Published online Nov 12, 2014. doi: 10.5499/wjr.v4.i3.80
Revised: September 25, 2014
Accepted: October 14, 2014
Published online: November 12, 2014
Periodontitis or Periodontal disease (PD) and Rheumatoid arthritis (RA) are two the most common chronic inflammatory diseases. Periodontitis is a biofilm associated destructive inflammatory disease of the periodontium caused by specific microorganisms. Rheumatoid arthritis is an autoimmune condition and is identified by elevated serum autoantibody titre directed against citrullinated peptides or rheumatoid factor. Periodontitis may involve some elements of autoimmunity. Recent studies have established that PD and RA show a common pathway and could be closely associated through a common dysregulation and dysfunction in inflammatory mechanism. The enzyme peptidyl arginine deiminase (PAD), expressed by Porphyromonas gingivalis (P. gingivalis) is responsible for the enzymatic deimination of arginine residuals to citrulline resulting in protein citrullination and its increased accumulation in RA. Citrullination by PAD may act as a putative biologic link between PD and RA. Association of Human leukocytic antigen-DR4 antigen has been established both with RA and PD. Several interleukins and inflammatory mediators (ILs) and Nuclear factor kappa beta ligand are linked to these common chronic inflammatory diseases. Antibodies directed against heat shock protein (hsp 70 ab) of P. gingivalis, P. melanogenicus and P. intermedia are raised in PD as well as RA. Both the conditions share many pathological and immunological similarities. Bacterial infection, genetic susceptibility, altered immune reaction and inflammatory mediators considered responsible for RA are also associated with PD. So it is plausible that a biological link may exist between PD and RA. Therapies aimed at modifying the expression and effect of inflammatory mediators and effector molecules such as matrix metalloproteinases, proinflammatory cytokines and autoantibodies of structural proteins may probably reduce the severity of both RA and PD.
Core tip: Periodontal disease (PD) and Rheumatoid arthritis (RA) share many pathological and immunological similarities. Recent studies have established significant association between the two. Bacterial infection, genetic susceptibility, altered immune reaction and inflammatory mediators considered responsible for RA are also associated with PD. So it is plausible that a biological link may exist between PD and RA. Therapies aimed at reduction of inflammatory mediators and effector molecules can probably reduce the severity of both RA and PD.
- Citation: Joseph R, Raj MJ, Sundareswaran S, Kaushik PC, Nagrale AV, Jose S, Rajappan S. Does a biological link exist between periodontitis and rheumatoid arthritis? World J Rheumatol 2014; 4(3): 80-87
- URL: https://www.wjgnet.com/2220-3214/full/v4/i3/80.htm
- DOI: https://dx.doi.org/10.5499/wjr.v4.i3.80
Periodontal disease (PD) is an immuno inflammatory disease of the periodontium which comprises of both hard and soft tissues like gingiva, periodontal ligament, cementum and alveolar bone. It results from a complex interaction between gram negative organisms, their byproducts and the response of the host[1-4]. The resulting gingival inflammation leads to destruction of both the soft and hard tissues supporting the tooth[5]. The prevalence is said to be as high as 80% to 90%[6].
For periodontal tissues in a healthy state, a steady equilibrium exists between tissue destruction and repair. Periodontal destruction is initiated and progressed by specific periodontal microorganisms that colonize in plaque biofilm. Host microbial interaction determines the extent and severity of periodontal disease[7-9]. A large number of different species of bacteria can be identified in the dental plaque[10] but only a few of them are implicated in chronic periodontitis[11,12]. To prevent exacerbated reactions and destruction of host tissues, an appropriate tolerance mechanism is required by the host to recognize and identify nonpathogenic and pathogenic bacteria[13]. Pattern recognition receptors and microbe associated molecular patterns have a very significant role in periodontal inflammation and adaptive immune response[13,14].
The equilibrium established between anti-inflammatory and proinflammatory cytokines (IL-1α, IL-1β, TNF-α, IL-6[15], IL-7, IL-11, IL-17A, IL-17F, IFN-γ, IL-4, IL-10, IL-13, IL-16, IFN-α, TGF-β[16,17]) is responsible for the net inflammatory response. Increased levels of IL-1β, IL-12, IL-6, IL-17, TNF-α, and IFN-γ are reported in gingival tissues of chronic destructive periodontitis[18,19]. In periodontitis both Th1 (IFN-γ, IL-2, TNF-α) and Th2 (IL-4, IL-5, IL-6, IL-13) type cytokines are observed[20]. There is supporting evidence for the role of IL-17 and Th17 cells in periodontal disease[21]. IL-17 induces IL-6 and IL-8 secretion by gingival fibroblasts and also upregulates MMP - Matrix Metalloproteinases (MMP-1) and MMP-3 in these cells[22,23]. IL-17 also induces IL-1β and TNF-α secretion from macrophages and gingival epithelial cells[22,23]. Inflammatory cytokines are produced as a result of activation of toll like receptors of oral epithelial cells by the lipopolysaccharide of the gram negative periodontal pathogens[24]. Recently it has been reported that pathogenesis of many systemic diseases are associated with these inflammatory mediators. The pathways bridging periodontal infection and systemic health include transient bacteremia via metastatic infection, injury and inflammation resulting from immunological response induced by periodontal pathogens[25].
Recent studies have demonstrated that chronic periodontitis acts as a risk factor for systemic diseases like diabetes mellitus, cardiovascular disease, adverse pregnancy outcomes, rheumatoid arthritis etc[26-28].
Rheumatoid arthritis (RA) is a chronic inflammatory disease of articular joint with unknown etiology marked by a symmetric, peripheral polyarthritis and often results in joint damage and physical disability. The pathogenic hallmark of RA is synovial inflammation and proliferation, focal bone erosion and thinning of articular cartilage. Articular cartilage is an avascular tissue composed of a specialized matrix of collagen, proteoglycans and other proteins. Chondrocytes contribute to the unique cellular component. Cartilage is a highly responsive tissue that reacts to inflammatory mediators and mechanical factors, which in turn alters the balance between cartilage anabolism and catabolism. The structural damage to the mineralized cartilage and subchondral bone is mediated by osteoclasts[29].
Worldwide prevalence of RA, an autoimmune condition is approximately 1%[30]. It is diagnosed as chronic inflammatory polyarthritis when five or more joints are affected[29]. On close observation, a number of similarities seem to exist between the supporting periodontal structures and articular joint (Table 1).
| Supporting periodontal structures | Articular joint |
| Periodontal structures comprise of cementum, alveolar bone, periodontal ligament, gingival crevicular fluid and gingiva | Articular joints comprise of articular cartilage, bone, ligaments, synovial cavity, synovial fluid, and synovial capsule |
| Cementum is an avascular tissue | Articular cartilage is an avascular tissue |
| Periodontal ligament is a thin connective tissue that surrounds the root connecting it to the alveolar bone | Synovial tissue is a thin layer of connective tissue. It consists primarily of two cell types- type A synoviocytes (macrophage derived) and type B synoviocyte (fibroblast derived) |
| Periodontal ligament is collagenous and consists of epithelial rests of malassez, fibroblasts, osteoblasts and ground substances (hyaluronic acid and proteoglycans-fibronectin and laminin) | Synovial fibroblasts are the most abundant and produce the structural components of the joints including collagen, fibronectin and laminin |
| Gingival crevicular fluid is an infiltrate of blood | Synovial fluid is an infiltrate of blood |
Periodontitis is a destructive chronic inflammatory disease of the periodontium caused by biofilm associated specific microorganisms[31-33]. Rheumatoid arthritis is an autoimmune condition and is characterized by elevated serum autoantibody titre directed against citrullinated peptides or rheumatoid factor (RF)[34,35]. Autoantibodies such as RF and anti-citrullinated protein/peptide antibody (ACPA) may be found in the sera of RA patients long before clinical onset of disease[27]. Periodontitis may also involve some elements of autoimmunity[36]. Autoantibodies and specific T cells against host molecules, such as type 1 collagen, have been detected in periodontal disease[23]. Recent studies have established statistically significant association between PD and RA[37-41]. The likelihood of PD among patients with RA is high. Also a higher prevalence of RA has been reported among patients with moderate to severe PD[41]. Joseph R. reported more periodontal destruction in RA group, pointing to a positive association between these diseases[42]. When comparing patients with RA and those with PD, many similarities have been reported in terms of serum cytokine and gene expression profiles, increased levels of serum matrix metalloproteinases, reactive oxygen species, lipid mediators, and neutrophil associated enzymes[5,43-46]. It has further been proposed that polymorphisms relating to genes encoding inflammatory cytokines might confer susceptibility to RA and PD[47-49]. Table 2 depicts some similarities observed in the pathogenesis of RA and PD.
| PD | RA |
| Chronic immunoinflammatory disease | Chronic immunoinflammatory disease |
| Periodontal pathogen is the main etiological agent with some element of autoimmunity | Bacteria/peptide as an adjunct antigen in autoantibody production |
| HLA-DR antigen association | HLA-DR antigen association |
| Inflammatory infiltrate mainly consists of B cells, plasma cells, PMN, T cell, dendritic cell, and macrophages | Inflammatory infiltrate consists of T cell, B cell, plasma cell, dendritic cell, mast cell, macrophages, and few granulocytes |
| Increases level of IL-1, TNF-α, PGE2, MMPs, NF-κB, RANK/RANKL/OPG, osteoclast activation | Increases level of IL-1, TNF-α, PGE2, MMPs NF-ĸβ, RANK/RANKL/OPG, osteoclast activation |
| Th1, ↑ ed Th2 and Th 17 | Th1 = Th2 and Th 17 |
| Role of nitric oxide | Role of nitric oxide |
| Genetic and environmental influences | Genetic and environmental influences |
| Bacterial DNA of anaerobes and high antibody titres against heat shock protein of P.gingivalis, P. Melanogenicus and P. Intermedia[95] | Bacterial DNA of anaerobes and high antibody titres against heat shock protein of P. gingivalis, P. Melanogenicus and P. Intermedia[95] |
Periodontal pathogens like Porphyromonas gingivalis (P. gingivalis) can invade the blood vessels and endothelial cells and lead to persistent bacteremia. It has the ability to invade primary chondrocytes of knee joints. As a result, cell cycle progression gets delayed, ultimately leading to accelerated apoptosis of these chondrocytes[50]. The virulence of P. gingivalis is mainly associated with its trypsin like proteolytic activity and ability to produce arginine and lysine -specific cysteine endopeptidase like gingipain R and gingipain K respectively[51]. Gingipain aids in evasion of host defense, tissue destruction and infection[52,53]. It leads to activation of MMPs (1, 3 and 9) and degradation of host proteins (laminin, fibronectin and collagen)[54]. Being the only identified bacterium with expression of peptidyl arginine deiminase (PAD), P. gingivalis and PAD represent a notable pathogenic element of RA[55-58]. PAD catalyses the deimination of arginine residuals to citrulline, a form of post-translational protein modification[59] which leads to an irreversible translation of arginine to citrulline[56,59]. However one important difference is that PAD expressed by P. gingivalis and human PAD are not exactly homologous[56,59]. It has been reported that in RA there is an increased citrullination of structure proteins[60]. This probably accounts for the fact that P. gingivalis titre significantly correlates with ACPA titre in RA patients[56,61-65].
The most potent disease risk gene in RA and PD is the genome on the human leukocytic antigen (HLA) region[66]. HLA-DR4 antigen is associated with both RA and PD. This genetic association between these chronic inflammatory conditions also points to the biologic link between them[65,67-69]. Hitchon and colleagues have reported an association between P. gingivalis and the presence of ACPA in a population with predominant RA-predisposing HLA-DRB1 alleles. This gene-environment interaction may contribute to the breaking up of self-tolerance to citrullinated proteins. It could also amplify autoimmune reactions which could predispose to RA[70].
The synovial fluid of RA patients is rich in proinflammatory cytokines and many interleukins, (IL-1, IL-6, IL-8, IL-15, and IL-17) as well as NF-κB ligand (RANKL) which can be linked with RA[71,72]. Similar profile of inflammatory mediators has been identified in chronic periodontitis[35,73,74]. Elevated serum levels of TNF-α is associated with both these chronic inflammatory diseases[74,75]. Lipopolysaccharides and other bacterial byproducts stimulate the release of TNF-α and it upregulates the release of prostaglandin E2 (PGE2) and MMPs that stimulate osteoclast activation. These inflammatory processes ultimately lead to bone resorption in both RA and PD[76].
It has been suggested that treatment of periodontitis in patient with RA improved their response to RA therapy[77-80]. Treatment of RA with disease modifying antirheumatic drugs (DMARDS) improves their periodontal condition due to its host modulatory effect, thus masking the gingival inflammation and actual periodontal destruction[81-83]. Similarly, reduction in the systemic inflammation by the additional effect of periodontal therapy may also have been masked by DMARDs[35]. Al-Katma et al[84] assessed the role of scaling and root planning (SRP) on RA and demonstrated that there was an improvement in RA scores in the test group as compared to the control group. Advances in treatment of RA have identified novel therapeutic targets such as anticytokine therapy. Anti-TNF-α therapy used to control RA may also be beneficial in the management of periodontitis[85-88]. Ortiz et al[77] assessed the additional effect of non-surgical periodontal therapy (NSPT) in RA patients under anti-TNF-α therapy and reported that regardless of the medications, supportive periodontal therapy had a positive result on the clinical features of RA. In the absence of periodontal treatment anti-TNF-α therapy alone had no relevant outcome on the periodontal condition[77].
First of all, PD and RA share many pathological and immunological similarities. A cyclic nature of disease activity is seen in both RA and PD. There is evidence to suggest that PD could act as a potential risk factor for RA[24,89,90]. Similarly, RA subjects have significantly increased clinical attachment loss (CAL)[19-21]. Increased levels of antibodies to periodontopathic bacteria are reported to have been identified in sera and synovial tissues of patients with RA[63,70,81,91]. Correlation of serum level IgG antibodies to P. gingivalis with anticyclic citrullinated peptide indicates that serum protein citrullination via peptidyl arginine deiminase of P. gingivalis drives RA responses[63,70]. Citrullination by PAD may act as a biologically plausible mechanistic link between PD and RA. Furthermore the presence of RA might predispose individuals to PD[92,93]. Clinical trials suggest that treatment of PD has a significant effect on RA severity and vice versa[84,94].
Second, it is suspected that IL-1 gene polymorphism affects the cytokine protein in RA and PD. HLA DR4 antigen is associated with both the conditions which points to the biological link between the two[67].
Third, it is reported that antibodies against heat shock protein (hsp 70 ab) of P. gingivalis, P. melanogenicus and P. intermedia are elevated not only in supporting periodontal tissues but also in synovial tissue of articular joints of RA patients[91,95].
Fourth point, both RA and PD have shown raised titres of IL-10, IL-1α , IL-1β, MMPs, TNF-α ,LT-α and low titres of IL-1α and IL-6[45]. A common inflammatory marker dysfunction seems to be associated with both the articular joint and supporting periodontal tissue.
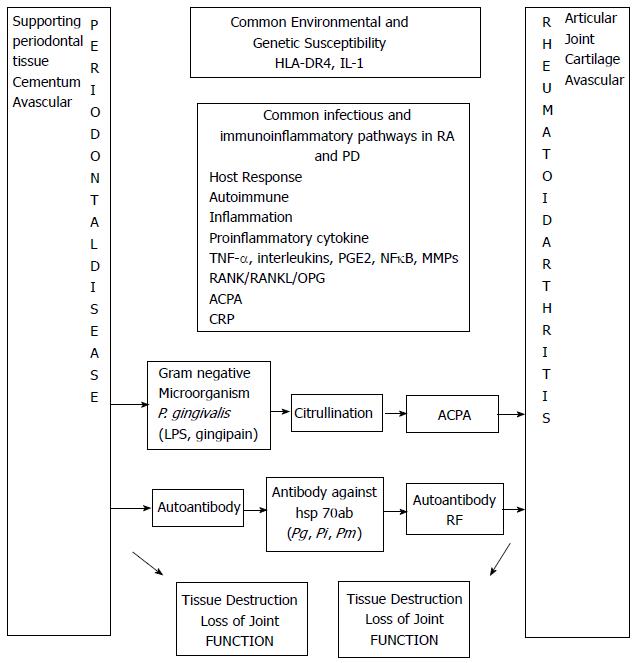
Bacterial infection, genetic susceptibility, altered immune reaction and inflammatory mediators considered responsible for RA are also associated with PD. So it is plausible that a biological link may exist between PD and RA (Figure 1).
Several studies have revealed that the prevalence of PD was high in patients with RA[40,42] and that PD severity was greater in RA patients[45]. Mercado et al[39] (2001) demonstrated a relation between RA and severity of periodontitis in a case control clinical study. Pischon et al[40] (2009) in a cross sectional clinical study showed significantly more CAL in RA subjects as compared to non-RA subjects. They have concluded that oral hygiene may account for this association to some extent. Kobayashi et al[49] (2007) reported that IL-1 and FCγR gene polymorphism have potential risk for RA and periodontitis.
Martinez-Martinez et al[96] (2009) in a case series clinical study on subjects with refractory RA and periodontitis found that P. intermedia, P. gingivalis, and T. denticola were the most predominant gram negative bacteria identified in synovial fluid, which substantiates the concept of anti-CCP and citrullinated structure protein. Dissick et al[41] (2010) in a case control study demonstrated that RA+ patients have more moderate to severe periodontitis. They have reported that females and smokers are at more risk in the RA+/periodontitis complex
Okada et al[81] (2011) demonstrated that corticosteroids, anti-rheumatic drugs, NSAIDs and TNF-α antagonists therapy improved the clinical features of periodontitis in RA patients. Presence of anti-PG IgG antibodies in RA+ patients may influence the serum RF level and periodontal health status[81]. Mayer et al[83] (2009) in a case control study concluded that TNF-α levels correlated with overall CAL and that inhibition of proinflammatory cytokines may account for the reduction of periodontal parameters. In another case control study, Ribeiro et al[94] (2005) evaluated the role of NSPT on RA status and found that RF decreased after periodontal intervention. The effects of NSPT in subjects with and without RA was studied by Pinho Mde et al[97] (2009) and they stated that that the relation between RA and periodontal disease activity is unclear. The effect of NSPT on RA patients under anti-TNF-α was studied by Ortiz et al[77] (2009) who inferred that NSPT had a positive effect on the clinical parameters of RA. Okada et al[98] in (2013) suggested that periodontal treatment decreases the levels of antibodies to P. gingivalis and citrulline in patients with RA and Periodontitis. They concluded that these observations may reflect the role of P. gingivalis in the protein citrullination which is related to the pathogenesis of RA[98]. Kaur et al[78] (2014) in a systematic review and meta analysis reported that non surgical periodontal therapy could lead to improvement in clinical and biochemical disease activity in RA.
Quirke et al[99] in 2013 reported that P. gingivalis is seemingly distinctive among periodontal pathogens in having PPAD (P. gingivalis peptidylarginine deiminase) with potential to evoke autoimmune response. They opined that the peptidyl citrulline specific immune response to PPAD might break tolerance in RA and could be a target for therapy[99]. Agnihotri et al[100] in (2014) reviewed the link between RA and PD in the elderly and inferred that thorough understanding of the link between the two chronic inflammatory diseases might be beneficial in rendering better health care protection and betterment of the life style of aged individuals.
The relationship between RA and PD can be attributed to common dysfunction and dysregulation in inflammatory mechanisms. Apparently, the common factors are bacterial lipopolysaccharides and inflammatory mediators. Development of specific autoantibodies by citrullination of protein by P. gingivalis may be the connecting link between RA and PD. Therapies aimed at suppression of inflammatory mediators and effector molecules such as MMP, proinflammatory cytokines and autoantibodies of structural proteins may probably reduce the severity of both RA and PD.
P- Reviewer: Ardila CM, Haraszthy V, Kanzaki H S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9-11. [PubMed] [Cited in This Article: ] |
| 3. | Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821-878. [PubMed] [Cited in This Article: ] |
| 4. | Cantore S, Mirgaldi R, Ballini A, Coscia MF, Scacco S, Papa F, Inchingolo F, Dipalma G, De Vito D. Cytokine gene polymorphisms associate with microbiogical agents in periodontal disease: our experience. Int J Med Sci. 2014;11:674-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. 2005;76:2066-2074. [PubMed] [Cited in This Article: ] |
| 6. | Saini R, Marawar PP, Shete S, Saini S. Periodontitis, a true infection. J Glob Infect Dis. 2009;1:149-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Van Dyke TE. Role of the neutrophil in oral disease: receptor deficiency in leukocytes from patients with juvenile periodontitis. Rev Infect Dis. 1985;7:419-425. [PubMed] [Cited in This Article: ] |
| 9. | Genco RJ, Van Dyke TE, Levine MJ, Nelson RD, Wilson ME. 1985 Kreshover lecture. Molecular factors influencing neutrophil defects in periodontal disease. J Dent Res. 1986;65:1379-1391. [PubMed] [Cited in This Article: ] |
| 10. | Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770-3783. [PubMed] [Cited in This Article: ] |
| 11. | Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-144. [PubMed] [Cited in This Article: ] |
| 12. | Socransky SS, Haffajee AD, Ximenez-Fyvie LA, Feres M, Mager D. Ecological considerations in the treatment of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis periodontal infections. Periodontol 2000. 1999;20:341-362. [PubMed] [Cited in This Article: ] |
| 13. | Beutler B. Toll-like receptors: how they work and what they do. Curr Opin Hematol. 2002;9:2-10. [PubMed] [Cited in This Article: ] |
| 14. | Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479-485. [PubMed] [Cited in This Article: ] |
| 15. | Lamster IB, Novak MJ. Host mediators in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. Crit Rev Oral Biol Med. 1992;3:31-60. [PubMed] [Cited in This Article: ] |
| 16. | Graves DT, Delima AJ, Assuma R, Amar S, Oates T, Cochran D. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol. 1998;69:1419-1425. [PubMed] [Cited in This Article: ] |
| 17. | Lappin DF, MacLeod CP, Kerr A, Mitchell T, Kinane DF. Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clin Exp Immunol. 2001;123:294-300. [PubMed] [Cited in This Article: ] |
| 18. | Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliński K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003;30:1046-1052. [PubMed] [Cited in This Article: ] |
| 19. | Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007;78:1545-1550. [PubMed] [Cited in This Article: ] |
| 20. | Matsuki Y, Yamamoto T, Hara K. Detection of inflammatory cytokine messenger RNA (mRNA)-expressing cells in human inflamed gingiva by combined in situ hybridization and immunohistochemistry. Immunology. 1992;76:42-47. [PubMed] [Cited in This Article: ] |
| 21. | Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817-828. [PubMed] [Cited in This Article: ] |
| 22. | Newman MG, Takei HH, Klokkevold PR, Carranza FA. Clinical Periodontology 10th ed. New Delhi: Elsevier Science Health Science Division 2006; 239. [Cited in This Article: ] |
| 23. | Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza’s Clinical Periodontology 11th edition (Elsevier Health Sciences, 2011: 281-282). Available from: http://www.clinicalperiodontology.com/. [Cited in This Article: ] |
| 24. | Tietze K, Dalpke A, Morath S, Mutters R, Heeg K, Nonnenmacher C. Differences in innate immune responses upon stimulation with gram-positive and gram-negative bacteria. J Periodontal Res. 2006;41:447-454. [PubMed] [Cited in This Article: ] |
| 25. | Akar H, Akar GC, Carrero JJ, Stenvinkel P, Lindholm B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6:218-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Kuo LC, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122:417-433. [PubMed] [Cited in This Article: ] |
| 27. | Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334. [PubMed] [Cited in This Article: ] |
| 28. | Mercado F, Marshall RI, Klestov AC, Bartold PM. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000;27:267-272. [PubMed] [Cited in This Article: ] |
| 29. | Longo D, Fauci A, Kasper D, Hauser S, Jameson J. Harrisons Manual of Medicine, 18th ed. New York: McGraw-Hill Education 2012; 2738. [Cited in This Article: ] |
| 30. | Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62:460-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Slots J, Rams TE. New views on periodontal microbiota in special patient categories. J Clin Periodontol. 1991;18:411-420. [PubMed] [Cited in This Article: ] |
| 32. | Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322-331. [PubMed] [Cited in This Article: ] |
| 33. | Wolff L, Dahlén G, Aeppli D. Bacteria as risk markers for periodontitis. J Periodontol. 1994;65:498-510. [PubMed] [Cited in This Article: ] |
| 34. | McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429-442. [PubMed] [Cited in This Article: ] |
| 35. | Rutger Persson G. Rheumatoid arthritis and periodontitis - inflammatory and infectious connections. Review of the literature. J Oral Microbiol. 2012;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14-40. [PubMed] [Cited in This Article: ] |
| 37. | Greenwald RA, Kirkwood K. Adult periodontitis as a model for rheumatoid arthritis (with emphasis on treatment strategies). J Rheumatol. 1999;26:1650-1653. [PubMed] [Cited in This Article: ] |
| 38. | Kässer UR, Gleissner C, Dehne F, Michel A, Willershausen-Zönnchen B, Bolten WW. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997;40:2248-2251. [PubMed] [Cited in This Article: ] |
| 39. | Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779-787. [PubMed] [Cited in This Article: ] |
| 40. | Pischon N, Pischon T, Kröger J, Gülmez E, Kleber BM, Bernimoulin JP, Landau H, Brinkmann PG, Schlattmann P, Zernicke J. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 41. | Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, Mikuls TR, Amdur RL, Richards JS, Kerr GS. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Joseph R, Rajappan S, Nath SG, Paul BJ. Association between chronic periodontitis and rheumatoid arthritis: a hospital-based case-control study. Rheumatol Int. 2013;33:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Mercado FB, Marshall RI, Bartold PM. Inter-relationships between rheumatoid arthritis and periodontal disease. A review. J Clin Periodontol. 2003;30:761-772. [PubMed] [Cited in This Article: ] |
| 44. | de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 306] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 45. | Havemose-Poulsen A, Sørensen LK, Stoltze K, Bendtzen K, Holmstrup P. Cytokine profiles in peripheral blood and whole blood cell cultures associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2005;76:2276-2285. [PubMed] [Cited in This Article: ] |
| 46. | Kobayashi T, Murasawa A, Komatsu Y, Yokoyama T, Ishida K, Abe A, Yamamoto K, Yoshie H. Serum cytokine and periodontal profiles in relation to disease activity of rheumatoid arthritis in Japanese adults. J Periodontol. 2010;81:650-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Havemose-Poulsen A, Sørensen LK, Bendtzen K, Holmstrup P. Polymorphisms within the IL-1 gene cluster: effects on cytokine profiles in peripheral blood and whole blood cell cultures of patients with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2007;78:475-492. [PubMed] [Cited in This Article: ] |
| 48. | Kobayashi T, Ito S, Kuroda T, Yamamoto K, Sugita N, Narita I, Sumida T, Gejyo F, Yoshie H. The interleukin-1 and Fcgamma receptor gene polymorphisms in Japanese patients with rheumatoid arthritis and periodontitis. J Periodontol. 2007;78:2311-2318. [PubMed] [Cited in This Article: ] |
| 49. | Kobayashi T, Murasawa A, Ito S, Yamamoto K, Komatsu Y, Abe A, Sumida T, Yoshie H. Cytokine gene polymorphisms associated with rheumatoid arthritis and periodontitis in Japanese adults. J Periodontol. 2009;80:792-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Pischon N, Röhner E, Hocke A, N’Guessan P, Müller HC, Matziolis G, Kanitz V, Purucker P, Kleber BM, Bernimoulin JP. Effects of Porphyromonas gingivalis on cell cycle progression and apoptosis of primary human chondrocytes. Ann Rheum Dis. 2009;68:1902-1907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Ogrendik M. Rheumatoid arthritis is linked to oral bacteria: etiological association. Mod Rheumatol. 2009;19:453-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. 2003;74:111-118. [PubMed] [Cited in This Article: ] |
| 53. | Stathopoulou PG, Galicia JC, Benakanakere MR, Garcia CA, Potempa J, Kinane DF. Porphyromonas gingivalis induce apoptosis in human gingival epithelial cells through a gingipain-dependent mechanism. BMC Microbiol. 2009;9:107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Jie Bao G, Kari K, Tervahartiala T, Sorsa T, Meurman JH. Proteolytic Activities of Oral Bacteria on ProMMP-9 and the Effect of Synthetic Proteinase Inhibitors. Open Dent J. 2008;2:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 1999;67:3248-3256. [PubMed] [Cited in This Article: ] |
| 56. | Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311-318. [PubMed] [Cited in This Article: ] |
| 57. | Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, Gawron K, Mizgalska D, Marcinska KA, Benedyk M. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013;9:e1003627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 58. | Abdullah SN, Farmer EA, Spargo L, Logan R, Gully N. Porphyromonas gingivalis peptidylarginine deiminase substrate specificity. Anaerobe. 2013;23:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155-163. [PubMed] [Cited in This Article: ] |
| 60. | Wegner N, Lundberg K, Kinloch A, Fisher B, Malmström V, Feldmann M, Venables PJ. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 61. | Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78-111. [PubMed] [Cited in This Article: ] |
| 62. | Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, Markt J, McGowan D, Kerr GS, Redman RS. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 63. | Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, Holers VM, Kuhn KA, O’Dell JR. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 64. | Liao F, Li Z, Wang Y, Shi B, Gong Z, Cheng X. Porphyromonas gingivalis may play an important role in the pathogenesis of periodontitis-associated rheumatoid arthritis. Med Hypotheses. 2009;72:732-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Mikuls TR, Thiele GM, Deane KD, Payne JB, O’Dell JR, Yu F, Sayles H, Weisman MH, Gregersen PK, Buckner JH. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64:3522-3530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 66. | Kinloch AJ, Alzabin S, Brintnell W, Wilson E, Barra L, Wegner N, Bell DA, Cairns E, Venables PJ. Immunization with Porphyromonas gingivalis enolase induces autoimmunity to mammalian α-enolase and arthritis in DR4-IE-transgenic mice. Arthritis Rheum. 2011;63:3818-3823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Marotte H, Farge P, Gaudin P, Alexandre C, Mougin B, Miossec P. The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann Rheum Dis. 2006;65:905-909. [PubMed] [Cited in This Article: ] |
| 68. | Bonfil JJ, Dillier FL, Mercier P, Reviron D, Foti B, Sambuc R, Brodeur JM, Sedarat C. A “case control” study on the rôle of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO). J Clin Periodontol. 1999;26:77-84. [PubMed] [Cited in This Article: ] |
| 69. | Kapitány A, Zilahi E, Szántó S, Szücs G, Szabó Z, Végvári A, Rass P, Sipka S, Szegedi G, Szekanecz Z. Association of rheumatoid arthritis with HLA-DR1 and HLA-DR4 in Hungary. Ann N Y Acad Sci. 2005;1051:263-270. [PubMed] [Cited in This Article: ] |
| 70. | Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, van der Woude D, Markland J, Robinson D, Elias B, Newkirk M. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010;37:1105-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 71. | Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31:927-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Fardellone P, Séjourné A, Paccou J, Goëb V. Bone remodelling markers in rheumatoid arthritis. Mediators Inflamm. 2014;2014:484280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Bozkurt FY, Berker E, Akkuş S, Bulut S. Relationship between interleukin-6 levels in gingival crevicular fluid and periodontal status in patients with rheumatoid arthritis and adult periodontitis. J Periodontol. 2000;71:1756-1760. [PubMed] [Cited in This Article: ] |
| 74. | Cetinkaya B, Guzeldemir E, Ogus E, Bulut S. Proinflammatory and anti-inflammatory cytokines in gingival crevicular fluid and serum of patients with rheumatoid arthritis and patients with chronic periodontitis. J Periodontol. 2013;84:84-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, Ishikawa T, Hanaoka E, Yamashita K, Yamashita M. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord. 2011;12:144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 76. | Xue M, McKelvey K, Shen K, Minhas N, March L, Park SY, Jackson CJ. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology (Oxford). 2014;Jun 29; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 77. | Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, Panneerselvam A, Askari A. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80:535-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 78. | Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2014;Apr 28; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Monsarrat P, Vergnes JN, Cantagrel A, Algans N, Cousty S, Kémoun P, Bertrand C, Arrivé E, Bou C, Sédarat C. Effect of periodontal treatment on the clinical parameters of patients with rheumatoid arthritis: study protocol of the randomized, controlled ESPERA trial. Trials. 2013;14:253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Erciyas K, Sezer U, Ustün K, Pehlivan Y, Kisacik B, Senyurt SZ, Tarakçioğlu M, Onat AM. Effects of periodontal therapy on disease activity and systemic inflammation in rheumatoid arthritis patients. Oral Dis. 2013;19:394-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Okada M, Kobayashi T, Ito S, Yokoyama T, Komatsu Y, Abe A, Murasawa A, Yoshie H. Antibody responses to periodontopathic bacteria in relation to rheumatoid arthritis in Japanese adults. J Periodontol. 2011;82:1433-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Han JY, Reynolds MA. Effect of anti-rheumatic agents on periodontal parameters and biomarkers of inflammation: a systematic review and meta-analysis. J Periodontal Implant Sci. 2012;42:3-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Mayer Y, Balbir-Gurman A, Machtei EE. Anti-tumor necrosis factor-alpha therapy and periodontal parameters in patients with rheumatoid arthritis. J Periodontol. 2009;80:1414-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol. 2007;13:134-137. [PubMed] [Cited in This Article: ] |
| 85. | Kobayashi T, Yokoyama T, Ito S, Kobayashi D, Yamagata A, Okada M, Oofusa K, Narita I, Murasawa A, Nakazono K. Periodontal and Serum Protein Profiles in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitor Adalimumab. J Periodontol. 2014;May 26; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 86. | Üstün K, Erciyas K, Kısacık B, Sezer U, Pehlivan Y, Öztuzcu S, Gündoğar H, Onat AM. Host modulation in rheumatoid arthritis patients with TNF blockers significantly decreases biochemical parameters in periodontitis. Inflammation. 2013;36:1171-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Queiroz-Junior CM, Bessoni RL, Costa VV, Souza DG, Teixeira MM, Silva TA. Preventive and therapeutic anti-TNF-α therapy with pentoxifylline decreases arthritis and the associated periodontal co-morbidity in mice. Life Sci. 2013;93:423-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Savioli C, Ribeiro AC, Fabri GM, Calich AL, Carvalho J, Silva CA, Viana VS, Bonfá E, Siqueira JT. Persistent periodontal disease hampers anti-tumor necrosis factor treatment response in rheumatoid arthritis. J Clin Rheumatol. 2012;18:180-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Scher JU, Bretz WA, Abramson SB. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors? Curr Opin Rheumatol. 2014;26:424-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 90. | Scher JU, Abramson SB. Periodontal disease, Porphyromonas gingivalis, and rheumatoid arthritis: what triggers autoimmunity and clinical disease? Arthritis Res Ther. 2013;15:122. [PubMed] [Cited in This Article: ] |
| 91. | Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, Jonsson R. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006;24:656-663. [PubMed] [Cited in This Article: ] |
| 92. | Ramamurthy NS, Greenwald RA, Celiker MY, Shi EY. Experimental arthritis in rats induces biomarkers of periodontitis which are ameliorated by gene therapy with tissue inhibitor of matrix metalloproteinases. J Periodontol. 2005;76:229-233. [PubMed] [Cited in This Article: ] |
| 93. | Queiroz-Junior CM, Madeira MF, Coelho FM, Costa VV, Bessoni RL, Sousa LF, Garlet GP, Souza Dda G, Teixeira MM, Silva TA. Experimental arthritis triggers periodontal disease in mice: involvement of TNF-α and the oral Microbiota. J Immunol. 2011;187:3821-3830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Ribeiro J, Leão A, Novaes AB. Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol. 2005;32:412-416. [PubMed] [Cited in This Article: ] |
| 95. | Modi DK, Chopra VS, Bhau U. Rheumatoid arthritis and periodontitis: biological links and the emergence of dual purpose therapies. Indian J Dent Res. 2009;20:86-90. [PubMed] [Cited in This Article: ] |
| 96. | Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, Rizo-Rodríguez JC, Little JW, Loyola-Rodríguez JP. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 2009;36:1004-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 97. | Pinho Mde N, Oliveira RD, Novaes AB, Voltarelli JC. Relationship between periodontitis and rheumatoid arthritis and the effect of non-surgical periodontal treatment. Braz Dent J. 2009;20:355-364. [PubMed] [Cited in This Article: ] |
| 98. | Okada M, Kobayashi T, Ito S, Yokoyama T, Abe A, Murasawa A, Yoshie H. Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J Periodontol. 2013;84:e74-e84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, Ytterberg AJ, Zubarev RA, Potempa J, Culshaw S. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 2014;73:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 100. | Agnihotri R, Gaur S. Rheumatoid arthritis in the elderly and its relationship with periodontitis: a review. Geriatr Gerontol Int. 2014;14:8-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |