Published online Jul 18, 2022. doi: 10.5500/wjt.v12.i7.163
Peer-review started: December 23, 2021
First decision: February 15, 2022
Revised: March 8, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 18, 2022
Kidney transplantation (KT) is the treatment of choice for patients with end-stage renal disease, providing a better survival rate and quality of life compared to dialysis. Despite the progress in the medical management of KT patients, from a purely surgical standpoint, KT has resisted innovations during the last 50 years. Recently, robot-assisted KT (RAKT) has been proposed as an alternative approach to open surgery, especially due to its potential benefits for fragile and immunocompromised recipients. It was not until 2014 that the role of RAKT has found value thanks to the pioneering Vattikuti Urology Institute-Medanta collaboration that conceptualized and developed a new surgical technique for RAKT following the Idea, Development, Exploration, Assessment, Long-term follow-up recommendations for introducing surgical innovations into real-life practice. During the last years, mirroring the Vattikuti-Medanta technique, several centers developed RAKT program worldwide, providing strong evidence about the safety and the feasibility of this procedure. However, the majority of RAKT are still performed in the living donor setting, as an “eligible” procedure, while only a few centers have realized KT through a robotic approach in the challenging scenario of cadaver donation. In addition, despite the spread of minimally-invasive (predominantly robotic) surgery worldwide, many KTs are still performed in an open fashion. Regardless of the type of incision employed by surgeons, open KT may lead to non-negligible risks of wound complications, especially among obese patients. Particularly, the assessment for KT should consider not only the added surgical technical challenges but also the higher risk of postoperative complications. In this context, robotic surgery could offer several benefits, including providing a better exposure of the surgical field and better instrument maneuverability, as well as the possibility to integrate other technological nuances, such as the use of intraoperative fluorescence vascular imaging with indocyanine green to assess the ureteral vascularization before the uretero-vesical anastomosis. Therefore, our review aims to report the more significant experiences regarding RAKT, focusing on the results and future perspectives.
Core Tip: Kidney transplantation (KT) is the treatment of choice for patients with end-stage renal disease, providing a better survival rate and quality of life compared to dialysis. Despite the progress in the medical management of KT patients, from a purely surgical standpoint KT has resisted innovations during the last 50 years. Recently, robot-assisted KT (RAKT) has been proposed as an alternative approach to open surgery especially thanks to its potential benefits for fragile and immunocompromised recipients. Therefore, our review aims to report the more significant experiences regarding RAKT, focusing on the results and future perspectives.
- Citation: Li Marzi V, Pecoraro A, Gallo ML, Caroti L, Peris A, Vignolini G, Serni S, Campi R. Robot-assisted kidney transplantation: Is it getting ready for prime time? World J Transplant 2022; 12(7): 163-174
- URL: https://www.wjgnet.com/2220-3230/full/v12/i7/163.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i7.163
Kidney transplantation (KT) is the treatment of choice for patients with end-stage renal disease, providing a better survival rate and quality of life compared to dialysis[1]. Despite the progress in the medical management of KT patients, from a purely surgical standpoint, KT has resisted innovations during the last 50 years[2]. Indeed, open surgery remains the gold standard approach for KT according to the latest European Association of Urology (EAU) guidelines[3]. Recently, minimally invasive surgery (MIS) [and in particular robot-assisted KT (RAKT)] has been proposed as an alternative approach to open surgery, particularly due to its potential benefits for fragile and immunocompromised recipients in terms of peri- and postoperative outcomes, length of hospital stay, postoperative pain, wound infection rate, and cosmetic results[4]. While the spread of a pure laparoscopic approach was limited by the complexity of the procedure and by long learning curves, robotic surgery in this setting helps overcome these limitations thanks to the three-dimensional vision, high magnification, elimination of hand tremor, and the opportunity to take advantage from the Endo-wrist technology.
In 2021, RAKT has become a reality at selected referral centers worldwide in the setting of KT from living donors (LD), with several reports showing favorable outcomes at a short- and mid-term follow-up. Yet, expanding the indications for RAKT from deceased donors (DD) is still challenging due to specific technical and logistical issues[5]. Herein we provide a comprehensive overview of the history of RAKT, focusing on the evolution of the techniques proposed by different groups worldwide, as well as on the specific challenges associated with the expansion of this approach for KT from DDs.
During the last decades, selected referral centers have implemented MIS in the field of KT from LDs. As such, a pure laparoscopic approach and subsequently RAKT were performed as progressive steps to minimize the surgical morbidity of KT while ensuring favorable functional and perioperative outcomes. Rosales et al[6] reported their first experience with a pure laparoscopic approach, introducing the kidney through a Pfannenstiel incision and using topical ice slush and cold saline to keep a low graft temperature. KT was completed with a median overall operative time of 240 min (53 min for vascular sutures), with a blood loss of 300 cm3 and a hospital stay of 14 d. No surgical complications were reported.
Then, Modi et al[7] published a larger experience of 72 patients treated with laparoscopic KT from LDs. The authors described the use of a Pfannenstiel incision and four left-sided abdominal ports, and compared laparoscopic KT to open KT. The authors found that laparoscopic KT was associated with a longer overall operative time [223.8 min vs 175.7 min (P = 0.07), respectively] and a similar estimated glomerular filtration rate (eGFR) value at 3, 6, 12, and 18 mo. The mean wound length was 5.5 and 17.8 cm (P = 0.0001) and the analgesic requirement was 1.4 and 3.2 mg morphine equivalent in first 24 h (P = 0.005) in the laparoscopic KT and open KT groups, respectively. While other groups have attempted to use a laparoscopic approach to perform KT from LDs, the spread of a pure laparoscopic approach among transplant centers was limited by several issues, such as the prolonged rewarming time (that could negatively impact graft outcomes), the complexity of surgical procedure, and the longer learning curve for surgeons, which represents a barrier to the widespread adoption of the technique across centers.
Therefore, thanks to the progressive spread of robotic surgery for the treatment of urological diseases[8], and given the persistence of the unmet clinical need of introducing MIS in the field of KT, the technique of RAKT was progressively codified and developed by selected centers in United States, India, and Europe[9-13] as shown in Table 1. In particular, Hoznek et al[14] performed the first KT assisted by a robot using an open incision and taking advantage of the robotic arms to perform the vascular anastomoses. For the first time, this experience demonstrated that vascular anastomoses for KT could be performed through the robotic platform.
| Ref. | Topic |
| Hoznek et al[14], 2002 | First procedure performed through da Vinci robot (Intuitive Surgical, Inc., Mountain View, California) to complete vascular dissection and anastomosis as well as ureterovesical anastomosis |
| Rosales et al[6], 2010 | First laparoscopic transplantation of a kidney from a living, related donor, performed April 16, 2009 |
| Boggi et al[13], 2011 | First European robotic kidney transplantation |
| Giulianotti et al[15], 2010 | First robotic kidney transplant in a morbidly obese patient |
| Menon et al[9], 2014 | First standardization of RAKT according to IDEAL principals. Phase 0 (simulation) studies included the establishment of techniques for pelvic cooling, graft placement in a robotic prostatectomy model, and simulation of the robotic kidney transplantation procedure in a cadaveric model. Phase 1 (innovation) studies began in January 2013 and involved treatment of a highly selective small group of patients (n = 7), using the principles utilized in the phase 0 studies, at a tertiary referral center |
| Menon et al[10], 2014 | Prospective study of 50 consecutive patients who underwent live-donor RAKT at Medanta Hospital following a 3-yr planning/simulation phase at the Vattikuti Urology Institute according to IDEAL principals |
| Sood et al[11], 2014 | Monitoring patient safety during the learning phase of RAKT and determine when it could be considered learned using the techniques of statistical process control |
| Breda et al[12], 2018 | First multicenter prospective observational study performed by the ERUS RAKT working group |
| Vignolini et al[5], 2019 | Report of the development of the first RAKT program from deceased donors |
| Territo et al[29], 2018 | Update of the multicenter prospective observational study performed by the ERUS RAKT working group |
| Campi et al[26], 2019 | Report of a monocentric RAKT experience with extraperitonelization of the graft according to the Vattikuti-Medanta technique, allowing a safe access for diagnostic and therapeutic percutaneous procedures during the postoperative period |
| Gallioli et al[19], 2020 | Analyse of the learning curve for RAKT. At least 35 cases are needed to achieve reproducibility in terms of timing, complications, and functional results |
| Vignolini et al[25], 2019 | First preliminary experience with 6 patients operated from January 2017 to April 2018 using indocyanine green fluorescence videography to assess graft and ureteral reperfusion |
| Musquera et al[17], 2021 | The results of the RAKT experience performed in 10 European centers by members of the ERUS-RAKT group |
While the first preliminary experience with a purely robotic KT was reported by Giulianotti et al[15], it was not until 2014 that the role of RAKT has been valued thanks to the pioneering Vattikuti Urology Institute-Medanta collaboration that conceptualized and developed a new surgical technique for RAKT following the Idea, Development, Exploration, Assessment, Long-term (IDEAL) follow-up recommendations for introducing surgical innovations into real-life practice[9-11,16].
Such a technique, described in detail in the following sections of the review, allowed to overcome the main limitations of a pure laparoscopic approach (i.e., long exposure of the graft to high temperatures during vascular anastomosis; technical challenges associated with performance of anastomoses laparoscopically leading to long learning curves, etc). This experience provided robust evidence showing the advantages of the robotic technology for minimally-invasive KT, and the foundation for the spread of a structured step-by-step technique for robotic KT to other referral KT centers worldwide[9,10].
A further major step in this direction was made by the EAU Robotic Urology Section (ERUS), which created a specific working group to prospectively collect data from patients undergoing RAKT from LDs at several European Institutions[12]. Breda et al[12] reported the results of a large multicenter prospective study by the ERUS-RAKT working group, confirming the feasibility and safety of RAKT and highlighting the reproducibility of the procedure by multiple surgeons with experience in both open KT and robotic urologic surgery. In this study, excellent perioperative and functional outcomes of RAKT were reported. An updated analysis from the ERUS-RAKT prospective registry including almost 300 patients provided evidence on the favorable mid-term outcomes of RAKT from LDs[17]. Lastly, the feasibility and safety of RAKT from DDs were explored by the team of the University of Florence[5]. This preliminary experience raised the bar for RAKT and led to a renowned enthusiasm for this technique also in the broader setting of DDs. In fact, the University of Florence experience confirmed that RAKT can be successfully performed in the complex setting of DDs despite specific logistical and technical challenges. Of note, expanding the indications for RAKT to DDs is a key unmet need for the transplant community, aiming to increase the number of recipients who may benefit from MIS.
IDEAL phase 0-1: The introduction of the Vattikuti-Medanta technique for RAKT with regional hypothermia following the IDEAL recommendations represents a milestone for the development and spread of RAKT worldwide[16]. The IDEAL phase 0-1 involved the preliminary ideation of a new procedure/technique that could provide benefits for patients. After this, authors could use animal models or cadavers to evaluate and modify the initial procedure to optimize results during real clinical cases[9].
First, to reduce the exposure of the graft to longer ischemia time, the authors tested a new technique to keep the graft temperature low within the pelvis, introducing 240-300 mL of ice slush in the abdomen during > 300 robot-assisted laparoscopic radical prostatectomies. In addition, based on previous experiences in robot-assisted laparoscopic radical prostatectomy and robotic partial nephrectomy, they employed the GelPOINT® device (Applied Medical Resources Corp, Rancho Santa Margarita, CA, United States) to provide an easy access to the intraperitoneal environment, allowing safe positioning of the graft into the surgical field, as well as of the ice slush to achieve renal hypothermia[18]. Later, to simulate a real procedure, four autotransplantations with such a robotic approach were performed in two cadavers. During the first procedure, the authors replicated the Giulianotti technique, highlighting relevant difficulties in performing the ureterovesical anastomosis without undocking the robot platform[15]. As such, for the following procedures, the cadaver was placed in a lithotomic position with a 15°-20° Trendelenburg tilt, and the robot was positioned between the patient’s legs mirroring the configuration for robot-assisted laparoscopic radical prostatectomy[9].
IDEAL phase 2A: Patient and trocar positioning: The ideal phase 2A aimed to evaluate the safety and the efficacy of the new procedure in a few patients in a small prospective study[16]. The absolute contraindications were the presence of significant atherosclerosis plaques at the level of the iliac vessels, prior bilateral KTs, previous major abdominal surgery, second transplant, simultaneous dual or multiple organ transplant, and second transplantation. After confirming the feasibility of RAKT in a cadaver model with the introduction of specific technical nuances, Menon et al[10] reported their first experience with RAKT from LDs in carefully selected patients.
In particular, the recipient was positioned as previously described[9]. A 4-5 cm periumbilical incision was performed for the GelPOINT® device. The port configuration included: (1) One 12-mm port for the camera and one 8-mm port for the assistant, placed within the GelPOINT device (to minimize the abdominal incisions); (2) Three 8-mm ports for the robotic arms; and (3) One 12-mm assistant port placed in the right iliac fossa. The da Vinci robotic platform (Intuitive Surgical, Sunnyvale, CA, United States) was docked between the patient’s legs. After skeletonization of external iliac vessels, the surgeon created an extraperitoneal pouch over the psoas muscle to allocate the graft after completion of the vascular anastomoses. The graft was placed in a gauze jacket filled with ice and then introduced into the pelvis using the GelPOINT device. Subsequently, 180-240 mL of ice slush were introduced in the pelvis through modified Toomey syringes to achieve adequate regional hypothermia.
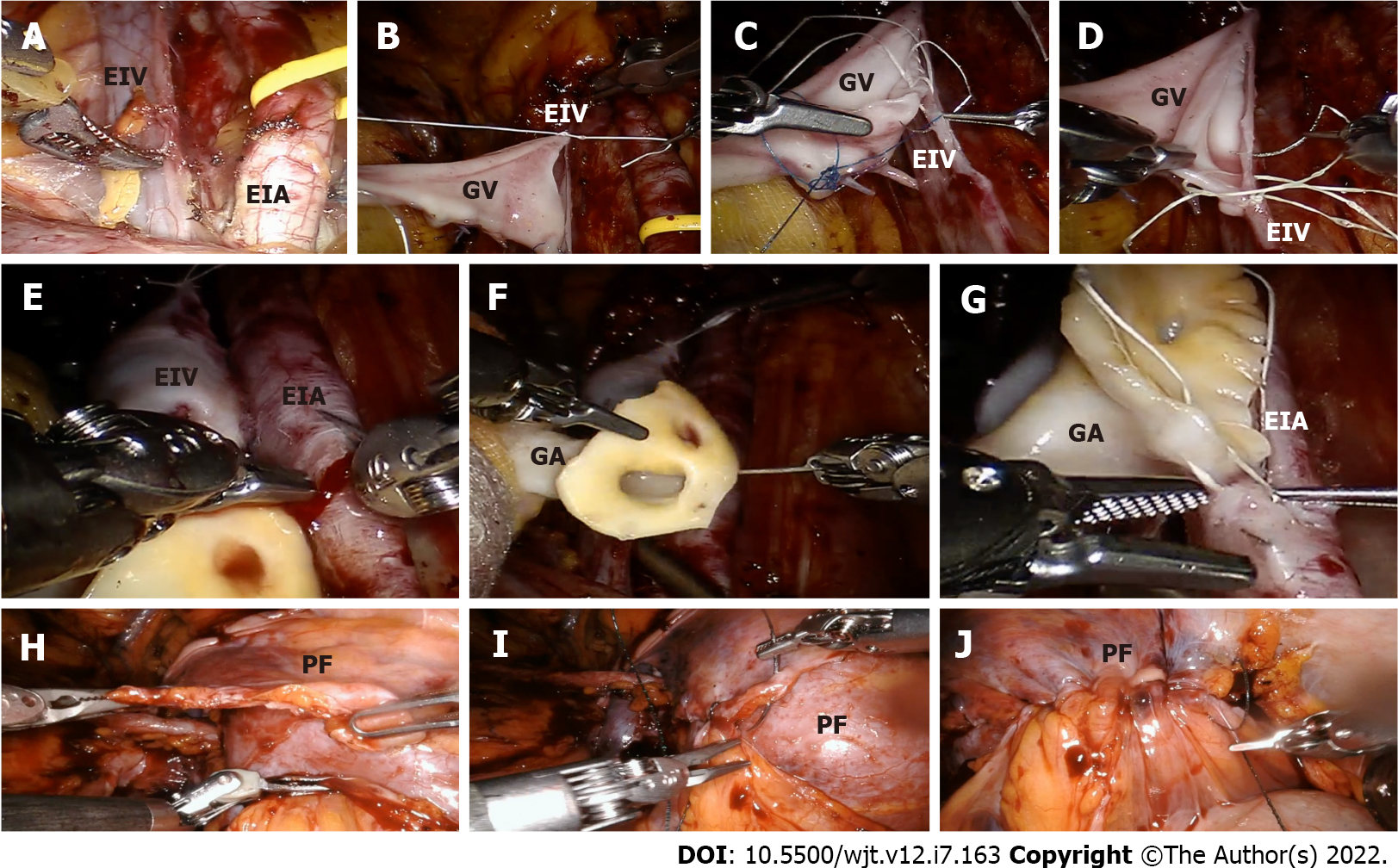
A distal bulldog clamp followed by a proximal clamp was placed on the external iliac vein. Then, a longitudinal venotomy with cold scissors was performed, and an end-to-side anastomosis between the graft renal vein and the external iliac vein was completed in an end-to-side fashion using a running ePTFE suture (Gore-Tex CV-6; W. L. Gore & Associates Inc, Flagstaff, AZ, United States)[10]. Before the suture had been finished, the lumen of the external iliac vein was flushed with heparinized solution through a 4.8 Fr ureteric catheter introduced through the assistant port. In the end, the graft vein was clamped, and the previously placed bulldog clamps were released and positioned proximally and then distally on the external iliac artery. Initially, the arteriotomy was made with cold scissors; thereafter, a laparoscopic aortic punch (Teleflex-Medical Inc, Research Triangle Park, NC, United States) was employed to create a circular hole. A continuous end-to-side anastomosis was realized between the external iliac and the graft artery using the Gore-Tex CV-6 suture.
At completion of the arterial anastomosis, the graft renal vessels were clamped and the external iliac artery declamped. If no signs of bleeding were observed, all clamps were removed to revascularize the graft. The graft was inspected for color, turgor, and on-table diuresis, and gently placed in the extraperitoneal pouch (closed by approximating the previously prepared peritoneal flaps) taking care not to stretch the vascular anastomoses. Lastly, the uretero-vesical anastomosis was performed according to a modified Lich-Gregoire technique using a 4-0 polydiaxone suture (Ethicon Inc, Cincinnati, OH, United States). A 6 Fr, 16-cm double-J stent, introduced through the assistant port, was placed into the ureter before completing the anastomosis. During this phase, developing an adequate detrusor tunnel was relevant to provide an anti-reflux mechanism. The stent was generally removed 3 wk after RAKT in the outpatient clinic.
In 2018 the ERUS RAKT working group reported their first multicenter prospective study on RAKT from LD enrolling 120 enrolled patients[12]. All European centers followed a standardized operative protocol based on the Vattikuti-Medanta experience with the introduction of a few technical nuances. The patient was placed in a lithotomy position with a 20°-30° Trendelenburg tilt. After the introduction of the GelPOINT through a linear periumbilical incision of 6 cm, the other four ports were placed, in the same position reported by Menon et al[10]. However, in 4 female recipients, the introduction of the graft was provided through a transvaginal GelPOINT. In all cases, a 2 cm incision of the GelPOINT cap was made to guarantee the introduction of ice slush with a modified Toomey tip syringe. After placing the clamp on the external iliac vein and the realization of the venotomy using Potts scissors, an end-to-side anastomosis between the graft vein and the external iliac vein was made with a 6/0 Gore-Tex® CV-6 TTc-9 or THc-12 needle. The suture was tied to secure the posterior wall of the anastomosis at the proximal angle and then it was completed until the distal to avoid stenosis. For the artery, the bulldog clamps placement on the external iliac artery were finalized to perform a preliminary incision with cold scissors, completed using a laparoscopic aortic punch. In the beginning, both vascular anastomoses require passing the needle in the external iliac vessel in an outside-inside direction and then inside-outside through the graft vessel. However, while during the venous anastomosis the knot was tied immediately, and only then the needle was passed outside-inside through the renal vein to start the running suture, during the artery anastomosis, the knot was created to a loop left outside after the passage of the needle through the graft vessel outside-inside. Finally, the vesicoureteral anastomosis was realized following the principles of the Lich-Gregoir technique over a pre-placed 4.8-Fr, 12-cm double-J stent[12].
During the procedure, an adequate management of vascular anastomosis was mandatory to reduce the risk of severe postoperative complications. In particular, avoiding intimal injury through a careful manipulation of graft vessels was a key step during RAKT. In addition, as suggested by Gallioli et al[19], a complete learning curve could be useful to achieve reproducible intra- and postoperative outcomes. Finally, the exclusion criteria to perform RAKT have been modified during the last years, but the main issues are currently represented by severe calcification at the level of the iliac vessels and previous bilateral KT[17].
After the development of RAKT from LD, some centers tried to widen the indications for RAKT, including grafts from DDs[20]. The main contraindications in these series were: (1) The presence of atherosclerotic plaques at the level of the iliac vessels; (2) Previous multiple major abdominal surgery; (3) Absolute contraindications for robotic surgery; and (4) Previous bilateral KT. In this context, the transplant multidisciplinary team must deal with specific issues from both organizational and technical standpoints due to the “emergency scenario” and the time-dependent nature of the intervention. To the best of our knowledge, the largest experience of RAKT from DD was reported by our group proposing specific technical nuances to improve surgical technique while ensuring maximal patient and graft safety[5,19].
The harvesting procedure is performed according to established protocol[21]. In case of grafts from donors after circulatory death, a hypothermic machine perfusion device is employed for graft preservation before RAKT. During the bench surgery, the graft is perfused with Celsior® solution. Then, the anterior margin of the vein is shaped by cutting a small part of venous tissue to provide better visualization of its posterior margin. In addition, if a right-sided graft is available, increasing the length of the right vein using an inferior vena cava patch is always considered; yet, RAKT using right-sided grafts from DDs appears feasible even without a caval patch thanks to the advantages of the robotic platform, as demonstrated for RAKT in LD setting[22].
Of note, if severe atherosclerotic plaques are observed at the level of the aortic Carrel’s patch, the surgeon may remove them, realizing the arterial anastomosis without the patch. In case of multiple vessels, the surgeon usually reconstructs them to perform a single anastomosis (i.e., using a side-to-side anastomosis between two renal arteries in a “pantaloon” fashion), as shown in several experiences[23,24]; alternatively, a small polar artery can be anastomosed to the inferior epigastric artery with a separate arterial anastomosis[10]. When the kidney is prepared, it is placed into a gauze jacket filled with ice to provide less traumatic handling and to maintain graft hypothermia. Finally, a 5-Fr, 12-cm double-J stent is routinely pre-placed into the ureter during bench surgery to facilitate the subsequent uretero-vesical anastomosis.
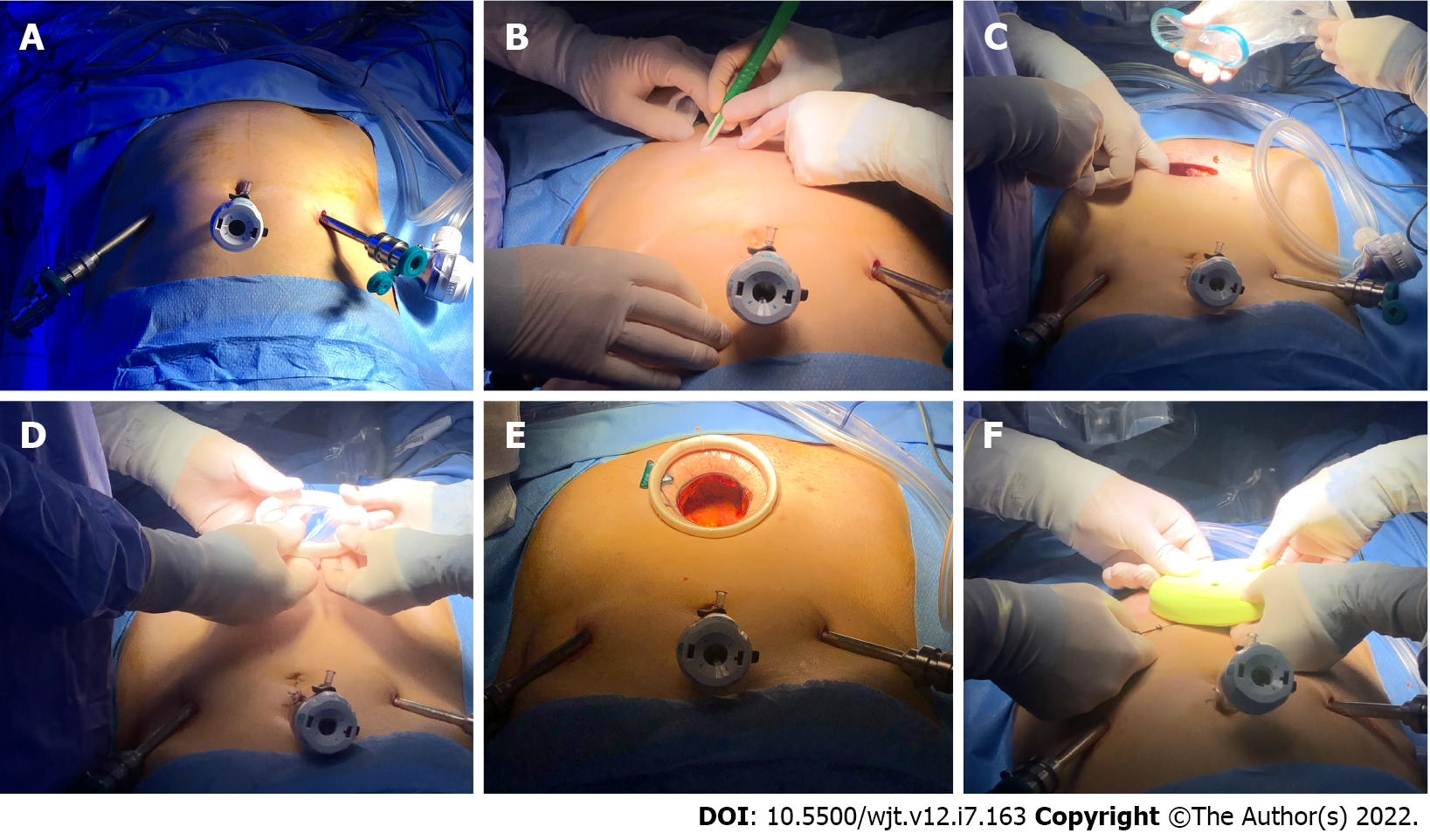
At our institution, all RAKTs followed the principles of the Vattikuti-Medanta technique with the progressive introduction of specific nuances during the learning curve[10]. Specifically: (1) A Pfannestiel rather than a periumbilical incision is used for the GelPOINT® (or the Alexis® Wound Protectors/ Retractors, Applied Medical Resources Corp, United States) placement improving the aesthetic results and providing closer access to the iliac vessels (Figure 1); (2) The GelPOINT® device is placed only after adequate preparation of iliac vessels, bladder, and extraperitoneal pouch to reduce the potential risk of bladder injury; (3) The venotomy is realized with curve scissors and then a two-continuous suture is completed for the posterior and anterior plate of the venous anastomosis. First, the anterior part is performed from 12 to 6 o’clock position knotting at 6 o’clock, and then the posterior one is completed from 6 to 12 o’clock position; and (4) The arteriotomy is realized with cold scissors without the use of a laparoscopic aortic punch. In addition, considering the higher risk of atherosclerotic plaques at the level of the external iliac arteries for recipient in DD setting, the anastomosis is performed using two running sutures (in Gore-Tex 5/0 instead of 6/0). After the realization of the posterior plate using a running suture from 12 to 6 o’clock position, without knotting at the end, the anterior wall is completed with another running suture from 6 to 12 o’clock position. Then, the two ends are tied together at 6 o’clock. This technique establishes the correct tension of the anastomosis considering the characteristics of both the graft and iliac vessels. If the Carrel’s patch is suitable, it can be removed, mirroring the anastomosis during RAKT in LD setting.
Regarding the assessment of graft and ureter reperfusion, our group proposed the use of intraoperative indocyanine green fluorescence videography to complement the intraoperative visual and ultrasound-based evaluation of the graft after completion of the vascular anastomoses[25]. In any case, the graft is allocated in the previously prepared extraperitoneal pouch by reapproximating the two peritoneal flaps prepared at the beginning of the procedure (Figure 2): This step has been shown to offer a safe access for diagnostic and therapeutic percutaneous procedures during the postoperative period, as reported by Campi et al[26] without any type of postprocedural complications.
During the last 10 years, several studies have been reported showing the feasibility and safety of RAKT in the LD setting. Menon et al[10] published their experience of the first 25 RAKTs, reporting a mean console, warm ischemia, arterial, and venous anastomotic times of 135 min, 2.4 min, 12 min, and 13.4 min, respectively. In addition, no delayed graft function (DGF) or early surgical postoperative complications were observed, while at 6 mo of follow-up two patients underwent re-exploration, and one patient died of congestive heart failure. Subsequently, Sood et al[27] published a preliminary comparison of 50 and 175 patients who had undergone RAKT and open KT, respectively. No difference in terms of early postoperative functional outcomes was reported (median creatinine 1.2 and 1.3 mg/dL, in RAKT and open KT group, respectively). No DGF was observed, while one patient in the RAKT group and four in the open KT underwent post-transplant dialysis. In addition, during the early follow-up, three deaths were observed (one in the RAKT group and two in the open KT, respectively). Recently, the final results of this experience (IDEAL phase 2B) have been published[28]. Particularly, 126 patients undergone RAKT and 378 open KT (1:3 matched cohort) were included, reporting a lower rate of wound infections (0% vs 4%, P = 0.023), symptomatic lymphoceles at 36 mo (0% vs 7%, P = 0.003), DGF (0% vs 2.3%, P = 0.081), and reduced postoperative pain with the robotic approach. At a median follow-up of 24.7 and 23.2 mo, for RAKT and open KT group respectively, no differences in terms of graft survival were observed {95.2% [95% confidence interval (CI): 86-99.3] vs 96.3% (95%CI: 93.1-99.4), P = 0.266}.
Another relevant experience was reported by Breda et al[12], presenting the preliminary results of ERUS RAKT working group from 120 patients who underwent RAKT. In this multicenter prospective observational study, the median operative and vascular suture time was 250 and 38 min, respectively. The median estimated blood loss was 150 mL and no major intraoperative complications were reported. Two patients needed open conversion and in five cases (4.2%), surgical management was requested for intraperitoneal hematoma. The median eGFR was 58.0 mL/min on postoperative day 30. Territo et al[29] updated this study, reporting the results of 291 RAKTs from LD and highlighting a shorter operative time after the first 120 cases (265 min vs 230 min, P = 0.005). The mean overall surgical and re-warming time was 244 (70.5) and 53.16 (15.27) min, respectively. In all, five (2%) were lost due to thrombosis and one due to acute rejection. Two patients had arterial stenosis, three had incisional hernias, six had ureteric stenosis, and nine had lymphoceles. Finally, Musquera et al[17] described the mid-terms outcomes of 291 RAKT from LDs procedures. Overall, 22 cases of early major postoperative complications (defined as Clavien-Dindo Complication > 2) were recorded, while after more than 90 d from RAKT, 16 cases of major postoperative complications were observed, including one patient who died for pulmonary thromboembolism, two cases of arterial stenosis, three of incisional hernias, two of ureteric stenosis, one of angioplasty, and seven of lymphoceles. However, regarding the functional outcomes, the authors reported a progressive improvement of the eGFR (60 mL/min/1.73 m2 at last follow-up). The median hospital stay ranged between 7 and 14 d[12,17], but it could be influenced by several items, such as hospital policies and patient-related factors. Despite the favorable results, several issues still limit the spread of RAKT from living (and deceased) donors worldwide, including the technical and logistical complexity of the procedure, as well as limited evidence regarding its learning curve. Sood et al[11] analyzed the learning curve of RAKTs with regional hypothermia from LDs, stratifying the recipients into three groups according to the robotic and open KT experience of the surgeons. Of note, they observed that the learning curve for RAKT was minimal for surgeons who had prior robotic and KT experience. These results were confirmed by Ahlawat et al[30] who described a short learning curve in RAKT for experienced surgeons in KT and robotic surgery, achieving optimal skills within ten cases. However, the authors suggested that further improvements could be observed for the first 20-25 cases.
Later, Gallioli et al[19] published the results of a multicenter study, including the five highest-volume centers of the ERUS RAKT working group. They demonstrated that the Trifecta, defined as no major intra/postoperative complications, no delayed graft function, and rewarming time < mean + 2 SD (= 48.6 min), was achieved in 75% of cases after a minimum of 35 procedures. Notably, all graft losses took place during the first ten RAKTs, raising concerns regarding potential technical errors during the very first cases of the robotic series, and highlighting the need of proper modular training for surgeons wishing to start their experience with RAKT. In brief, the authors suggested that at least 35 procedures could be necessary to achieve reproducibility in surgical time, complications rate, and functional results. In conclusion, while further prospective studies are needed to define the differences in the learning curve of open and robotic KT (adjusting for all patient- and provider-specific factors), centers that are interested in developing RAKT programs may benefit from existing courses on RAKT (i.e., Orsi Academy, Belgium) and from the expertise gained by multicenter collaborations such as the ERUS-RAKT working group. Standardized proficiency-based training curricula are warranted.
Since its inception, RAKTs has been primarily developed as an “elective” procedure in the LD setting. Considering the limited available evidence, as well as several logistical challenges, many teams might have concerns regarding the feasibility and safety of RAKT in a more complex scenario, such as that of DDs. Indeed, RAKT from DDs is a challenging procedure, demanding great efforts from organizational standpoints and with only few preliminary experiences worldwide[15,17,19,31-33]. Particularly, Vignolini et al[5] published the results of their structured RAKT program, based on a previous solid experience in open KT and RAKT from LDs.
The authors defined 5 essential phases to determine the technical and logistical feasibility of performing RAKT in case of DDs. Initially, the availability of the dedicated surgical team must be ensure, while the recipient is admitted to the Nephrology Unit to perform careful anesthesiologic and preoperative work-up. Then, the availability of the robotic operating room must be verified, aiming to start RAKT within 16 h from the organ procurement surgery, in order to keep the overall ischemia time < 24 h[34]. Finally, a careful graft evaluation on the bench is critical to individualize the indication for RAKT (i.e., open KT is preferred in case of multiple vessels which cannot be reconstructed to perform a single vascular robotic anastomosis).
Despite these specific challenges, preliminary experiences coming from selected referral centers worldwide provided the proof of the concept that RAKT (in experienced hands) can be safely perform from DDs, with favorable short- and mid-term outcomes, even during the pandemic[5,19,35,36]. To the best of our knowledge, our experience represents the largest series so far on RAKT from unselected DDs[19,37]. At a median follow-up of 16 mo [interquartile range (IQR): 7-22], recipients showed good functional results with a median eGFR of 57 mL/min/1.73 m2 (IQR: 45-76); only two patients needed dialysis treatment at the last follow-up. The safety profile of RAKT from DDs in terms of major (Clavien-Dindo grade ≥ 3) surgical complications was also promising. These favorable preliminary findings were confirmed by an updated analysis comparing RAKT and open KT from DDs at our center[37]. Overall, there were no significant differences between the RAKT and open KT cohorts in terms of baseline donor-, graft- and recipient-related characteristics, except for a significantly higher proportion of pre-emptive recipients in the RAKT cohort (40.0% vs 4.9%, P = 0.0001), a significantly lower American Society of Anesthesiologists score among patients undergoing RAKT (2 vs 3, P = 0.033). The re-warming and the vascular anastomosis time did not significantly differ between RAKT and open KT (47 min vs 28 min, P = 0.2; 15 min vs 18 min, P = 0.2, respectively).
There were no significant differences between RAKT and open KT in terms of median hospital stay (13 d) as well as the major postoperative complication rate. However, the RAKT group was associated with a significantly lower blood transfusion rate (14.3% vs 22.2%, P = 0.008). At the last follow-up, no differences were observed between the two groups in terms of mid-term graft function. Despite lack of randomization, our experience provides further evidence supporting the non-inferiority of RAKT as compared to open KT from DDs, provided careful patient selection, adequate surgical training, and availability of a framework allowing performance of RAKT even in “non-elective” conditions (i.e., weekends, night, etc). However, our technique is not devoid of limitations. In particular, all candidates for RAKT are evaluated with a computed tomography (CT) scan before surgery to identify atherosclerotic plaques at the level of the external iliac vessels; that remains an absolute contraindication for the procedure. In addition, in case of atherosclerotic lesions of the renal vessels, the characteristics of polypropylene needle could provide advantages compared to Gore-Tex, but it is not suitable for robotic surgery. For these reasons, a carefully preoperative evaluation of patients is needed to tailor the surgical approach taking into consideration the patients’ characteristics, especially when the procedure will be carried out as an emergency.
Despite the development and spread of minimally invasive (predominantly robotic) surgery worldwide, many KTs are still performed in an open fashion. Regardless of the type of incision employed by surgeons, open KT may lead to non-negligible risks of wound complications[38], especially among obese patients. In addition, considering the fragility of KT recipients, there is certainly a window of opportunity for new surgical techniques to minimize the morbidity of KT allowing faster recover and better patient-reported outcomes[39]. As such, RAKT has the potential to reduce specific KT-related surgical complications, such as wound dehiscence/infection, symptomatic lymphoceles, postoperative pain, as well as to minimize the length of hospitalization. RAKT might also improve the cosmetic result of KT. All these potential advantages of RAKT are most promising for overweight/obese recipients[40], who represent a patient population at a higher risk of postoperative adverse events. As universally known, the obese “pandemic” is nowadays spread in developed countries, affecting a large part of the population. Although obesity is not considered an absolute contraindication for KT, European and United States data have shown that this condition is associated with a reduced chance of receiving transplantation[12]. The assessment of obese recipients for KT should consider not only the added surgical technical challenges but also the higher risk of postoperative complications, while remaining the best treatment option[41,42]. In this context, robotic surgery could offer several benefits, providing a better exposure of the surgical field and a better instrument maneuverability.
However, the optimal indications as well as the ideal body mass index (BMI) to perform RAKT is still under debate. Recently, some experiences regarding the outcomes for obese patients and morbidity obese ones (BMI ≥ 30 and 35 kg/m2, respectively) have been reported, highlighting benefits in terms of postoperative wound infection if compared to open KT[40-43]. In addition, Spaggiari et al[44] have recently published the results about the simultaneous realization of RAKT and sleeve-gastrectomy, improving the patients’ compliance and outcomes. The available evidence suggests potential advantages, even in terms of learning curve. As previously reported, a surgeon’s background has a limited impact on his ability to perform RAKT; what really matter is the previous surgeons’ exposure to robotic surgery and open KT[11]. However, considering the major exposure to minimally invasive surgery and expertise in ureteral diseases, urologists may have advantages, if compared to other specialties (e.g., general surgeons, transplant surgeons), as well as the skills to manage significant postoperative complications (e.g., ureteral stricture).
On this regard, while Musquera et al[17] reported two patients treated through open ureteral reimplantation for stenosis, Campi et al[37] reported two cases of endoscopic management for ureteral complications in a DD setting. Therefore, the best surgical approach to treat urological complications should be evaluated in light of patients’ and related-problems characteristics (endoscopic, minimally invasive surgery, or an open approach). Despite the fact that the development of a RAKT program from DD could be extremely challenging from both a technical and organizational standpoint, Campi et al[37] proposed the realization of a dedicated pathway, avoiding any impact on donors’ management from both a clinical and organizational standpoint, even in the DD setting. To move the field forward, specific challenges of RAKT (especially in the DD setting) must be overcome. These include the need of a dedicated, highly qualified surgical team (trained in robotic surgery), and higher direct costs as compared to open KT. While an estimated increased cost of 15000 USD per RAKT has been reported if compared to open approach[31], the higher availability of platforms will hopefully reduce the costs of robotic technology, mitigating the financial downside of RAKT in the future. This might potentially allow a more significant penetrance of the robotic technology among KT centers in Europe and worldwide.
In conclusion, the vast majority of RAKTs so far have been performed using grafts from LDs in carefully selected recipients and have been shown to achieve optimal early and mid-term outcomes (which are at least non-inferior to those of open KT based on the current literature). Yet, to date, no randomized controlled trial has been conducted comparing RAKT to the gold-standard open approach. As such, several clinical and research questions (such as the reproducibility of RAKT outside referral high-volume centers) remain unanswered. In addition, only a few preliminary experiences have been reported on the outcomes of RAKT from DDs. In this scenario, critical steps need to be taken to implement the technique and the logistics aiming to increase the number of recipients who may benefit from minimally invasive surgery and “making RAKT ready for the prime time”. Large randomized prospective multicenter studies are eagerly warranted to address these unmet clinical needs, defining the best indications and limits of robotic surgery for KT.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ciancio G, United States; Guler Cimen S, Turkey S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Hariharan S, Israni AK, Danovitch G. Long-Term Survival after Kidney Transplantation. N Engl J Med. 2021;385:729-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 201] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 2. | Alcaraz A, Peri L, Izquierdo L, Musquera M. Is Robotic Kidney Transplant the Near Future? Eur Urol. 2017;72:218-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Breda A, Budde K, Figueiredo A, Lledó García E, Olsburgh J, Regele H, Boissier R, Hevia V, Rodríguez Faba O, Zakri RH. EAU Guidelines on Renal Transplantation. Proceedings of the EAU Annual Congress; 2021 Jul 8-12; Leeds, Milan. Arnhem: EAU Guidelines Office, 2021: 484-505. [Cited in This Article: ] |
| 4. | Herrell SD, Smith JA Jr. Laparoscopic and robotic radical prostatectomy: what are the real advantages? BJU Int. 2005;95:3-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Vignolini G, Campi R, Sessa F, Greco I, Larti A, Giancane S, Sebastianelli A, Gacci M, Peris A, Li Marzi V, Breda A, Siena G, Serni S. Development of a robot-assisted kidney transplantation programme from deceased donors in a referral academic centre: technical nuances and preliminary results. BJU Int. 2019;123:474-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Rosales A, Salvador JT, Urdaneta G, Patiño D, Montlleó M, Esquena S, Caffaratti J, Ponce de León J, Guirado L, Villavicencio H. Laparoscopic kidney transplantation. Eur Urol. 2010;57:164-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Modi P, Pal B, Modi J, Singla S, Patel C, Patel R, Padhy S, T K, Patel K, Rizvi J, Sharma S, Sharma V, Modi M, Shah VR, Trivedi HL. Retroperitoneoscopic living-donor nephrectomy and laparoscopic kidney transplantation: experience of initial 72 cases. Transplantation. 2013;95:100-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Rassweiler JJ, Autorino R, Klein J, Mottrie A, Goezen AS, Stolzenburg JU, Rha KH, Schurr M, Kaouk J, Patel V, Dasgupta P, Liatsikos E. Future of robotic surgery in urology. BJU Int. 2017;120:822-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Menon M, Abaza R, Sood A, Ahlawat R, Ghani KR, Jeong W, Kher V, Kumar RK, Bhandari M. Robotic kidney transplantation with regional hypothermia: evolution of a novel procedure utilizing the IDEAL guidelines (IDEAL phase 0 and 1). Eur Urol. 2014;65:1001-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Menon M, Sood A, Bhandari M, Kher V, Ghosh P, Abaza R, Jeong W, Ghani KR, Kumar RK, Modi P, Ahlawat R. Robotic kidney transplantation with regional hypothermia: a step-by-step description of the Vattikuti Urology Institute-Medanta technique (IDEAL phase 2a). Eur Urol. 2014;65:991-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Sood A, Ghani KR, Ahlawat R, Modi P, Abaza R, Jeong W, Sammon JD, Diaz M, Kher V, Menon M, Bhandari M. Application of the statistical process control method for prospective patient safety monitoring during the learning phase: robotic kidney transplantation with regional hypothermia (IDEAL phase 2a-b). Eur Urol. 2014;66:371-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Breda A, Territo A, Gausa L, Tuğcu V, Alcaraz A, Musquera M, Decaestecker K, Desender L, Stockle M, Janssen M, Fornara P, Mohammed N, Siena G, Serni S, Guirado L, Facundo C, Doumerc N. Robot-assisted Kidney Transplantation: The European Experience. Eur Urol. 2018;73:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Boggi U, Vistoli F, Signori S, D'Imporzano S, Amorese G, Consani G, Guarracino F, Melfi F, Mussi A, Mosca F. Robotic renal transplantation: first European case. Transpl Int. 2011;24:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Hoznek A, Zaki SK, Samadi DB, Salomon L, Lobontiu A, Lang P, Abbou CC. Robotic assisted kidney transplantation: an initial experience. J Urol. 2002;167:1604-1606. [PubMed] [Cited in This Article: ] |
| 15. | Giulianotti P, Gorodner V, Sbrana F, Tzvetanov I, Jeon H, Bianco F, Kinzer K, Oberholzer J, Benedetti E. Robotic transabdominal kidney transplantation in a morbidly obese patient. Am J Transplant. 2010;10:1478-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J; Balliol Collaboration, Aronson JK, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Feldman LS, Flum DR, Maddern GJ, Nicholl J, Reeves BC, Seiler CM, Strasberg SM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Ergina PL, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Meakins J, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1181] [Cited by in F6Publishing: 1215] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 17. | Musquera M, Peri L, Ajami T, Campi R, Tugcu V, Decaestecker K, Stockle M, Fornara P, Doumerc N, Vigues F, Barod R, Desender L, Territo A, Serni S, Vignolini G, Sahin S, Zeuschner P, Banga N, Breda A, Alcaraz A. Robot-assisted kidney transplantation: update from the European Robotic Urology Section (ERUS) series. BJU Int. 2021;127:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Rogers CG, Ghani KR, Kumar RK, Jeong W, Menon M. Robotic partial nephrectomy with cold ischemia and on-clamp tumor extraction: recapitulating the open approach. Eur Urol. 2013;63:573-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Gallioli A, Territo A, Boissier R, Campi R, Vignolini G, Musquera M, Alcaraz A, Decaestecker K, Tugcu V, Vanacore D, Serni S, Breda A. Learning Curve in Robot-assisted Kidney Transplantation: Results from the European Robotic Urological Society Working Group. Eur Urol. 2020;78:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Vignolini G, Greco I, Sessa F, Gemma L, Pecoraro A, Barzaghi P, Grosso A, Corti F, Mormile N, Martiriggiano M, Berni A, Firenzuoli N, Gacci M, Giancane S, Sebastianelli A, Li Marzi V, Serni S, Campi R. The University of Florence Technique for Robot-Assisted Kidney Transplantation: 3-Year Experience. Front Surg. 2020;7:583798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Peris A, Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Franci A, Rugna M, De Vito L, Ticali PF, Li Marzi V, Migliaccio ML. Implementing a donation after circulatory death program in a setting of donation after brain death activity. Minerva Anestesiol. 2018;84:1387-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Serni S, Pecoraro A, Sessa F, Gemma L, Greco I, Barzaghi P, Grosso AA, Corti F, Mormile N, Spatafora P, Caroassai S, Berni A, Gacci M, Giancane S, Tuccio A, Sebastianelli A, Li Marzi V, Vignolini G, Campi R. Robot-Assisted Laparoscopic Living Donor Nephrectomy: The University of Florence Technique. Front Surg. 2020;7:588215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Siena G, Campi R, Decaestecker K, Tuğcu V, Sahin S, Alcaraz A, Musquera M, Territo A, Gausa L, Randon C, Stockle M, Janssen M, Fornara P, Mohammed N, Guirado L, Facundo C, Doumerc N, Vignolini G, Breda A, Serni S. Robot-assisted Kidney Transplantation with Regional Hypothermia Using Grafts with Multiple Vessels After Extracorporeal Vascular Reconstruction: Results from the European Association of Urology Robotic Urology Section Working Group. Eur Urol Focus. 2018;4:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Nataraj SA, Zafar FA, Ghosh P, Ahlawat R. Feasibility and Functional Outcome of Robotic Assisted Kidney Transplantation Using Grafts With Multiple Vessels: Comparison to Propensity Matched Contemporary Open Kidney Transplants Cohort. Front Surg. 2020;7:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Vignolini G, Sessa F, Greco I, Cito G, Vanacore D, Cocci A, Sessa M, Grandi V, Pili A, Giancane S, Gacci M, Sebastianelli A, Li Marzi V, Breda A, Campi R, Serni S. Intraoperative assessment of ureteral and graft reperfusion during robotic kidney transplantation with indocyanine green fluorescence videography. Minerva Urol Nefrol. 2019;71:79-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Campi R, Vignolini G, Savi E, Sessa F, Agostini S, Serni S. Robotic kidney transplantation allows safe access for transplant renal biopsy and percutaneous procedures. Transpl Int. 2019;32:1333-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Sood A, Ghosh P, Jeong W, Bhandari M, Ahlawat R, Menon M. 715 Robotic kidney transplantation with regional hypothermia: Results from a prospective two-arm non-randomized controlled trial (Ideal phase 2b). Eur Urol Suppl. 2016;15:e715. [DOI] [Cited in This Article: ] |
| 28. | Ahlawat R, Sood A, Jeong W, Ghosh P, Keeley J, Abdollah F, Kher V, Olson P, Farah G, Wurst H, Bhandari M, Menon M. Robotic Kidney Transplantation with Regional Hypothermia versus Open Kidney Transplantation for Patients with End Stage Renal Disease: An Ideal Stage 2B Study. J Urol. 2021;205:595-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Territo A, Gausa L, Alcaraz A, Musquera M, Doumerc N, Decaestecker K, Desender L, Stockle M, Janssen M, Fornara P, Mohammed N, Siena G, Serni S, Sahin S, Tuǧcu V, Basile G, Breda A. European experience of robot-assisted kidney transplantation: minimum of 1-year follow-up. BJU Int. 2018;122:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Ahlawat RK, Tugcu V, Arora S, Wong P, Sood A, Jeong W, Bhandari M, Menon M. Learning Curves and Timing of Surgical Trials: Robotic Kidney Transplantation with Regional Hypothermia. J Endourol. 2018;32:1160-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Oberholzer J, Giulianotti P, Danielson KK, Spaggiari M, Bejarano-Pineda L, Bianco F, Tzvetanov I, Ayloo S, Jeon H, Garcia-Roca R, Thielke J, Tang I, Akkina S, Becker B, Kinzer K, Patel A, Benedetti E. Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant. 2013;13:721-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Tsai MK, Lee CY, Yang CY, Yeh CC, Hu RH, Lai HS. Robot-assisted renal transplantation in the retroperitoneum. Transpl Int. 2014;27:452-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Doumerc N, Beauval JB, Rostaing L, Sallusto F. A new surgical area opened in renal transplantation: a pure robot-assisted approach for both living donor nephrectomy and kidney transplantation using transvaginal route. Transpl Int. 2016;29:122-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Delsuc C, Faure A, Berthiller J, Dorez D, Matillon X, Meas-Yedid V, Floccard B, Marcotte G, Labeye V, Rabeyrin M, Codas R, Chauvet C, Robinson P, Morelon E, Badet L, Hanf W, Rimmelé T. Uncontrolled donation after circulatory death: comparison of two kidney preservation protocols on graft outcomes. BMC Nephrol. 2018;19:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Tzvetanov IG, Spaggiari M, Tulla KA, Di Bella C, Okoye O, Di Cocco P, Jeon H, Oberholzer J, Cristoforo Giulianotti P, Benedetti E. Robotic kidney transplantation in the obese patient: 10-year experience from a single center. Am J Transplant. 2020;20:430-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Li Marzi V, Campi R, Pecoraro A, Peris A, Serni S. Feasibility and Safety of Kidney Transplantation from Deceased Donors during the COVID-19 Pandemic: Insights from an Italian Academic Centre. Actas Urol Esp (Engl Ed). 2020;44:708-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Campi R, Pecoraro A, Li Marzi V, Tuccio A, Giancane S, Peris A, Cirami CL, Breda A, Vignolini G, Serni S. Robotic Versus Open Kidney Transplantation from Deceased Donors: A Prospective Observational Study. Eur Urol Open Sci. 2022;39:36-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Nanni G, Tondolo V, Citterio F, Romagnoli J, Borgetti M, Boldrini G, Castagneto M. Comparison of oblique versus hockey-stick surgical incision for kidney transplantation. Transplant Proc. 2005;37:2479-2481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Wagenaar S, Nederhoed JH, Hoksbergen AWJ, Bonjer HJ, Wisselink W, van Ramshorst GH. Minimally Invasive, Laparoscopic, and Robotic-assisted Techniques Versus Open Techniques for Kidney Transplant Recipients: A Systematic Review. Eur Urol. 2017;72:205-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Prudhomme T, Beauval JB, Lesourd M, Roumiguié M, Decaestecker K, Vignolini G, Campi R, Serni S, Territo A, Gausa L, Tugcu V, Sahin S, Alcaraz A, Musquera M, Stockle M, Janssen M, Fornara P, Mohammed N, Del Bello A, Kamar N, Sallusto F, Breda A, Doumerc N. Robotic-assisted kidney transplantation in obese recipients compared to non-obese recipients: the European experience. World J Urol. 2021;39:1287-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Kim SD, Kim JI, Moon IS, Park SC. Comparison of Minimal Skin Incision Technique in Living Kidney Transplantation and Conventional Kidney Transplantation. Chin Med J (Engl). 2016;129:917-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Malinka T, Banz VM, Wagner J, Candinas D, Inderbitzin D. Incision length for kidney transplantation does not influence short- or long-term outcome: a prospective randomized controlled trial. Clin Transplant. 2013;27:E538-E545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Spaggiari M, Lendacki FR, Di Bella C, Giulianotti PC, Benedetti E, Oberholzer J, Tzvetanov I. Minimally invasive, robot-assisted procedure for kidney transplantation among morbidly obese: Positive outcomes at 5 years post-transplant. Clin Transplant. 2018;32:e13404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Spaggiari M, Di Cocco P, Tulla K, Kaylan KB, Masrur MA, Hassan C, Alvarez JA, Benedetti E, Tzvetanov I. Simultaneous robotic kidney transplantation and bariatric surgery for morbidly obese patients with end-stage renal failure. Am J Transplant. 2021;21:1525-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |