Published online Sep 24, 2014. doi: 10.5500/wjt.v4.i3.196
Revised: March 16, 2014
Accepted: June 27, 2014
Published online: September 24, 2014
AIM: To test whether intra-articular injection of porcine adipose-derived stem cells (ADSCs) can treat canine osteoarthritis (OA).
METHODS: To enroll in this study dogs must have stifle joint OA that had lasted ≥ 3 mo and been treated with OA medication without significant improvement. Three dogs fulfilled these criteria and were thus subjects for ADSCs treatment. ADSCs were isolated from abdominal adipose tissue of a 2-mo-old female Yorkshire pig. Their stem cell marker expression was examined by immunofluorescence staining. For treatment, 5 million ADSCs were injected into the diseased joint of each dog. In the next 48 h, the patient was observed for signs of inflammatory and allergic reactions. The patient was then discharged to the owner and, at 2, 6, and 12 wk, followed up with orthopedic assessment, owner questionnaire, X-ray imaging, and force-plate gait analysis.
RESULTS: Porcine ADSCs expressed mesenchymal stem cell markers CD90 and CD105. Injection of porcine ADSCs into canine stifle joints did not cause any inflammatory or allergic reactions. Orthopedic evaluation found improvements in two dogs, particularly at the longest time point. Owners’ evaluation found increased capacity and decreased pain in all three dogs’ activities such as walking and running. Radiographic evaluation did not find statistically significant differences before and after treatment. Force-plate analysis found significant improvements in all three dogs after treatment.
CONCLUSION: Xenotransplantation of ADSCs for the treatment of OA is feasible. Further studies are needed to validate this novel treatment modality, which can then be implemented for the routine treatment of OA in veterinary medicine.
Core tip: Intra-articular injection of autologous adipose-derived stem cells (ADSCs) has been shown to be effective in treating osteoarthritis in dogs. However, most veterinary clinics lack the equipment and expertise for ADSC isolation. The present study showed that intra-articular injection of porcine ADSCs into the diseased stifle joint of three dogs improved their mobility and activity and lessened their pain. The injection of porcine ADSCs elicited no signs of inflammation or immunological reaction. Thus, porcine ADSCs can substitute for autologous canine ADSCs, and such a treatment strategy can drastically reduce treatment cost.
- Citation: Tsai SY, Huang YC, Chueh LL, Yeh LS, Lin CS. Intra-articular transplantation of porcine adipose-derived stem cells for the treatment of canine osteoarthritis: A pilot study. World J Transplant 2014; 4(3): 196-205
- URL: https://www.wjgnet.com/2220-3230/full/v4/i3/196.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i3.196
Osteoarthritis (OA), also known as degenerative joint disease, is the most common cause of chronic pain in companion animals and afflicts more than 10 million dogs in the United States[1]. Current treatments, which commonly involve behavior modification and use of non-steroidal anti-inflammatory drugs (NSAIDs), are non-curative and are rather aimed at alleviating discomfort, decelerating tissue damage, and/or improving joint functionality[2]. However, chronic use of NSAIDs is associated with gastric ulceration/perforation and renal and hepatic damages[2]. As such, alternative treatment modalities without these adverse side effects are highly desirable.
Pathologically OA is characterized by the progressive degradation of articular cartilage, subchondral bone remodeling, and synovial inflammation[3,4]. While the exact cause of these pathological changes remains unknown, it is believed to involve the overproduction of pro-inflammatory cytokines (e.g., interleukin-1β), tissue-destructive proteinases (e.g., MMP-13), and catabolic mediators (e.g., nitric oxide) from chondrocytes and synovial cells[3,4]. As such, current pharmaceutical development for OA treatment largely aimed at preventing the production or action of these harmful molecules[3,4].
Mesenchymal stem/stromal cells (MSCs), first identified in bone marrow, are now found in virtually all tissues[5]. They are classified as multipotent, that is, able to differentiate into several cell lineages such as osteocytes, chondrocytes, adipocytes, and myocytes. This multipotency was originally the scientific basis for using MSCs as therapeutic agents for a wide variety of diseases, but decade-long research has resulted in a shift of emphasis from differentiation to paracrine actions as the main mechanism for MSC’s therapeutic efficacy[6]. Specifically, when transplanted into diseased tissues, MSCs communicate with local cells through secretion of a wide array of cytokines and growth factors[7]. They can also promote the migration of endogenous repairing cells to injury sites and suppress immunoreactions[7]. Together, these actions help restore physiological balance and enhance healing. In veterinary medicine, MSCs have been used to treat not only OA but also tendon injury, bone fracture, spinal cord injury, and liver disease[8].
Adipose-derived stem/stromal cells (ADSCs) are MSCs isolated from adipose tissue, and their therapeutic potential has been demonstrated in numerous preclinical and clinical studies[9-11]. Due to their abundant tissue source, low donor site morbidity, and ease of isolation, ADSCs have become increasingly the preferred MSCs for therapeutic application[9]. In veterinary medicine, there have been numerous claims in the mainstream media and in the Internet about ADSC’s “miraculous” therapeutic efficacy. In the scientific literature, there have been four studies that used ADSCs to treat canine OA[12-15], and the results all indicated ADSC’s efficacy in ameliorating OA symptoms.
The isolation of ADSCs from patients requires certain equipment, reagents, and expertise that are lacking in most veterinary clinics. As such, clinical application of autologous ADSCs in veterinary medicine is currently a costly and time-consuming process. On the other hand, if the therapeutic ADSCs can be obtained in a ready-for-injection form, their clinical applicability will increase substantially. To this end, it has been shown that ADSCs possess immunosuppressive capability and can be xenogeneically transplanted in immunocompetent recipients without the use of immunosuppressants[16]. Therefore, we hypothesized that ADSCs can be applied in a xenogeneic fashion, thereby eliminating the veterinarian’s burden to perform adipose tissue harvest and ADSC isolation. In the present study we show that porcine ADSCs can effectively treat canine OA.
ADSCs were isolated as described previously[17] from the abdominal fat of a 2-mo-old female Yorkshire pig. Briefly, the adipose tissue was rinsed with phosphate-buffered saline (PBS) containing 1% penicillin and streptomycin, minced into small pieces, and then incubated in a solution containing 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO, United States) for 1 h at 37 °C with vigorous shake. The top lipid layer was removed and the remaining liquid portion was centrifuged at 220 g for 10 min at room temperature. The pellet was treated with 160 mmol/L NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), filtered through a 40-μm cell strainer (BD Biosciences, Bedford, MA, United States), and plated at a density of 1 × 106 cells in a 10-cm dish. After reaching 80% confluence, the cells were harvested, aliquoted, and stored in liquid nitrogen at a density of 5 × 105 cells per mL of freezing media (DMEM, 20% FBS, and 10% DMSO) per vial. One week prior to the scheduled stem cell (SC) injection, the frozen cells were thawed and cultured until the necessary cell number for intra-articular injection was reached.
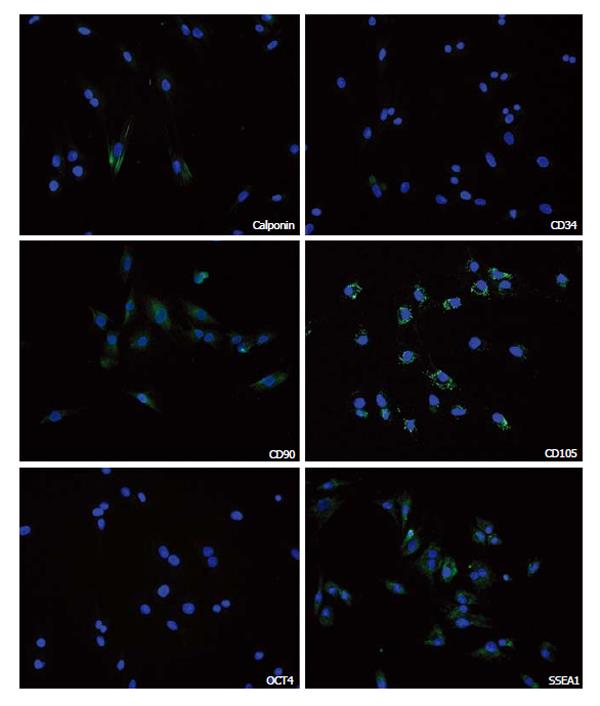
ADSCs (1 × 105 cells/well) were seeded on coverslips and placed in 6-well plates with 3 mL/well of DMEM and 10% FBS. After 24 h of incubation, the cells were fixed in cold methanol for 5 min at 4 °C, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% normal equine serum in PBS for 1 h at room temperature. The cells were then incubated with a primary antibody for 1 h at room temperature. After washing with PBS three times, the cells were incubated with FITC-conjugated secondary antibody for 1 h at room temperature. After three washes with PBS, the cells were further stained with 4-, 6-diamidino-2-phenylindole (DAPI, for nuclear staining) for 5 min. The stained cells were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments Inc., Melville, NY). Primary antibodies were against CD34 (sc7324), CD105 (sc18838), calponin (ab46794), SSEA-1 (ab16285), Oct4 (ab19857), and CD90 (ab225). Antibodies with “sc” and “ab” catalog numbers were purchased from Santa Cruz Biotech (Santa Cruz, CA, United States) and Abcam Inc. (Cambridge, MA, United States), respectively.
To enroll in this study the dogs must have chronic and stable OA of the stifle joints as defined by exhibition of the following symptoms: chronic lameness, exercise intolerance, difficulty getting up after a prolonged rest, and pain in the diseased limb when maneuvered. In addition, the disease must have lasted 3 mo or longer and been treated with OA medication without significant improvement. A total of three dogs fulfilled these criteria and thus enrolled in this study.
To exclude the involvement of systemic diseases, patients underwent physical examination and hematologic evaluation, including blood cell count, serum albumin, total protein, glucose, liver index, and kidney index. To affirm OA in the stifle joint, X-ray imaging was conducted to identify osteophyte formation and subchondral bone sclerosis/remodeling. Exclusion criteria included history of surgery, autoimmune or inflammatory arthritis, and other systemic diseases or non-orthopedic disorders (such as neurological diseases) that could affect gait and thus led to misdiagnosis.
To avoid the effects of OA medications on SC treatment evaluation, patients were required to stop taking steroids and non-steroidal analgesics for 3 and 2 wk, respectively, prior to SC injection. Use of these medications was also prohibited during the treatment and follow-up periods (3 mo total). In addition, patients were required to maintain stable body weight, as fluctuation in body weight might affect SC treatment evaluation.
Prior to SC injection, the owner of each patient dog was informed of the potential benefits and risks of this treatment. The owner was then required to sign a consent form, agreeing to closely observe the patient during the entire treatment and follow-up periods, to report any unusual behavior or symptom, and to return to the clinic for checkup at specified times.
Anesthesia was induced by iv injection of propofol (4 mg/kg) and maintained by isoflurane inhalation. Physiological monitoring included heart rate, electrocardiography, blood oxygen, blood pressure, and respiration. After shaving and disinfection of the stifle joint area, the anesthetized dog was placed on its side with the affected limb facing up. A 1-inch-long 24G needle attached to a syringe was inserted through the joint capsule lateral to the patellar ligament and toward the femoral condyle. A small amount of synovial fluid was withdrawn for analysis to rule out non-OA inflammation or autoimmune disease. Stem cells (5 million in 1 mL of PBS) were then injected via the same needle. The patient was allowed to wake up from anesthesia and further observed for approximately 45 min before being discharged with 3 d of cephalexin (20 mg/kg orally). Phone calls were made to the owner 6, 24, and 48 h later to inquire about any adverse reactions.
Treatment efficacy was evaluated at 2, 6, and 12 wk by orthopedic assessment, owner questionnaire, X-ray imaging, and force-plate gait analysis. Orthopedic assessment was conducted according to McCarthy et al[18] and used a scoring system shown in Table 1. Owner’s questionnaire and radiographic assessment were conducted according to Moreau et al[19] and used scoring systems shown in Table 2. Force-plate analysis was conducted according to Moreau et al[19]. In this study the force plate was a 10 m × 1.2 m × 0.19 m wooden walkway and in the middle of which embedded a pressure sensor (model OR6-7-1000, Advanced Mechanical Technology, Inc. Watertown, MA, United States) (Figure 1). During each analysis the dog was leash-walked by its owner from one end toward the other end of the walkway. The walking speed (between 1.9 and 2.2 m/s) was recorded by two sets of photoelectric cells, and a difference of < 0.2 m/s between the two recorded speeds was considered a valid speed. The walk was repeated until three valid speeds were recorded for each leg. During each walk the values of peak vertical force (PVF) and vertical impulse (VI) were obtained. PVF, normalized for body weight, is expressed as N kg- in percentile. VI, normalized for time and body weight, is expressed as Nskg-. Values obtained in the three trials (walks with valid speed) were averaged for statistical analysis.
| Criterion | Clinical evaluation | Score |
| Lameness | Walks normally | 1 |
| Slightly lame when walking | 2 | |
| Moderately lame when walking | 3 | |
| Severely lame when walking | 4 | |
| Reluctant to rise and will not walk more than five paces | 5 | |
| Joint mobility | Full range of motion | 1 |
| Mild limitation (10%-20%) in range of motion; no crepitus | 2 | |
| Mild limitation (10%-20%) in range of motion; with crepitus | 3 | |
| Moderate limitation (20%-50%) in range of motion; with crepitus | 4 | |
| Severe limitation (> 50%) in range of motion; with crepitus | 5 | |
| Pain on palpation | None | 1 |
| Mild signs: dog turns head in recognition | 2 | |
| Moderate signs: dog pulls limb away | 3 | |
| Severe signs: dog vocalizes or becomes aggressive | 4 | |
| Dog will not allow palpation | 5 | |
| Weight-bearing | Equal on all limbs standing and walking | 1 |
| Normal standing; favors affected limb when walking | 2 | |
| Partial weight-bearing standing and walking | 3 | |
| Partial weight-bearing standing; non-weight-bearing walking | 4 | |
| Non-weight-bearing standing and walking | 5 | |
| Overall score of | Not affected | 1 |
| clinical condition | Mildly affected | 2 |
| Moderately affected | 3 | |
| Severely affected | 4 | |
| Very severely affected | 5 |
| Walking | Getting up1 | Running | Climbing stairs | Playing or exercising | |
| Activities | |||||
| No difficulty | 1 | 1 | 1 | 1 | 1 |
| Slight and occasional difficulty | 2 | 2 | 2 | 2 | 2 |
| Frequent slight difficulty | 3 | 3 | 3 | 3 | 3 |
| Constant and obvious difficulty | 4 | 4 | 4 | 4 | 4 |
| Unable to perform | 5 | 5 | 5 | 5 | 5 |
| Signs of pain | |||||
| No sign of pain | 1 | 1 | 1 | 1 | 1 |
| Occasional pain, but with no link to a specific activity | 2 | 2 | 2 | 2 | 2 |
| Pain after this activity | 3 | 3 | 3 | 3 | 3 |
| Constant pain | 4 | 4 | 4 | 4 | 4 |
Data was analyzed with SPSS Statistics (SPSS Inc., Chicago, IL, United States of America), using one-way ANOVA followed by Tukey-Kramer post hoc comparison. Statistical significance was set at P < 0.05.
Porcine ADSCs were immunostained for smooth muscle marker calponin, hematopoietic stem cell marker CD34, embryonic stem cell markers Oct4 and SSEA-1, and mesenchymal stem cell markers CD90 and CD105. The results shown in Figure 2 are consistent with ADSCs of other mammalian species[16,20].
Three dogs, all having unilateral stifle OA, were enrolled in this study. Their characteristics are listed in Table 3.
| Case 1 | Case 2 | Case 3 | |
| Breed | Labrador Retriever | Golden Retriever | Beagle |
| Age (yr) | 2 | 5 | 2 |
| Sex | Male | Female | Male |
| Weight (kg) | 34 | 35 | 14 |
| Joint affected | Left stifle | Left stifle | Right stifle |
| Disease duration | > 1 yr | 5 mo | 6 mo |
Synovial fluid analysis (Table 4) indicated that the three canine patients’ ailing stifle joints were not due to non-OA inflammation or autoimmune disease.
| Case 1 | Case 2 | Case 3 | |
| Color | Light yellow | Light yellow | Light yellow |
| Turbidity | Clear | Clear | Clear |
| Viscosity | > 2.5 cm | > 2.5 cm | < 2.5 cm |
| Nucleated cell count (/μL) | 2160 | 1088 | 1433 |
| Neutrophil | 2% | 2% | 10% |
| Monocyte | 22% | 0% | 20% |
| Lymphocyte | 75% | 93% | 68% |
| Macrophage | 11% | 5% | 2% |
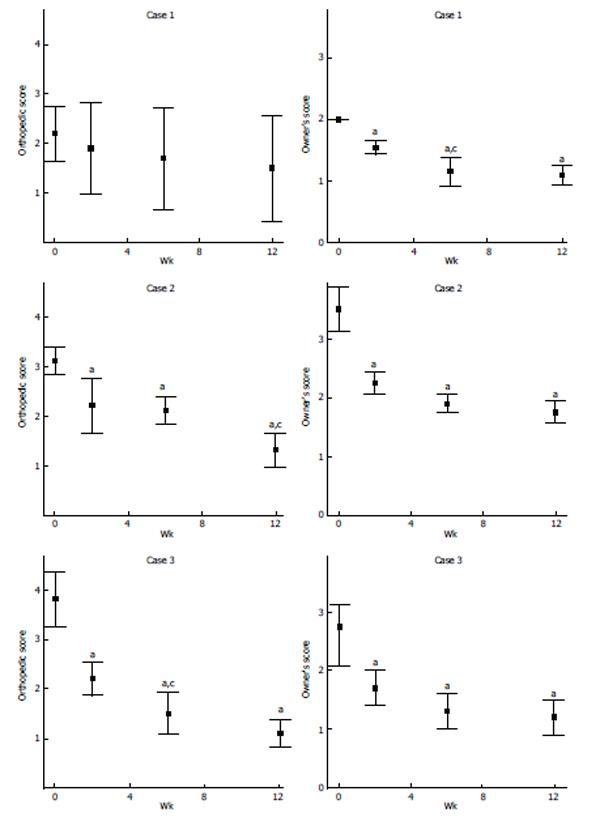
On a scale of 1 to 5, with 1 being the best, the patient’s OA symptoms such as lameness, joint mobility, pain on palpation, and weight bearing, were evaluated. The results presented in Figure 3 indicate an overall improvement.
At 6, 24, and 48 h after SC injection, the owners were contacted by phone. At 6 h, all three owners reported more pronounced lameness than before SC injection. However, at 24 and 48 h, all three owners reported having returned to at least the pre-treatment level of weight bearing. The owners also reported no sign of inflammation on the treated joint, nor any sign of allergic reaction.
For long-term follow-up, the owners were asked to evaluate their pets’ ability to perform activities (e.g., walking, running, and jumping) and degree of discomfort. The scoring was on based on scales of 1 to 5 and 1 to 4 (1 being the best) for activity and discomfort, respectively. The results presented in Figure 3 indicate an overall improvement.
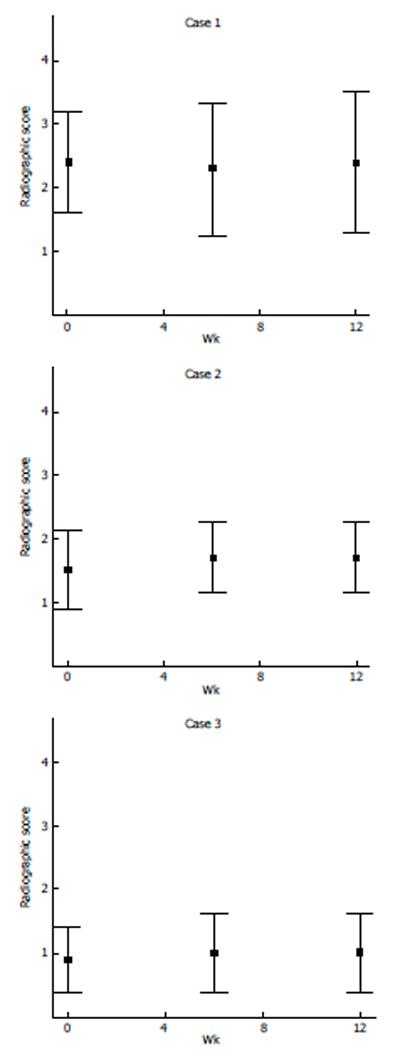
X-ray images of the diseased stifle joint of each dog were evaluated by one radiologist and four surgeons. The scores of these 5 independent evaluations were averaged and presented in Figure 4. Overall, there was no significant difference before and after treatment.
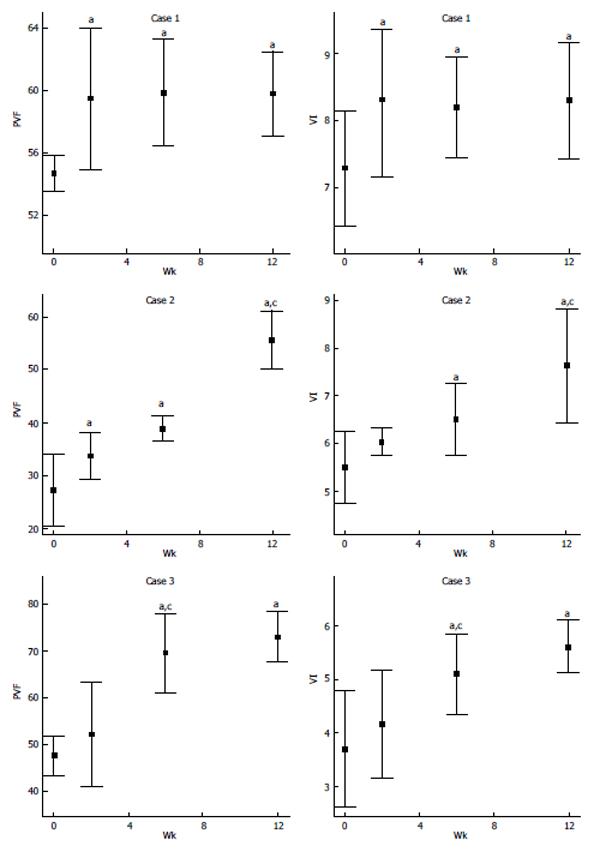
As shown in Figure 5 and Table 5, both PVF and VI values were significantly higher (better joint function) at all three time points after SC injection when compared to before injection. Furthermore, in cases 2 and 3, the highest values occurred at the 12th week.
| Pre-injection | 2 wk post-injection | 6 wk post-injection | 12 wk post-injection | |
| Case 1 | ||||
| PVF-experimental limb | 54.72 | 59.52 | 59.84 | 59.76 |
| PVF-contralateral limb | 66.02 | 63.53 | 65.89 | 68.67 |
| VI-experimental limb | 7.3 | 8.3 | 8.4 | 8.3 |
| VI-contralateral limb | 8.4 | 9.7 | 9.2 | 9.5 |
| Case 2 | ||||
| PVF-experimental limb | 27.42 | 33.77 | 39 | 55.62 |
| PVF-contralateral limb | 73.43 | 68.33 | 77.8 | 73.92 |
| VI-experimental limb | 5.5 | 6.1 | 6.5 | 7.7 |
| VI-contralateral limb | 11 | 11.1 | 13.3 | 11.5 |
| Case 3 | ||||
| PVF-experimental limb | 47.59 | 52.13 | 69.51 | 73.02 |
| PVF-contralateral limb | 109.72 | 105.24 | 85.95 | 87.29 |
| VI-experimental limb | 3.7 | 4.2 | 5 | 5.6 |
| VI-contralateral limb | 9 | 9.3 | 8.2 | 8.4 |
Several studies have demonstrated the efficacy of intra-articular MSC injection for the treatment of OA[7,21]. In veterinary medicine there have been four such studies using ADSCs to treat canine OA. First, Black et al[13] conducted a randomized, double-blinded, and placebo-controlled study that involved 21 dogs with chronic OA of the hip. A minimum of 23 g of adipose tissue was excised from each dog and shipped to the principal authors’ company Vet-Stem for ADSC isolation. The isolated cells were then shipped back to the veterinary clinic, where the veterinarian performed the intra-articular injection. Dogs in the treatment group were each injected with 4.2 to 5 million of autologous ADSCs whereas dogs in the control group PBS. Therapeutic efficacy was evaluated by orthopedic assessment and owner questionnaire at 1, 2, and 3 mo post-treatment. The results show that ADSC therapy significantly improved scores for lameness, pain, and range of motion.
In a second study, also by Black et al[12], 14 dogs were treated with ADSCs for chronic OA of the elbow. The preparation of ADSCs was similar to the first study, and 3 to 5 million autologous ADSCs were injected into each OA elbow. Treatment evaluation was conducted the same way as before, and the results again indicated ADSC’s therapeutic efficacy. One notable difference this time is that all recruited dogs were treated with ADSCs, citing owners’ objection to placebo treatment.
The third study involved 4 dogs with chronic OA of the elbow[14]. The dogs were treated with intra-articular injection of 3 to 5 million autologous ADSCs and evaluated one month later by veterinarian and owner assessments. The results show that all 4 dogs had improved joint functionality and reduced lameness.
The forth study used force-plate analysis to assess the effectiveness of autologous ADSCs in treating hip OA in 8 dogs[15]. The results show that both PVF and VI were significantly improved at 180 d post-treatment.
While the abovementioned studies all demonstrated ADSC’s therapeutic efficacy, the adoption of this novel OA treatment into clinical practice faces many challenges, including: (1) most veterinary clinics lack the equipment and expertise for ADSC isolation; (2) excision of adipose tissue causes donor site morbidity; (3) individually-made ADSC isolation is costly and time-consuming; and (4) at least two veterinarian appointments are needed for adipose tissue procurement and ADSC injection. However, these obstacles can be overcome if the therapeutic ADSCs are from a xenogeneic source, and to this end, numerous studies have demonstrated the feasibility of xenotransplantation with ADSCs[16].
In the present study, the injection of porcine ADSCs into the stifle joint of 3 dogs did not cause any inflammatory or allergic reaction, and its therapeutic efficacy was comparable to the aforementioned autologous treatments. As the radiographic assessment, which was conducted independently by 5 investigators, did not find structural improvement in the treated joints, it appears that the improved scores in force-plate, orthopedic, and owner’s assessments were due to ADSC’s anti-inflammatory and/or immunomodulatory effects. Similar negative radiographic findings in OA patients treated with NSAIDs have been reported previously[22]. The present study is also similar to the three aforementioned veterinarian studies in that the presence of the injected ADSCs in the treated joint could not be confirmed due to the fact that these patients are companion dogs, not experimental canine models, and as such, could not be sacrificed for histological examination.
The present study is pilot in nature and was intended as a proof of concept. When further validated in trials with larger case numbers, it can potentially lead to the development of a simple and inexpensive treatment for OA and other degenerative diseases in veterinary medicine. However, owner’s reluctance to participate and/or objection to placebo treatment will remain the most challenging issues going forward with such trials. It is thus our hope that the publication of this pilot study will encourage more participation from both veterinarians and pet owners.
Osteoarthritis (OA) is a prevailing canine disease that negatively impacts the patient’s quality of life. Current treatments for OA most commonly involve the use of non-steroidal anti-inflammatory drugs that can cause gastric ulceration and renal and hepatic damages. As such, alternative treatments without these adverse side effects are highly sought after. Toward this end, intra-articular injection of autologous adipose-derived stem cells (ADSCs) has been shown to be highly effective. However, the isolation of ADSCs from patients requires certain equipment, reagents, and expertise that are lacking in most veterinary clinics. Thus, the present study aimed at testing whether ADSCs can be applied in a xenogeneic fashion. In particular, porcine ADSCs, which can be manufactured on a commercial scale, were shown to be highly effective in treating canine OA.
Mesenchymal stem/stromal cells (MSCs), including ADSCs, have been consistently shown to have the ability to suppress immunity. Therefore, when transplanted into an allogeneic or xenogeneic recipient, they are well tolerated and able to perform functions similar to autologously transplanted MSCs, such as the secretion of anti-inflammatory and immunomodulatory molecules. These unique properties of MSCs are apparently responsible for the therapeutic efficacy of porcine ADSCs for canine OA, as demonstrated in the present study.
Previous studies have shown that intra-articular injection of autologous ADSCs was efficacious in treating canine OA. However, such a treatment is time-consuming and burdensome for both the veterinarian and the pet owner. In contrast, by using porcine ADSC instead of autologous ADSC, as demonstrated in the present study, intra-articular injection of ADSC will become an inexpensive and expeditious treatment for canine OA.
From the authors’ results, it appears that intra-articular injection of porcine ADSCs is a safe and effective treatment for canine OA. However, due to the small sample size in the current study, this innovative therapeutic approach will require further validation with treatment on additional patients. It is also imperative that long-term safety and effectiveness be demonstrated before this treatment modality can become a routine veterinary practice.
Xenotransplantation is transplantation of tissue or cell from one species to another. Force-plate analysis employs a plate or platform in which an embedded pressure sensor measures the ground reaction forces generated by a walking animal. A limb with OA generates less ground reaction forces than a normal limb, and as OA symptoms improve, so do ground reaction forces.
This paper investigates the effect of intra-articular injection of adipose-derived stem cells for treating canine OA. The aim of the study is highly important and authors do a good job to describe its significance in the paper. Overall, the manuscript is well-written.
P- Reviewer: Fernandez-Gutierrez B, Saarakkala S, Schinhan M, Seijas R S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Johnston SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 1997;27:699-723. [PubMed] [Cited in This Article: ] |
| 2. | Rychel JK. Diagnosis and treatment of osteoarthritis. Top Companion Anim Med. 2010;25:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1443] [Cited by in F6Publishing: 1724] [Article Influence: 123.1] [Reference Citation Analysis (1)] |
| 4. | Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115-2126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1404] [Cited by in F6Publishing: 1402] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 5. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1715] [Cited by in F6Publishing: 1652] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 6. | Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 594] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 7. | Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Ribitsch I, Burk J, Delling U, Geißler C, Gittel C, Jülke H, Brehm W. Basic science and clinical application of stem cells in veterinary medicine. Adv Biochem Eng Biotechnol. 2010;123:219-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21:1783-1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF, Lin CS. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Lin G, Xin Z, Zhang H, Banie L, Wang G, Qiu X, Ning H, Lue TF, Lin CS. Identification of active and quiescent adipose vascular stromal cells. Cytotherapy. 2012;14:240-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200. [PubMed] [Cited in This Article: ] |
| 13. | Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272-284. [PubMed] [Cited in This Article: ] |
| 14. | Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Vilar JM, Morales M, Santana A, Spinella G, Rubio M, Cuervo B, Cugat R, Carrillo JM. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet Res. 2013;9:131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012;21:2770-2778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | McCarthy G, O’Donovan J, Jones B, McAllister H, Seed M, Mooney C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet J. 2007;174:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Moreau M, Dupuis J, Bonneau NH, Desnoyers M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 21. | Coleman CM, Curtin C, Barry FP, O’Flatharta C, Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010;21:1239-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Dieppe P, Cushnaghan J, Jasani MK, McCrae F, Watt I. A two-year, placebo-controlled trial of non-steroidal anti-inflammatory therapy in osteoarthritis of the knee joint. Br J Rheumatol. 1993;32:595-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |