arteries of aorta or carotids. Subsequently it can result in enlargement of arteries and compensatory intima-media thickening, or arteriosclerosis. If either is present, they could be more directly correlated with future CVD risk (Figure 2)[44,45].
One of the first studies to demonstrate anaemia as an independent risk for CVD outcomes was carried out in the ARIC studies. It found anaemia to be associated with an adjusted hazard ratio of 1.41 for CVD in the entire cohort[46].
Jurkouitz et al[47] looked at 13329 patients and found a significant link between the haemoglobin (Hb) level and the serum creatinine (S-cr). In anaemic patients, a high S-cr level can result in the risk of coronary events rising by 2.7 fold when compared to a normal S-cr. This is independent of risk factors that include age, gender and race[47].
Levin et al[48] focused on the association between anaemia and LVH. 246 participants with a creatinine clearance of 25 to 75 mL/min (0.42 to 1.25 mL/s) underwent echocardiographic imaging done at baseline and 12 mo to specifically investigate LV growth. The results showed that each 0.5-g/dL (5-g/L) drop in Hb level led to a 32% increased odds of LV growth[48].
Further anaemia and echocardiographic studies carried out by Foley et al[41], demonstrated that each 1 g/dL drop in Hb was independently linked with the presence of left ventricular dilatation on repeat echocardiogram and the subsequent development of new/recurrent heart failure. Furthermore, every 1 g/dL drop in the haemoglobin level was independently linked with mortality when patients were on dialysis therapy[41].
Weiner et al[49] looked at the combination of both LVH and anaemia in patients at the earlier stages of renal dysfunction (eGFR 30-60), revealing that patients who had LVH as well as anaemia, had a risk of cardiac disease that increased by 4-fold compared with individuals who had neither anaemia nor LVH. However in those having LVH but no anaemia or anaemia but no LVH, the risk of CVD outcomes did not increase significantly[49].
The strong association between anaemia and cardiovascular complications has led to several studies investigating whether correction of haematocrit has any benefit on adverse cardiovascular outcomes. Besarab et al[50], looked at 1233 patients on haemodialysis with heart disease who were prescribed recombinant human erythropoietin (epoetin), looking at length of time till death or the first non-fatal myocardial infarction. Six hundred eighteen patients received sufficient doses to sustain a haematocrit of 42% and 615 to sustain a haematocrit of 30%. The study was halted due to the higher haematocrit target group having an almost significant mortality risk, and the fact that thrombotic vascular access events were also higher. No single unifying explanation was thought to be the cause[50].
The Cardiovascular Risk Reduction by Early Anaemia Treatment with Epoetin Beta (CREATE) trial looked at patients in the earlier stages of CKD (3 and 4) to try and achieve levels of normal; 130-150 g/L and low to normal; 105-115 g/L Hb. The normal Hb group was found to have an improved overall health and quality of life. LVMI was found in both groups to be stable, and thus treating the anaemia did not have an effect on the LVH progression[51]. This is supported by many other studies[52-54].
The Anaemia Working Group of European Renal Best Practice (ERBP) published a statement on its opinion of what Hb targets should be. Following results of the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Study they maintained that Hb values of 11-12 g/dL should be targeted in CKD patients, and without deliberately aiming for targets above 13 g/dL[55,56].
Correcting the anaemia associated with ESRD with recombinant human erythropoietin (r-EPO)/ iron supplementation is essential, and results in significant improvements of Hb levels as well as a reduction in the need for blood transfusion requirements[57,58]. Recent literature however, suggests excess iron as well as contributing to arrhythmias and heart failure may also have a role to play in the development of vascular calcification[59,60].
A cross sectional study by Bagheri et al[61] looked at 337 patients to evaluate the importance of iron stores in the risk evaluation of atherosclerotic disease. All patients had an angiogram of the coronary artery, and the study revealed that the iron serum level was significantly more raised in the severe atherosclerosis group than in the normal group[61].
It is thought that one of the ways in which iron contributes to vascular calcification is by enhancing the oxidative stress process through the induction of reactive oxygen species. This subsequently activates molecular mechanisms that result in vascular calcification[60].
Drüeke et al[62] looked at the role of I.V. iron therapy in 79 dialysis patients. They concluded that iron therapy (at dose 1.5-2 g per year) can lead to arterial wall damage in the early stages of atherosclerosis[62]. This was later supported by Reis et al[63] who showed a significant relationship between ferritin, I.V. iron dosage and common carotid artery intima-media thickness in 60 dialysis patients[63].
Further research is required to assess the effect that iron has on arterial calcification, however the potential of iron to cause oxidant injury and CVD should not be disregarded.
Homocysteine (HC) at high levels can be an independent risk factor for cardiovascular disease[64]. Levels of HC are elevated in renal failure and there is an inverse relationship between HC levels and GFR, such that more than 85% of patients on dialysis will experience a mild to moderate level of hyperhomocysteinemia[64,65].
The pathogenesis of hyperhomocysteinemia in renal dysfunction remains to be elucidated[64,66]. There is some evidence to suggest that high levels of homocysteine may be due to a reduced HC clearance and insufficient metabolism by less well functioning kidneys. However there is no direct evidence to support this[64].
Several epidemiological studies suggest that a high level of HC specifies a higher risk of CVD as well as stroke[67,68]. While the underlying mechanisms are yet to be defined, both in vitro/vivo studies suggest that production of potent reactive oxygen species (ROS) and decreased endothelial nitric oxide play a pivotal role. Thus high HC levels may facilitate oxidative damage at the vascular interface[69-71]. Other proposed mechanisms suggest that elevated HC causes proliferation of smooth muscle cells thus leading to increased oxidation of low-density lipoproteins[72]. Elevated HC is also associated with increased platelet aggregation and hence favouring a prothrombotic state[73]. This strongly links elevated levels of HC to the enhancement of atherosclerosis and other thrombotic events.
Several trials have looked at the efficacy of HC-lowering treatments on clinical outcomes. Two major studies, Homocysteine study (HOST)[74] and Heart Outcomes Prevention Evaluation-2 (HOPE-2)[75] looked for any benefits in certain vitamins including; folic acid, vitamin B6 and vitamin B12 supplements on overall CVD risk and mortality. Both studies found no significant benefit on CVD risk or all-cause mortality. Therefore based on these trials, there is not much to support using HC-lowering interventions for preventing cardiovascular outcomes.
Dysfunction in the metabolism of minerals occurs early in CKD. As GFR levels decline, there is a decrease in serum calcium (Ca) levels while parathyroid hormone (PTH) and phosphate levels become elevated[76]. An elevated level of serum phosphorus is highly prevalent in ESRD patients, and is a significant and independent risk factor of all cause and cardiovascular mortality[77]. A study by Block et al[77] found that phosphate levels greater than 6.5 mg/dL were associated with a far greater mortality risk (27%) when compared with levels of between 2.4-6.5 mg/dL[77].
Further studies by Kestenbaum et al[78] demonstrated that PO4 levels > 3.5 mg/dL are linked with an increased risk of death that is significant. Furthermore, for every 0.323 mmol/L serum phosphate increase, there was an increased risk of death by 23%[78].
In a study by Dhingra et al[79] it was suggested that even phosphate levels within the normal range can contribute to CVD in patients who have kidney function within the normal, to near-normal range. Furthermore phosphate levels above 1.1 mmol/L can increase the risk of CVD events by 55%, following adjustment for any traditional cardiovascular risk factors. The cholesterol and recurrent events study (CARE) enlisted 4159 patients who had a background of previous myocardial infarction concluded that there was a graded, independent relationship between baseline fasting serum phosphate level and the subsequent risk for all-cause mortality, the development of new heart failure, and coronary events[80].
Calcium-phosphate products are also associated with increased risk of cardiovascular morbidity and mortality in CKD patients. Ganesh et al[81] demonstrated that for every rise in serum calcium-phosphate product by 0.8 mmol2/l2, there was an increased sudden death risk of approximately 7% in those on long-term haemodialysis[81].
The underlying mechanism through which hyperphosphatemia and an increase in calcium-phosphate product leads to cardiovascular disease is not well established. One theory is that high phosphate levels exacerbate the atherosclerosis process by increased calcification and proliferation of smooth muscle[82].
Raggi et al[83] carried out a cross-sectional study of 205 patients on hemodialysis who had baseline electron-beam tomography (EBT) testing to evaluate both vascular/valvular calcification. The incidence and degree of valvular calcification was found to be remarkable with 45% of subjects having calcification of the mitral valve, and 34% having calcification of the aortic valve, compared with 3%-5% prevalence in the general population. More than 70% of patients had coronary artery calcification significant enough to be linked with a high risk of future MI and coronary death in the general population[83].
Goodman et al[84] screened for calcification using EBT in 39 young patients (age range 7 to 30 years of age) with ESRD on dialysis. It found evidence of coronary calcification in 14 of the 16 patients aged 20-30 years[84].
Current KDOQI guidelines advise that serum phosphate levels should be maintained at 0.8 mmol/L in stage 3-4 CKD and between 1.1 and 1.8 mmol/L in stage 5 CKD[8]. Several phosphate binders exist in the treatment of hyperphosphataemia, however the choice of binders is controversial.
Calcium-based binders have been shown in observational studies to be associated with arterial calcification[84,85]. Sevelamer is a non-absorbable agent that does not contain calcium and has been shown in a significant number of trials to be effective in lowering serum phosphate levels. It has also been shown to have beneficial effects on vascular calcification progression and bone disease[86].
Two large studies have compared Sevelamer with calcium-based binders. In the Renagel in New Dialysis (RIND) trial it found that calcium-based phosphate binders resulted in higher cases of mortality than compared to Sevelamer[87]. The Dialysis Clinical Outcomes Revisited (DCOR) study however showed that the difference in mortality was not significant. Conflicting results can be possibly explained by difference in patient population[88].
Not only is albuminuria a marker of renal damage, it is also an independent risk factor for CVD and leads to an increase in all cause mortality in diabetics, those with hypertension and in relatively unselected or general populations[89-93].
In the Heart Outcomes Evaluation (HOPE) trial results showed that in those with or without diabetes, albuminuria of any level can be a risk factor for CVD events. It also found microalbuminuria to result in an increased risk of future stroke, myocardial infarctions and death in both diabetic and non-diabetics without CKD.
Additionally, for every increase in the albumin:creatinine ratio (ACR) by 0.4 mg/mmol, there was a 5.9% increase in the HR hazard ratio (HR) of major CVD outcomes[91].
The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial studied 8206 patients in order to establish if the relationship between albuminuria and cardiovascular risk can be useful in predicting cardiovascular morbidity and mortality in hypertensive patients. It discovered that for every increase of the ACR by 10-fold, cardiovascular deaths increased up to 98% in non-diabetics without CKD[92].
These trials demonstrate that the relationship between albuminuria and experiencing a CV event is not entirely restricted to the microalbuminuria cut off range. The relationship between the ACR and CV disease can extend to at least as low as 0.5 mg/mmol and thus an ACR of 2.0 mg/mmol, the threshold for the screening of microalbuminuria may not be appropriate when considering the risk for CV outcomes[91,92].
Microalbuminuria has also been associated with several lipid abnormalities. In a study by Kahri et al[94], the lipid profiles in both those having microalbuminuria and normoalbuminuria were compared. It found that the high-density lipoproteins (HDL) that are known to be cardioprotective were 11.6-fold lower in microalbuminuric patients compared with normoalbuminuric patients[94].
The pathophysiology behind how microalbuminuria contributes to CVD remains to be fully understood, however studies suggest that microalbuminuria might reflect endothelial dysfunction[95,96]. As well as causing impaired arterial dilatory capacity[97], microalbuminuria has been shown to increase levels of several adhesion molecules including Von willeband factor (vWF), Vascular adhesion molecule-1, thrombomodulin, PAI-1, serum IV collagen and t-PA[87]. These all favour the formation of atherosclerosis[98].
It is now well established that the incidence of acute-phase inflammation and oxidative stress in patients with ESRD is high, which are both significant contributors to a high degree of CVD morbidity and mortality[99-101]. Oberg et al[102] confirmed presence of increased oxidative stress and acute-phase inflammation in early and advanced stages of CKD (3-5) compared to healthy subjects. Renal insufficiency is associated with increased levels of several different inflammatory and pro-coagulant biomarkers, the main two being CRP and IL-6, which are strong predictors of all-cause mortality and cardiovascular outcomes in ESRD[103-105]. Elevation of these markers as well as fibrinogen, PAP, factor VII-VIII, and D-dimer was apparent even in those who had no evidence of clinical or subclinical cardiovascular disease[106-108].
The extent to which GFR is related to biomarkers of inflammation is controversial. While one cross sectional study found that increased CRP was associated with decreased GFR, other studies have found serum CRP levels do not correlate with either GFR or disease progression[109].
A study by Friedman et al[110], which looked at both CRP and albumin in dialysis patients, found CRP to be a significant predictor of death and suggested that these patients need to have careful evaluation as well as monitoring irrespective of whether albumin concentration is in the normal range[111]. Evidence seems to suggest that CRP may also be responsible for numerous processes involved in propagating atherosclerosis, which includes plaque initiation, formation, and rupture[111].
Inflammatory and prothrombotic markers have been heavily linked with CVD and mortality in CKD patients. Shlipak et al[112] however, demonstrated that although CRP and IL-6 are linked with CVD, their collective impact on cardiovascular mortality is actually far less significant compared to the collective impact of the more traditional risk factors[112].
Numerous experimental studies have revealed that endothelium derived vasoactive mediator nitric oxide (NO) has a vital part to play in progressive kidney damage. Low levels of NO leads to endothelial cell injury and dysfunction, and plays a major role in potentiating atherosclerosis[113-115]. Animal studies in which NO synthase (NOS) was inhibited resulted in enhanced progression of the atherosclerotic process as well as causing impairment in the angiogenic response and loss of the capillary endothelium[116]. Endogenous NOS inhibitor, asymmetric dimethylarginine (ADMA) is thought to be significantly associated with the oxidative stress process through its inhibition of NO, and thus leading to endothelial dysfunction and vascular damage[117]. ADMA correlates with traditional and non-traditional risk factors, it is recognised as a strong indicator in atherosclerosis, and is a strong independent predictor of death and incident cardiovascular complications in both CKD and non-CKD patients[117-119].
In a cohort study of 131 patients with CKD, the correlation between levels of ADMA and the probability of progressing to ESRD and death was investigated. ADMA was found to be reliable in predicting event occurrence independently of other confounders, such as GFR, proteinuria and several others. Furthermore, ADMA was found to be inversely related to GFR and signifies an independent marker of risk for ESRD progression and mortality[117].
Several studies have looked at interventions in order to reduce the plasma levels of ADMA or its binding capability to NOS in an attempt to decrease any risk of CVD events in those suffering from CKD. Lerman et al[120] studied the effects of supplementing 26 patients with L-arginine, a precursor to NO in order determine its future therapeutic use. It found that following 6 mo of supplementation, it improved endothelium function in coronary vessels while also providing symptomatic relief and lowering levels of endothelin[120]. Further support of these results come from studies by Clarkson et al[121] who explained that L-arginine orally enhanced the peripheral endothelium-dependent dilation of hypercholesterolaemic patients, as well as Rector et al[122] who showed that L-arginine was helpful in heart failure subjects.
Other studies have looked at the antioxidant effects of acetylcysteine and Vitamin E. Both supplements have demonstrated a reduction in composite cardiovascular end points in haemodialysis patients[123,124].
Hypertension itself can act as a dominant risk factor for CVD in patients with CKD, and it is almost inevitable that CKD patients will have hypertension. The underlying mechanism considered most important in the elevation of blood pressure, is related to retention of sodium as well as stimulation of the renin-angiotensin system[125]. Sympathetic activation and elevated catecholamine release in CKD has also been linked[126,127]. Cardiac damage caused by hypertension in CKD patients is thought to be via LVH induction[128].
Two major studies have evaluated the relationship between renal function and mortality in hypertensive patients. In the Hypertension Detection and Follow-up Program Cooperative Group, 10490 patients where analysed to assess all-cause mortality. It demonstrated that in those who had a baseline creatinine of ≥ 1.7 mg/dL the mortality rate (8-year) was ≥ 3 times greater than that of all other patients[129].
The Hypertension Optimal Treatment (HOT) study supports these outcomes. They evaluated 18790 patients over 3.8 years, only 10% of whom had evidence of atherosclerotic plaques. It found that the relative risk for both mortality and CVD events was 1.65 and 1.58 respectively, in those with a GFR < 60 mL/min compared with those who had a GFR > 60 mL/min[130].
Despite strong evidence linking CVD mortality in hypertensive CKD patients, the ideal blood pressure (BP) targets in these patients remains a challenging area. The National Institute for Health and Clinical excellence (NICE) suggests that antihypertensive treatment be commenced in those < 80 years with declining renal function and hypertension-stage 1, to aim for a blood pressure of < 140 ⁄ 90 mmHg. The BP during haemodialysis/peritoneal dialysis period should not exceed > 160 mmHg[8,131]. However excessive BP lowering in these patients may prove to be detrimental due to the risk of exacerbating myocardial stunning[132-134]. There remains an insufficient number of RCT trials on optimum blood pressure control in CKD patients and it is important to address this issue.
While hyperuricemia is a well-recognised consequence of impaired renal function it is also linked with increasing hypertension risk, ESRD and unfavorable cardiovascular outcomes[135].
UA has been demonstrated as an independent risk factor for the onset of CKD, in a healthy population. Obermayer et al[136] reported that even with a modest rise in UA levels there was a two-fold increased risk of renal disease, whilst at 535 µmol/L or more the risk was three-fold.
Sedaghat et al[137] further supported this theory. They analysed 2601 subjects aged > 55 years who where followed up over a 6.5 year period. They exhibited that for each 60 μmol/L rise of uric acid, there was a decline in the eGFR by 0.19 mL/min. In hypertensive patients the decline in eGFR was more profound[137].
The detrimental effects of hyperuricemia are also well documented in CVD. In the health professionals follow-up study hyperuricemia was found to increase CVD mortality even more than compared with those who already had established heart disease[138]. This was further supported by long-term data from the NHANES I study that demonstrated that there was a proportionate rise in CVD mortality with UA levels[139].
Studies have looked at the use of xanthine oxidase (XO) inhibitors (Allopurinol, Febuxostat), as potential treatment to prevent further renal deterioration and to provide a cardioprotective effect. In one meta-analyses, allopurinol was found to produce a small but yet significant reduction of both systolic/diastolic blood pressure. This is further supported by randomised controlled trials, where again a significant blood pressure drop is achieved with UA-lowering agents[140].
Rekhraj et al[141] demonstrated that treating patients with diagnosed LVH and ischaemic heart disease with high dose allopurinol (600 mg/d) for 9 mo, resulted in a reduction of the LVM and end systolic volume as well as improving endothelial function[141]. This modest reduction in LVM was shown in the LIFE study to reduce mortality and cardiovascular outcomes by 13%[142,143].
In the context of CKD, a study by Goicoechea et al[144] demonstrated that in those patients receiving allopurinol for 12 mo there was a diminution in the deterioration of kidney function or the need for dialysis compared to placebo. (143) In another study allopurinol reduced eGFR in patients with, established CKD (stage 3) independent of age, sex or diabetes. Adverse cardiovascular events were also found to be reduced[144].
CKD is a major health concern globally and leads to high rates of morbidity, mortality and healthcare expenditure. Much of the morbidity and mortality associated with CKD is significantly attributable to cardiovascular outcomes. While we have tried to briefly analyse some of the current knowledge underlying the strong association between CKD and CV outcomes there still remains several non-traditional risk factors and pathophysiological mechanisms to be described.
Several markers have a clear association with current and subsequent CV outcomes including; reduced GFR, albuminuria, troponins, phosphate, vitamin D, FGF-23 and NT-proB-NP[145-152]. While these markers are present, there remains no routine screening for CVD in CKD patients, despite strong evidence supporting this. The screening and treatment of patients with abnormal markers cannot only reduce overall cardiovascular events and kidney failure, but could also prove to be cost effective.
Perhaps one reason for lack of implementation is due to the fact that these diagnostic screening tools are lacking in sensitivity and specificity to make them reliable, and are in need of more RCT-quality evidence in order to guide intervention.
Therapeutic interventions that aim at reducing traditional risk factors for CVD have not been shown to be effective at lowering the incidence of CVD events or mortality in those with CKD. More importantly, strategies in risk reduction have inadequately tackled non-traditional risk factors that have been exhibited as being essential in the progression of CVD. Furthermore there remains to be clear evidence with regards to the benefit of modifying these non-traditional risk factors.
In view of current knowledge it is perhaps favourable to investigate preventive strategies in early-stage chronic kidney disease and multifactorial interventions in late-stage chronic kidney disease.
Some of the excess CVD risk associated with CKD has been explained by a multifactorial range of myocardial and vascular insult including; uraemic cardiomyopathy, inflammation, oxidative stress, autonomic dysfunction and endothelial dysfunction. However, it is very likely that there are as yet undiscovered factors, and inter-relationships, which are possibly of great significance.
P- Reviewer: Chang CC, Chawla M, Fassett RG S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 786] [Cited by in F6Publishing: 786] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 2. | Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 1113] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 3. | Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1021] [Cited by in F6Publishing: 1101] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 4. | Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16-S23. [PubMed] [Cited in This Article: ] |
| 5. | Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 680] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 6. | Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606-1615. [PubMed] [Cited in This Article: ] |
| 7. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] [Cited in This Article: ] |
| 8. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;3:S1-130. [PubMed] [Cited in This Article: ] |
| 9. | Kannel WB, DAWBER TR, KAGAN A, REVOTSKIE N, STOKES J. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1166] [Cited by in F6Publishing: 1024] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 10. | Vlagopoulos PT, Sarnak MJ. Traditional and nontraditional cardiovascular risk factors in chronic kidney disease. Med Clin North Am. 2005;89:587-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Foley RN, Parfrey PS. Cardiac disease in chronic uremia: clinical outcome and risk factors. Adv Ren Replace Ther. 1997;4:234-248. [PubMed] [Cited in This Article: ] |
| 12. | Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 833] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 532] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 403] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol. 2000;11:912-916. [PubMed] [Cited in This Article: ] |
| 16. | McGregor E, Jardine AG, Murray LS, Dargie HJ, Rodger RS, Junor BJ, McMillan MA, Briggs JD. Pre-operative echocardiographic abnormalities and adverse outcome following renal transplantation. Nephrol Dial Transplant. 1998;13:1499-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Beaubien ER, Pylypchuk GB, Akhtar J, Biem HJ. Value of corrected QT interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am J Kidney Dis. 2002;39:834-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Lörincz I, Zilahi Z, Kun C, Mátyus J, Kakuk G. ECG abnormalities in hemodialysis. Am Heart J. 1997;134:1138-1140. [PubMed] [Cited in This Article: ] |
| 19. | Stewart GA, Gansevoort RT, Mark PB, Rooney E, McDonagh TA, Dargie HJ, Stuart R, Rodger C, Jardine AG. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. 2005;67:217-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Parfrey PS, Harnett JD, Griffiths SM, Taylor R, Hand J, King A, Barre PE. The clinical course of left ventricular hypertrophy in dialysis patients. Nephron. 1990;55:114-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 112] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 450] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 22. | Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46:320-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | De Leeuw PW, Thijs L, Birkenhäger WH, Voyaki SM, Efstratopoulos AD, Fagard RH, Leonetti G, Nachev C, Petrie JC, Rodicio JL. Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst-Eur trial. J Am Soc Nephrol. 2002;13:2213-2222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol. 2001;12:218-225. [PubMed] [Cited in This Article: ] |
| 25. | Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 977] [Cited by in F6Publishing: 925] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 26. | Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol. 2001;38:705-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Kiyosue A, Hirata Y, Ando J, Fujita H, Morita T, Takahashi M, Nagata D, Kohro T, Imai Y, Nagai R. Relationship between renal dysfunction and severity of coronary artery disease in Japanese patients. Circ J. 2010;74:786-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Goldsmith DJ, Covic A. Coronary artery disease in uremia: Etiology, diagnosis, and therapy. Kidney Int. 2001;60:2059-2078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Manske CL, Thomas W, Wang Y, Wilson RF. Screening diabetic transplant candidates for coronary artery disease: identification of a low risk subgroup. Kidney Int. 1993;44:617-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Weinrauch L, D’Elia JA, Healy RW, Gleason RE, Christleib AR, Leland OS. Asymptomatic coronary artery disease: angiographic assessment of diabetics evaluated for renal transplantation. Circulation. 1978;58:1184-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Braun WE, Phillips DF, Vidt DG, Novick AC, Nakamoto S, Popowniak KL, Paganini E, Magnusson M, Pohl M, Steinmuller DR. Coronary artery disease in 100 diabetics with end-stage renal failure. Transplant Proc. 1984;16:603-607. [PubMed] [Cited in This Article: ] |
| 32. | Lorber MI, Van Buren CT, Flechner SM, Cameron L, Leatherwood J, Walker WE, Smalling RW, Kahan BD. Pretransplant coronary arteriography for diabetic renal transplant recipients. Transplant Proc. 1987;19:1539-1541. [PubMed] [Cited in This Article: ] |
| 33. | Liu H, Yan L, Ma GS, Zhang LP, Gao M, Wang YL, Wang SP, Liu BC. Association of chronic kidney disease and coronary artery disease in 1,010 consecutive patients undergoing coronary angiography. J Nephrol. 2012;25:219-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | De Lima JJ, Sabbaga E, Vieira ML, de Paula FJ, Ianhez LE, Krieger EM, Ramires JA. Coronary angiography is the best predictor of events in renal transplant candidates compared with noninvasive testing. Hypertension. 2003;42:263-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Karthikeyan V, Ananthasubramaniam K. Coronary risk assessment and management options in chronic kidney disease patients prior to kidney transplantation. Curr Cardiol Rev. 2009;5:177-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69:1839-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Mark PB, Patel RK, Jardine AG. Screening for coronary artery disease before renal transplantation-rational or rationing? Transplantation. 2010;89:807-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Pavlović-Kentera V, Clemons GK, Djukanović L, Biljanović-Paunovic L. Erythropoietin and anemia in chronic renal failure. Exp Hematol. 1987;15:785-789. [PubMed] [Cited in This Article: ] |
| 39. | McGonigle RJ, Wallin JD, Shadduck RK, Fisher JW. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int. 1984;25:437-444. [PubMed] [Cited in This Article: ] |
| 40. | Comorbid conditions and correlations with mortality risk among 3, 399 incident hemodialysis patients. Am J Kidney Dis. 1992;20:32-38. [PubMed] [Cited in This Article: ] |
| 41. | Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 483] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. 1972;83:415-426. [PubMed] [Cited in This Article: ] |
| 43. | Gerry JL, Baird MG, Fortuin NJ. Evaluation of left ventricular function in patients with sickle cell anemia. Am J Med. 1976;60:968-972. [PubMed] [Cited in This Article: ] |
| 44. | Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15 Suppl 3:14-18. [PubMed] [Cited in This Article: ] |
| 45. | Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1102] [Cited by in F6Publishing: 1019] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 46. | Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, Levey AS. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 344] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 47. | Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919-2925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 49. | Weiner DE, Tighiouart H, Vlagopoulos PT, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1528] [Cited by in F6Publishing: 1452] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 51. | Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071-2084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1478] [Cited by in F6Publishing: 1429] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 52. | Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, Pereira BJ. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Roger SD, McMahon LP, Clarkson A, Disney A, Harris D, Hawley C, Healy H, Kerr P, Lynn K, Parnham A. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. J Am Soc Nephrol. 2004;15:148-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 54. | Ritz E, Laville M, Bilous RW, O’Donoghue D, Scherhag A, Burger U, de Alvaro F. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis. 2007;49:194-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Locatelli F, Aljama P, Canaud B, Covic A, De Francisco A, Macdougall IC, Wiecek A, Vanholder R. Target haemoglobin to aim for with erythropoiesis-stimulating agents: a position statement by ERBP following publication of the Trial to reduce cardiovascular events with Aranesp therapy (TREAT) study. Nephrol Dial Transplant. 2010;25:2846-2850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1506] [Cited by in F6Publishing: 1438] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 57. | Jones M, Ibels L, Schenkel B, Zagari M. Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int. 2004;65:757-767. [PubMed] [Cited in This Article: ] |
| 58. | Collins AJ, Li S, St Peter W, Ebben J, Roberts T, Ma JZ, Manning W. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol. 2001;12:2465-2473. [PubMed] [Cited in This Article: ] |
| 59. | Kuryshev YA, Brittenham GM, Fujioka H, Kannan P, Shieh CC, Cohen SA, Brown AM. Decreased sodium and increased transient outward potassium currents in iron-loaded cardiac myocytes. Implications for the arrhythmogenesis of human siderotic heart disease. Circulation. 1999;100:675-683. [PubMed] [Cited in This Article: ] |
| 60. | Neven E, De Schutter TM, Behets GJ, Gupta A, D’Haese PC. Iron and vascular calcification. Is there a link? Nephrol Dial Transplant. 2011;26:1137-1145. [PubMed] [Cited in This Article: ] |
| 61. | Bagheri B, Shokrzadeh M, Mokhberi V, Azizi S, Khalilian A, Akbari N, Habibi V, Yousefnejad K, Tabiban S, Nabati M. Association between Serum Iron and the Severity of Coronary Artery Disease. Int Cardiovasc Res J. 2013;7:95-98. [PubMed] [Cited in This Article: ] |
| 62. | Drüeke T, Witko-Sarsat V, Massy Z, Descamps-Latscha B, Guerin AP, Marchais SJ, Gausson V, London GM. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106:2212-2217. [PubMed] [Cited in This Article: ] |
| 63. | Reis KA, Guz G, Ozdemir H, Erten Y, Atalay V, Bicik Z, Ozkurt ZN, Bali M, Sindel S. Intravenous iron therapy as a possible risk factor for atherosclerosis in end-stage renal disease. Int Heart J. 2005;46:255-264. [PubMed] [Cited in This Article: ] |
| 64. | Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12:2181-2189. [PubMed] [Cited in This Article: ] |
| 65. | Arnadottir M, Hultberg B, Nilsson-Ehle P, Thysell H. The effect of reduced glomerular filtration rate on plasma total homocysteine concentration. Scand J Clin Lab Invest. 1996;56:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Bostom AG, Culleton BF. Hyperhomocysteinemia in chronic renal disease. J Am Soc Nephrol. 1999;10:891-900. [PubMed] [Cited in This Article: ] |
| 67. | Eikelboom JW, Lonn E, Genest J, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131:363-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 649] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 68. | Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D’Agostino RB, Wilson PW, Wolf PA. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med. 1999;131:352-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 69. | Harker LA, Slichter SJ, Scott CR, Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974;291:537-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 516] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 539] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 71. | Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, Loscalzo J. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91:308-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 582] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 72. | Tsai JC, Perrella MA, Yoshizumi M, Hsieh CM, Haber E, Schlegel R, Lee ME. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci USA. 1994;91:6369-6373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 518] [Cited by in F6Publishing: 501] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 73. | Durand P, Lussier-Cacan S, Blache D. Acute methionine load-induced hyperhomocysteinemia enhances platelet aggregation, thromboxane biosynthesis, and macrophage-derived tissue factor activity in rats. FASEB J. 1997;11:1157-1168. [PubMed] [Cited in This Article: ] |
| 74. | Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 75. | Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, Yusuf S, Lonn EM. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease--results of the renal Hope-2 study. Nephrol Dial Transplant. 2008;23:645-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1008] [Cited by in F6Publishing: 963] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 77. | Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1705] [Cited by in F6Publishing: 1610] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 78. | Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 853] [Cited by in F6Publishing: 814] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 79. | Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 600] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 80. | Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627-2633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 643] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 81. | Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131-2138. [PubMed] [Cited in This Article: ] |
| 82. | Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 415] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 83. | Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 796] [Article Influence: 36.2] [Reference Citation Analysis (2)] |
| 84. | Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1961] [Cited by in F6Publishing: 1910] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 85. | Guérin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014-1021. [PubMed] [Cited in This Article: ] |
| 86. | Ossareh S. Clinical and economic aspects of sevelamer therapy in end-stage renal disease patients. Int J Nephrol Renovasc Dis. 2014;7:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 524] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 88. | Suki WN. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients: results of a randomized clinical trial. J Ren Nutr. 2008;18:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Agewall S, Wikstrand J, Ljungman S, Fagerberg B. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol. 1997;80:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 133] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 483] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 91. | Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1708] [Cited by in F6Publishing: 1681] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 92. | Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlöf B, Devereux RB, Beevers G, de Faire U. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 395] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 93. | Pedrinelli R, Dell’Omo G, Di Bello V, Pellegrini G, Pucci L, Del Prato S, Penno G. Low-grade inflammation and microalbuminuria in hypertension. Arterioscler Thromb Vasc Biol. 2004;24:2414-2419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Kahri J, Groop PH, Elliott T, Viberti G, Taskinen MR. Plasma cholesteryl ester transfer protein and its relationship to plasma lipoproteins and apolipoprotein A-I-containing lipoproteins in IDDM patients with microalbuminuria and clinical nephropathy. Diabetes Care. 1994;17:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk CG, Tarnow L, Parving HH. Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest. 2008;68:731-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Stehouwer CD. Endothelial dysfunction in diabetic nephropathy: state of the art and potential significance for non-diabetic renal disease. Nephrol Dial Transplant. 2004;19:778-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869-1874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 98. | Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res. 1997;34:55-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 323] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 99. | Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12:1549-1557. [PubMed] [Cited in This Article: ] |
| 100. | Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. 1997;8:475-486. [PubMed] [Cited in This Article: ] |
| 101. | Maggi E, Bellazzi R, Falaschi F, Frattoni A, Perani G, Finardi G, Gazo A, Nai M, Romanini D, Bellomo G. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis? Kidney Int. 1994;45:876-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 186] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 515] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 103. | Stenvinkel P, Pecoits-Filho R, Lindholm B. Coronary artery disease in end-stage renal disease: no longer a simple plumbing problem. J Am Soc Nephrol. 2003;14:1927-1939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 104. | Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4096] [Cited by in F6Publishing: 4022] [Article Influence: 167.6] [Reference Citation Analysis (0)] |
| 105. | Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1521] [Cited by in F6Publishing: 1562] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 106. | Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118:956-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 705] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 107. | Tracy RP, Arnold AM, Ettinger W, Fried L, Meilahn E, Savage P. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly: results from the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:1776-1783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 180] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 109. | Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 110. | Friedman AN, Hunsicker LG, Selhub J, Bostom AG. C-reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney Int. 2005;68:773-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Iseki K, Tozawa M, Yoshi S, Fukiyama K. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant. 1999;14:1956-1960. [PubMed] [Cited in This Article: ] |
| 112. | Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 515] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 113. | Wever R, Boer P, Hijmering M, Stroes E, Verhaar M, Kastelein J, Versluis K, Lagerwerf F, van Rijn H, Koomans H. Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 1999;19:1168-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 114. | Passauer J, Pistrosch F, Büssemaker E, Lässig G, Herbrig K, Gross P. Reduced agonist-induced endothelium-dependent vasodilation in uremia is attributable to an impairment of vascular nitric oxide. J Am Soc Nephrol. 2005;16:959-965. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Hasdan G, Benchetrit S, Rashid G, Green J, Bernheim J, Rathaus M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61:586-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 116. | Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, Ritz E. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2005;16:2456-2461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 117. | Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449-2455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 118. | Valkonen VP, Päivä H, Salonen JT, Lakka TA, Lehtimäki T, Laakso J, Laaksonen R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 443] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 119. | Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 780] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 120. | Lerman A, Burnett JC, Higano ST, McKinley LJ, Holmes DR. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 258] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 121. | Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97:1989-1994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 301] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 122. | Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 194] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 123. | Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation. 2003;107:992-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 124. | Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 658] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 125. | Guyton AC, Coleman TG. Quantitative analysis of the pathophysiology of hypertension. 1969. J Am Soc Nephrol. 1999;10:2248-2258. [PubMed] [Cited in This Article: ] |
| 126. | Converse RL, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 787] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 127. | Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 128. | Locatelli F, Bommer J, London GM, Martín-Malo A, Wanner C, Yaqoob M, Zoccali C. Cardiovascular disease determinants in chronic renal failure: clinical approach and treatment. Nephrol Dial Transplant. 2001;16:459-468. [PubMed] [Cited in This Article: ] |
| 129. | Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, Schneider KA. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension. 1989;13:I80-I93. [PubMed] [Cited in This Article: ] |
| 130. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [PubMed] [Cited in This Article: ] |
| 131. | NICE. Hypertension, Guidance and guidelines. 2014; Available from: http: //www.nice.org.uk/guidance/cg127. [Cited in This Article: ] |
| 132. | Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73:759-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 133. | Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 134. | Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 135. | Zhang YF, He F, Ding HH, Dai W, Zhang Q, Luan H, Lv YM, Zeng HB. Effect of uric-acid-lowering therapy on progression of chronic kidney disease: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci. 2014;34:476-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407-2413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 137. | Sedaghat S, Hoorn EJ, van Rooij FJ, Hofman A, Franco OH, Witteman JC, Dehghan A. Serum uric acid and chronic kidney disease: the role of hypertension. PLoS One. 2013;8:e76827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 138. | Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 439] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 139. | Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404-2410. [PubMed] [Cited in This Article: ] |
| 140. | Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2013;15:435-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 141. | Rekhraj S, Gandy SJ, Szwejkowski BR, Nadir MA, Noman A, Houston JG, Lang CC, George J, Struthers AD. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol. 2013;61:926-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 142. | Okin PM, Devereux RB, Jern S, Julius S, Kjeldsen SE, Dahlöf B. Relation of echocardiographic left ventricular mass and hypertrophy to persistent electrocardiographic left ventricular hypertrophy in hypertensive patients: the LIFE Study. Am J Hypertens. 2001;14:775-782. [PubMed] [Cited in This Article: ] |
| 143. | Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 580] [Cited by in F6Publishing: 562] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 144. | Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 563] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 145. | Apple FS, Murakami MM, Pearce LA, Herzog CA. Multi-biomarker risk stratification of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and cardiac troponin T and I in end-stage renal disease for all-cause death. Clin Chem. 2004;50:2279-2285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 146. | Clerico A, Caprioli R, Del Ry S, Giannessi D. Clinical relevance of cardiac natriuretic peptides measured by means of competitive and non-competitive immunoassay methods in patients with renal failure on chronic hemodialysis. J Endocrinol Invest. 2001;24:24-30. [PubMed] [Cited in This Article: ] |
| 147. | Bode E, Wuppinger T, Bode T, Alber H, Ulmer H, Pachinger O, Mair J. Risk stratification in stable coronary artery disease: superiority of N-terminal pro B-type natriuretic peptide over high-sensitivity C-reactive protein, gamma-glutamyl transferase, and traditional risk factors. Coron Artery Dis. 2012;23:91-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 148. | Kanderian AS, Francis GS. Cardiac troponins and chronic kidney disease. Kidney Int. 2006;69:1112-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Freda BJ, Tang WH, Van Lente F, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40:2065-2071. [PubMed] [Cited in This Article: ] |
| 150. | Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellström D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O. Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol. 2008;158:125-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 151. | Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272-2279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 491] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 152. | Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1244] [Cited by in F6Publishing: 1119] [Article Influence: 69.9] [Reference Citation Analysis (0)] |