Published online Dec 28, 2020. doi: 10.5499/wjr.v10.i1.1
Peer-review started: June 12, 2020
First decision: October 21, 2020
Revised: October 26, 2020
Accepted: November 4, 2020
Article in press: November 4, 2020
Published online: December 28, 2020
There is a lack of studies and educational programs focused on biosimilars and shared decision-making among patients diagnosed with various rheumatic diseases.
To improve knowledge and awareness of biosimilars and shared decision-making among patients attending rheumatology practices in Colorado as well as to assess a rheumatology patient’s interest in discussing biosimilars as well as shared decision-making with others (e.g., medical professionals, family members, friends).
Our goal was to work with 80 rheumatology teams in Colorado. We developed and distributed 2000 multi-page brochures to each participating office and later conducted an online anonymous survey.
There were a total of 49 (2.5%) rheumatology patients who responded to our survey. After reading our educational booklet, many survey respondents identified the correct answer in most questions focused on biosimilars or shared decision-making. Our survey results suggest that patients attending rheumatology practices in Colorado are generally not involved in discussions with their providers regarding treatment plans or options. The improvement in scores after reading our educational materials was statistically significant for biosimilars and shared decision-making.
Overall, the level of knowledge and awareness of biosimilars and shared decision-making among patients attending rheumatology practices in Colorado was low. More educational programs as well as follow up trainings to measure changes in knowledge and awareness regarding biosimilars and shared decision-making among patients attending rheumatology practices are recommended.
Core Tip: What is already known about this subject? Clinical research indicates that the introduction of biosimilars will not compromise either efficacy or safety for patients with rheumatic diseases such as rheumatoid arthritis. Unfortunately, there is a lack of knowledge and awareness regarding biosimilars among these patients. Very limited research of rheumatology patients’ knowledge, and awareness of biosimilars and shared decision-making, as well as motivation to learn more about these subjects exists. What does this study add? Consistently with several other studies, many patients attending rheumatology practices in Colorado have a low level of knowledge and awareness of shared decision-making and, especially, biosimilars. Moreover, most of these patients do not engage in important discussions regarding treatment plans or options with their doctors. As our survey respondents gained knowledge about biosimilars and shared decision-making from our printing materials it seems that educational programs enriched with printed materials may impact patients’ knowledge and awareness of biosimilars as well as shared decision-making. How might this impact on clinical practice or future developments? As we have become aware of which style of learning our survey respondents prefer regarding biosimilars and shared decision-making, we believe that prospective projects will benefit not only from booklets, but also from online presentations and webinars. Based on our current study results, we recommend adding more survey questions focused on shared decision-making such as discussing treatment plans and options with doctors.
- Citation: Ismailov R, Simoens S, Khasanova Z. Greater awareness of biosimilars and shared decision-making among patients attending rheumatology practices in Colorado, United States: Real-world data. World J Rheumatol 2020; 10(1): 1-10
- URL: https://www.wjgnet.com/2220-3214/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5499/wjr.v10.i1.1
Rheumatoid arthritis (RA) causes premature death, disability, and lowers quality of life[1,2]. It is the third most common type of arthritis, behind osteoarthritis and gout, affecting approximately 1.3 million in the United States[1,2]. In the last decade treatment of RA has been improved by biologic therapies. However, many patients have limited access to these medications as they are expensive[3,4].
Market competition and access to more affordable treatment options will likely improve through the introduction of biosimilars to the United States market[5]. The US Healthcare system savings from the use of biosimilars in the next several years could be as much as $150 billion[6]. These funds could then be put toward treating more RA patients or improving healthcare overall[7,8]. Clinical research indicates that the introduction of biosimilars will not compromise either efficacy or safety for RA patients[7,8].
Both physicians and patients need to be educated about the rigorous process of evaluation that biosimilars undergo prior to approval. Unfortunately, according to recent research there is a lack of knowledge and awareness surrounding biosimilars among patients. For example, many patients have never heard of biosimilars, as found in a recent study conducted in the United States[9].
We recently improved local oncology patients' knowledge about biosimilars[10]. Many of our survey respondents chose the correct definition of biosimilars and nearly 80% identified the correct answer concerning various topics related to biosimilars (e.g., regulation, adverse reactions reporting and cost issues)[10]. As we mentioned in our previous study, the introduction of biosimilars can be implemented more effectively by shared decision-making. Shared decision-making improves patient satisfaction and leads to better understanding, improved communication between patient and provider, improved medication schedule adherence, and more positive clinical outcomes[11,12]. It is advocated by many organizations associated with healthcare such as the Institute of Medicine while others such as Medicare and Medicaid require shared decision-making for some screening and medical procedures[13,14].
The goal of our current research was to improve knowledge and awareness of biosimilars as well as various topics related to biosimilars. We also aimed to improve patient’s awareness of, and learn about their involvement in shared decision-making. In addition, we set out to evaluate a rheumatology patient’s interest in discussing biosimilars and shared decision-making with others, such as medical professionals, family members, or friends. Lastly, we planned to examine motivation levels in learning more about these topics in the future.
The proposed project aimed to work with 80 rheumatology teams in Colorado. They were identified through the Colorado Department of Health Care, Policy and Financing (https://www.colorado.gov/hcpf) as physician offices located within 100 miles of the greater Denver area in Colorado. Our aim was to work with the patients attending these practices/clinics and we intended to target at least 2000 patients. We developed and distributed our booklets [8.5’’ by 5.5’’ vertical, 8 pages total (4 pages cover and 4 inside)] to all participating clinics. We obtained exempt determination from the Integreview Institutional Review Board prior to the implementation of the project.
Our educational materials were designed with the idea in mind that patients attending rheumatology clinics in Colorado may have a very poor understanding of biosimilars and shared decision-making. The most recent data available concerning biosimilars and shared decision-making was used. Several volunteers, who did not have medical backgrounds, were asked to read through our educational materials to ensure their comprehensibility as well.
Each booklet contained an invitation to participate in an online anonymous survey. Our educational booklet also explained the voluntary nature of the survey and encouraged each patient read through it in its entirety prior to taking the survey. After the open period of the survey was over, we focused our efforts on the analysis of the survey results and on the development of a manuscript for publication.
The survey was open to all study participants for approximately 3 consecutive months. We used a popular online tool (Surveymonkey®, Palo Alto, CA, United States) which we successfully used in our previous educational initiatives focused on biosimilars[10,15]. This online survey tool has been used in other research projects as well[16,17].
There were 24 questions in our survey, 2 of which related to demographics, the rest of which focused on various topics related to biosimilars and shared decision-making. We asked our respondents to rate their level of knowledge regarding biosimilars as well as shared decision-making on a scale from 1 to 10 (1 being lowest and 10 being highest level of knowledge).
Our aim was to maintain simplicity while taking into account data from published peer-reviewed literature focused on biosimilars and shared decision-making. Additionally, a few volunteers read our survey to ensure readability and comprehensibility. A 10-point scale to measure motivation and interest levels was used. Responses were given on a scale of 10 (the strongest motivation or interest levels) to 1 (no motivation or interest).
Informed consent was obtained from all survey respondents prior to the beginning of the survey. Our survey was completely anonymous, did not ask sensitive or stigmatizing questions, and did not collect identifying information. All survey respondents were informed that their responses would be used in the development of a research report. We estimated that our survey would take about 10 min to finish based on response times from our volunteers.
Statistical analysis was implemented using the Statistical Package for Social Sciences (SPSS®) software (version 16, SPSS®, Inc, Chicago, IL, United States). A non-parametric Chi-square test was used to analyze categorical data. P values smaller than 0.05 were considered statistically significant. A one-sample Wilcoxon signed-rank test was applied for each of the subjects (e.g., biosimilars and shared decision-making) and the test criteria Z and P values were reported. The null hypothesis was tested stating that the difference between the patients’ scores before and after reading our educational booklet was not significant.
There were a total of 49 patients who responded to our survey. This corresponds to 2.5% of the actual response rate (49 out of 2000). Most of our survey respondents were 18 to 40 years old (46.9%) followed by 41 to 60 years old (42.9%). Only about 10% of respondents were 61 to 80 years old. More than two thirds of respondents were women (67.3%).
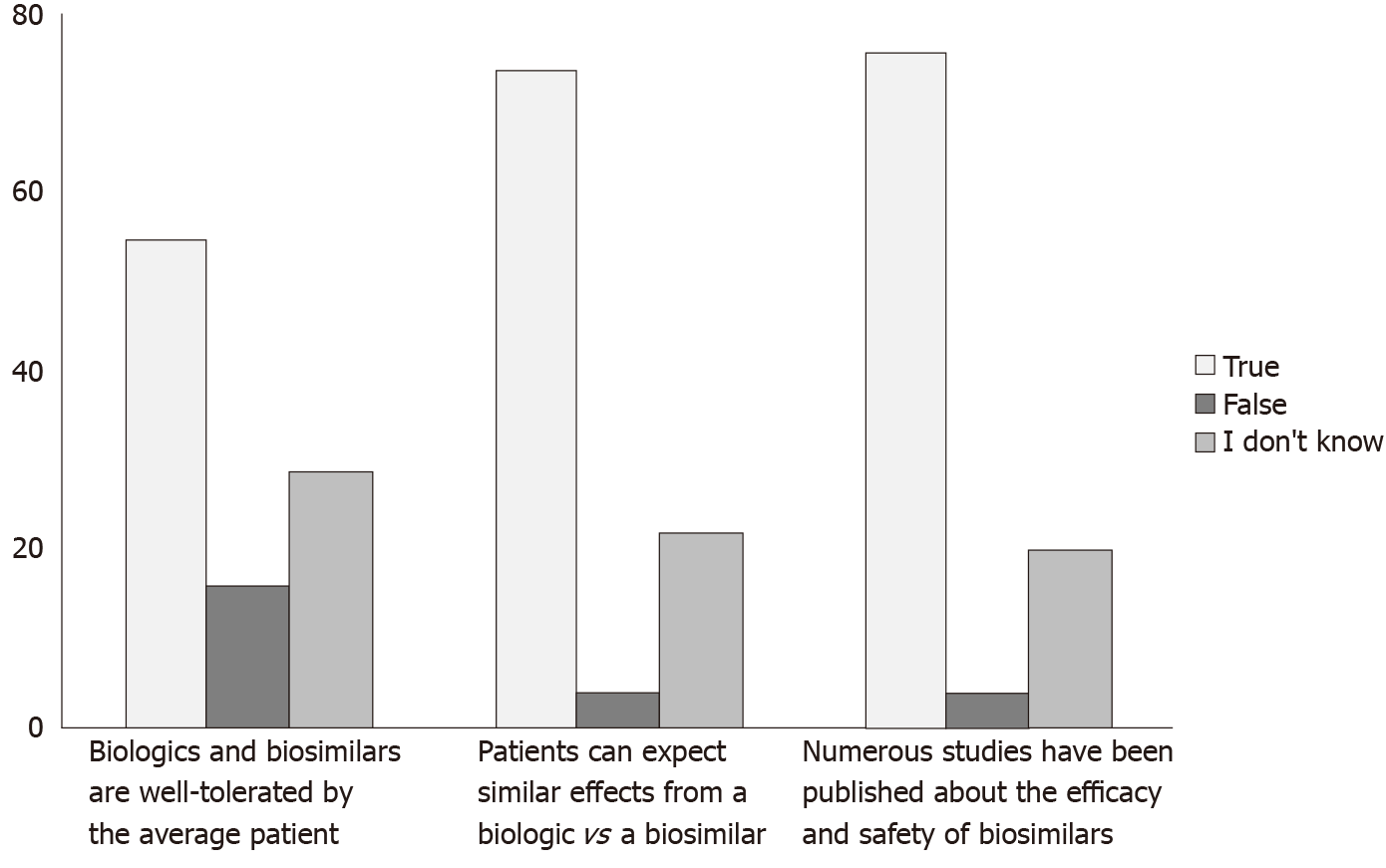
More than 70% of respondents identified the correct answer on 4 out of 5 questions focused on various topics related to biosimilar, including the definition (Figure 1). Almost 82% of survey respondents chose the correct answer on the question focused on Food and Drug Administration (FDA) regulatory policy concerning biosimilars. At the same time, only 55% of survey respondents found the correct answer to the question: “Biologics and biosimilars are well tolerated by the average patient”.
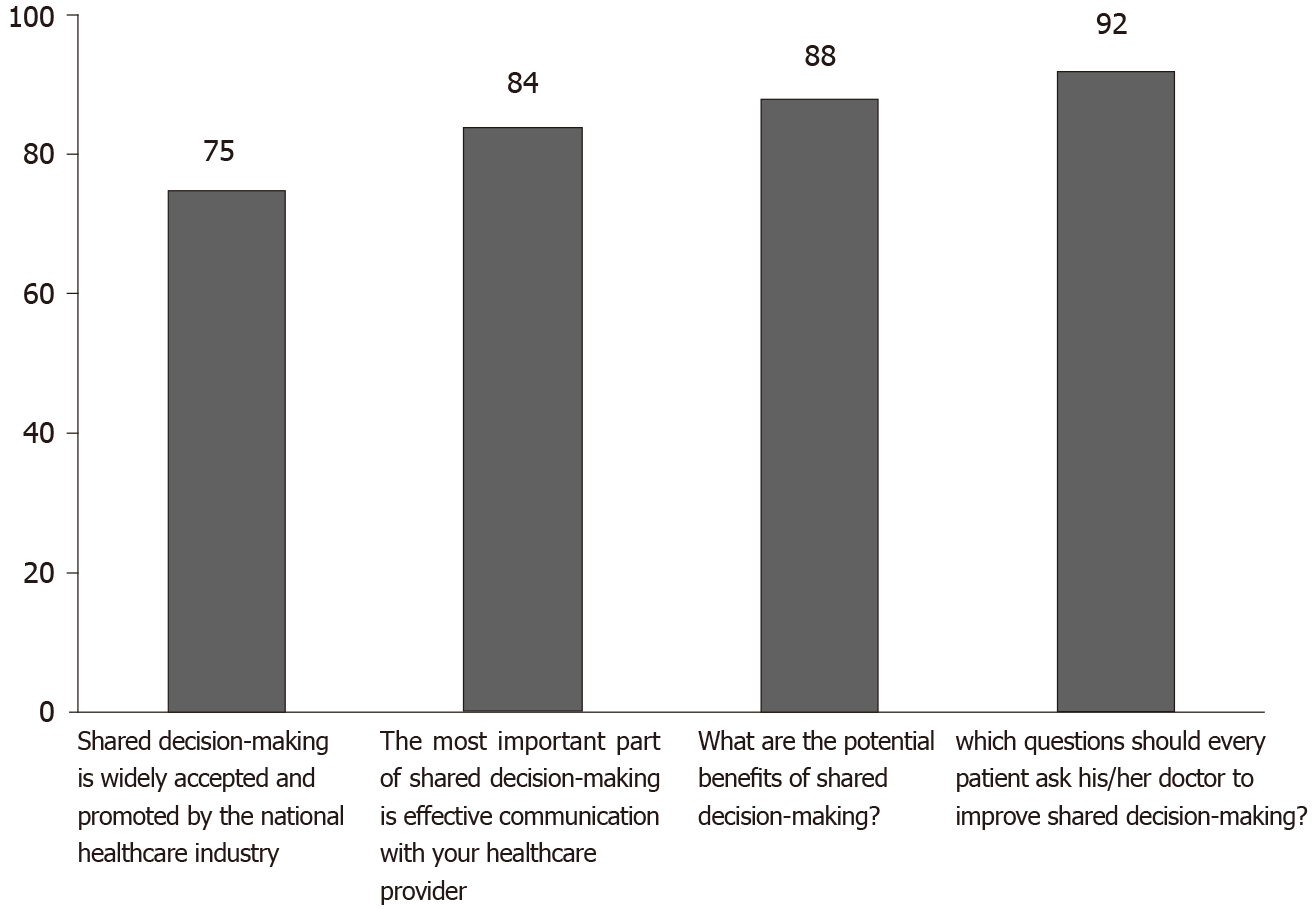
More than 80% of patients who responded to our survey identified the correct answer on questions concerning shared decision-making (Figure 2). The correct answer to the question focusing on the discussion of financial issues related to treatment was chosen by nearly nine out of ten patients. Nearly three quarters of survey respondents (73.5%) marked the right answer for the question: “Shared decision-making is widely accepted and promoted by the national healthcare industry”.
One out of five survey respondents indicated that their doctor never discusses a treatment plan with them and more than half of respondents answered that their doctor only does it rarely. Almost 80% of patients have never or rarely been asked by their doctor if they would like to help make a decision when there are a variety of treatment options. More than 60% of survey takers reported that their doctors have either never or rarely made assumptions about their income. In addition, only about 6% have usually or always been asked by their doctor if they would like to help make a decision when there are a variety of treatment options. Similarly, for only about 6% of respondents do their providers either usually or always discuss a treatment plan with them. Finally, for only about 6% of patients did their doctors make assumptions about their income.
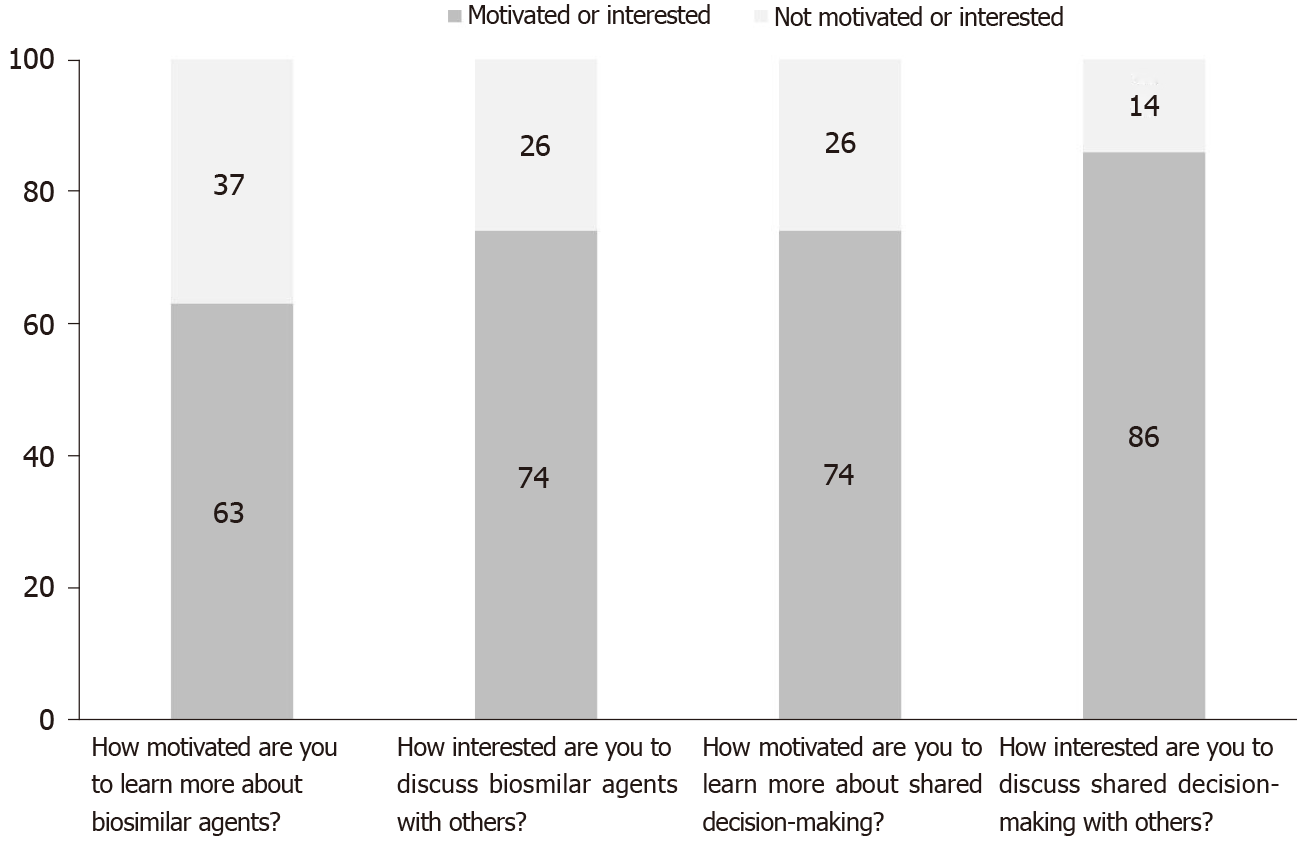
More than 60% of survey takers were motivated (motivation rank 6-10) to learn more about biosimilars and more than 70% were regarding shared decision-making (Figure 3). This compares to nearly one third of respondents who were not motivated (motivation rank 1-5, P value < 0.05). Similarly, more than 70% of patients were interested in discussing biosimilars with others (e.g., medical professionals, family members, friends) while nearly 30% were not (interest rank 1-5, P value < 0.05).
More than 60% of patients who took the survey rated their level of knowledge of biosimilars prior to reading our educational booklet as “1”. More than 40% rated it as “2” for shared decision-making. About two thirds of respondents rated their knowledge of biosimilars after reading our educational materials as “6” and nearly 70% rated it as “6” or “7” for shared decision-making. The improvement in scores after reading our educational materials was statistically significant for both subjects (e.g., biosimilars: Z = 6.162, P value < 0.001 and shared decision-making Z = 6.171, P value < 0.001).
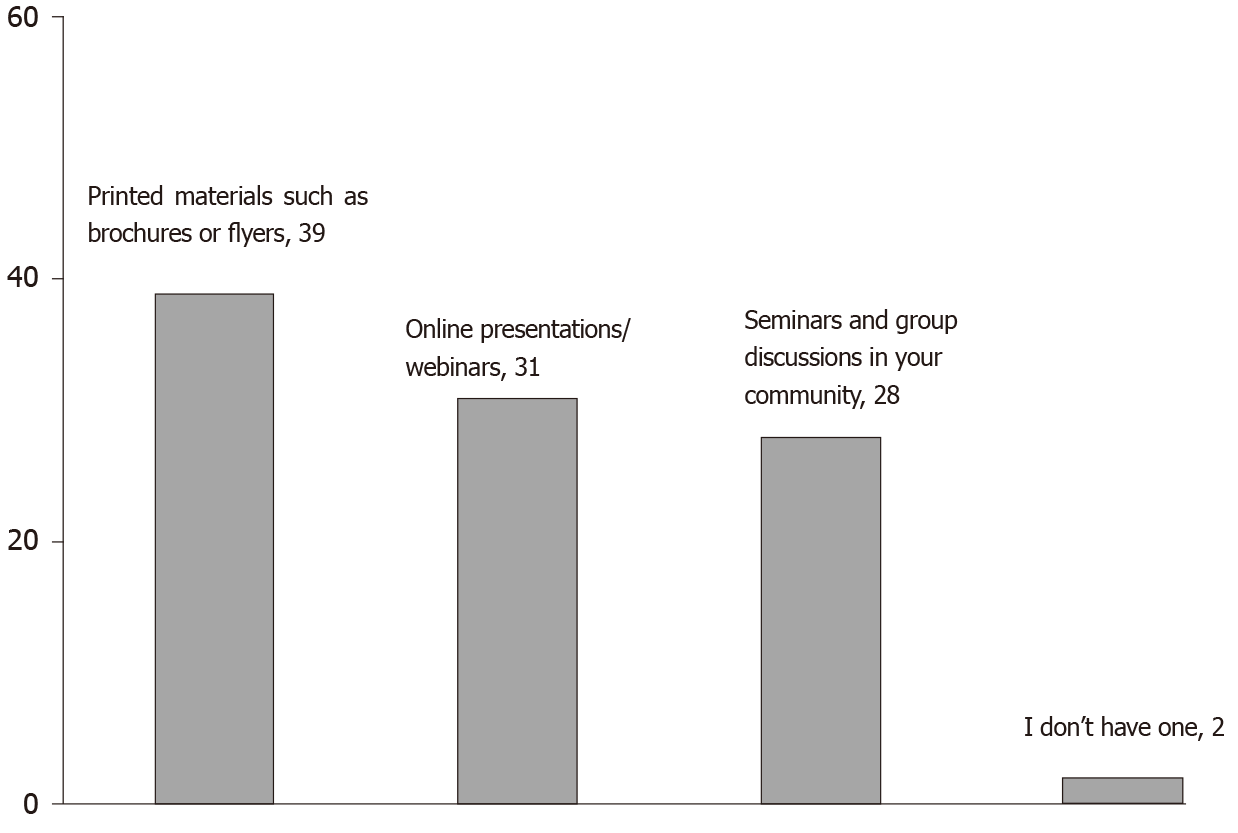
Printed materials such as brochures or flyers were the most popular style to learn more about biosimilars as well as shared decision-making (38.8%), followed by online presentations and webinars (30.6%) and seminars and group discussions (28.6%) (Figure 4).
Although biologic therapy of RA is often very effective, many patients do not have access to this therapy because of the high cost[5,7]. As a less expensive alternative, biosimilars could give RA patients access to more affordable treatment[1,2]. Unfortunately, recent efforts to educate patients regarding various concerns around biosimilars have not greatly succeeded. For instance, the respondents of the previous survey were rather skeptical about biosimilars; with only a quarter of those who took the survey having no specific concerns[18]. In another survey, roughly one out of five had no specific concerns[19]. Interestingly, respondents from a follow-up survey were significantly more likely to trust that biosimilars would have an positive impact on the treatment of autoimmune diseases[19].
Our results indicate that rheumatology patients initially had a low level of knowledge of biosimilars. Similarly, nearly 60% of survey respondents from a study of several hundred patients treated for rheumatic disease did not know what biosimilars were[20]. An analysis of a nationally representative sample in the United States showed that many participants had never heard about biosimilars[9]. In that same study, approximately one out of ten patients diagnosed with various autoimmune conditions had some impression of biosimilars. Only one out of five among those with autoimmune diseases, and who attended support groups, had a cursory general impression of biosimilars.
In contrast to our results, about every other RA patient from the anonymous survey conducted in Belgium had heard of biosimilars[4]. Moreover, most of the patients in that survey identified the correct answer regarding the definition of a biosimilar[4]. Similarly, most patients on biosimilars who participated in am anonymous web-survey in the United Kingdom thought biosimilars were similar copies of biologics as well[21].
Only about half of the survey takers in our study identified the correct answer to the question: “Biologics and biosimilars are well tolerated by the average patient”. We believe that such a low rate could be attributed, in part, to lack of awareness regarding biologics overall even though our educational booklet provided a clear answer to this question. Obviously, more patient education programs discussing both biologics and biosimilars are needed to alleviate such concerns.
Our current study results indicate that, overall, our participants had a better awareness of shared decision-making as compared to biosimilars before and after reading our booklet. Many survey respondents identified the right answer on questions concerning shared decision-making. One of the explanations is that the topic of shared decision-making is relatively easier to understand than biosimilars. There are many benefits of shared decision-making such as improved exchange of information, higher priority placed on taking action, and more discussion around treatment options[22,23]. In addition, a 5% savings on medical costs has been reported by patients who have been introduced to shared decision-making[24].
Our results indicate that our survey respondents have not generally been involved in discussions with their doctors regarding treatment plans or options. Moreover, most doctors do not make assumptions about our survey respondent’s income. Unfortunately, we do not know if patients who read our brochures but decided not to participate in our survey have also not involved in such discussions. Moreover, the survey responses received in our current study may not reflect the views of all patients attending rheumatology practices in Colorado. One explanation might be that patients with more negative experience were more likely to respond to our survey.
However, our results somewhat echo a previous questionnaire-based study which found that nearly every other patient preferred that their doctor decide treatment without their participation[23]. Moreover, about one out of five preferred to share the decision equally with their doctor and only a few wished to make the decision by themselves[23]. Increasingly, studies show a greater preference for shared decision-making with time, with a 21% leap in preference in studies conducted after 2000[22,23]. Obviously, both doctor and patient need to understand the importance of discussing new treatment options. This, in turn, will likely improve patient treatment adherence and will have a positive impact on all other treatment outcomes.
Nevertheless, most patients participating in our survey had an interest in discussing biosimilars with their doctors as well as family members and friends and were motivated to learn more about biosimilars. Interestingly, more patients in our study were willing to discuss and learn more about shared decision-making than were about biosimilars. Similarly, we found that most patients attending oncology/hematology practices in Colorado were also interested in discussing biosimilars with their doctor and others in our previous study[10]. As we mentioned previously, local patient organizations and groups could encourage the discussion focused on biosimilars. The previous study, which examined perception and knowledge of biosimilars among Belgian rheumatologists and rheumatoid arthritis patients, also stressed the importance of both patient organizations and doctors in patient education[4].
We found that knowledge and awareness of shared decision-making was higher after patients had read our educational materials. This result is important in light of the nocebo effect, which refers to a patient’s negative expectation towards switching from a reference biologic to its biosimilar[25]. The occurrence of the nocebo effect may translate into decreased adherence to therapy or even therapy discontinuation and, hence, has a negative impact on the cost-effectiveness of biologic therapy. Improved shared decision-making and patient empowerment has been proposed as a strategy to mitigate the risk of the nocebo effect[26].
As we observe the improvement in scores after reading our booklet, we also suggest that prospective educational initiatives should add various printing materials such as brochures. However, no previous studies exist which focus on preferred styles of learning more about biosimilars and shared decision-making among rheumatology patients. We in fact explored such learning styles in our study which focused on knowledge and awareness of biosimilars among patients attending oncology practices in Colorado[10]. Similar to our current study results, printed materials were the most popular style of learning identified.
However, in contrast to our previous results, rheumatology patients seem to prefer online presentations and webinars over seminars and group discussions in their community. One of the explanations could be the fact that our rheumatology patient cohort was generally younger than the oncology one. Future educational interventions should be focused on other similar issues such as which style of learning more about biosimilars and/or shared decision-making is preferred in other patient groups (e.g., children, seniors etc.) and other geographic areas. Another direction for prospective projects focused on this subject is whether a combination of learning styles (i.e., printed materials and live seminars) is more effective than a single learning approach for these particular subjects.
One of the strengths of the current study is that the response rate was higher compared to our previous patient-based initiative focused on biosimilars (2.5% vs 1.6%)[10]. At the same time, our project focused on educating oncology/hematology clinic team members, in Colorado, about biosimilars yielded more than 6% response rate[8]. One of the obvious explanations is that medical professionals are generally more interested in this subject and thus more motivated to take part in the survey. Moreover, we believe that many health professionals involved in the treatment of various autoimmune diseases and cancers are aware of the less than optimal outcomes associated with said treatments and thus are motivated to learn more about biosimilars. Another explanation for our relatively low response rate is that the subject itself (“biosimilars”) is a relatively new one as compared to other treatment options with the general public still largely unaware of this class of medications. As such, prospective studies focused on biosimilars might attract a higher response rate as more biosimilars enter the medical field.
We aimed to develop our booklet using language easily understood by non-medical professionals as we concluded from literature reviews that many patients probably had never heard about biosimilars or shared decision-making before. As such, our booklet and survey did not discuss topics that could raise a potential challenge for our participants, such as immunogenicity. We hope to conduct more prospective studies that would discuss more complex issues related to biosimilars.
As mentioned earlier, very limited research of rheumatology patients’ knowledge, and awareness of biosimilars and shared decision-making, as well as motivation to learn more about these subjects exists. More educational programs focused on biosimilars and shared decision-making should incorporate strengths and limitations from this study. For example, they might benefit from using various preferred learning styles (e.g., seminars, printed materials, and webinars). Such studies may also explore rheumatology patients’ perception and attitudes toward biosimilars and various topics related to shared decision-making. Based on our current study results, we recommend adding more survey questions focused on shared decision-making such as discussing treatment plans and options with doctors in various clinical settings (e.g., oncology, immunology, dermatology, etc.). Overall, more educational efforts focused on biosimilars and shared decision-making are warranted not only for rheumatology patients but also for patients in other clinical specialties such as gastroenterology, immunology, and oncology.
Based on our study results, many patients attending rheumatology practices in Colorado have a low level of knowledge and awareness of shared decision-making and, especially, biosimilars. On the other hand, most of these patients do not engage in important discussions regarding treatment plans or options with their doctors. As our survey respondents gained knowledge about biosimilars and shared decision-making from our booklet it seems that educational programs enriched with printed materials may impact both health care providers’ and patients’ knowledge and awareness of biosimilars as well as shared decision-making, as is consistent with our previous results. Moreover, patients attending rheumatology practices in Colorado were motivated to learn more about these topics and discuss them with others including their health care providers.
As we have become aware of which style of learning our survey respondents prefer regarding biosimilars and shared decision-making, we believe that prospective projects will benefit not only from booklets, but also from online presentation and webinars. Some complex topics such as immunogenicity should be incorporated by future educational programs as well. Follow up surveys measuring changes in knowledge and awareness regarding biosimilars and shared decision-making among patients attending rheumatology practices in Colorado remain highly needed. Future studies should explore knowledge and awareness of biosimilars and shared decision-making among members of rheumatology healthcare teams as well.
There are gaps in patients’ understanding of various topics related to biosimilars and shared decision-making.
On the other hand, there is limited published data focused on biosimilars and shared decision-making among patients attending rheumatology practices.
We aimed to increase knowledge and awareness of biosimilars and shared decision-making among patients diagnosed with various rheumatic diseases.
We developed patient-friendly print materials focused on biosimilars and shared decision-making and distributed them to each participating rheumatology office in Colorado. Subsequently, we administered a survey of patients from participating offices.
After reading our print materials, most patients identified the correct answer regarding biosimilars and shared decision-making. Moreover, many patients were motivated to learn more about biosimilars and shared decision-making. On the other hand, our results indicate that many rheumatology patients in Colorado are generally not involved in discussions with their providers regarding treatment plans or options.
Our educational project effectively increased the low baseline knowledge and awareness of biosimilars and shared decision-making among patients diagnosed with various rheumatic diseases.
Future studies should be able to use the strengths and limitations from our current project to conduct more educational programs focused on biosimilars and shared decision-making with the ultimate goal to improve treatment adherence.
Manuscript source: Unsolicited manuscript
Specialty type: Rheumatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elzawawy A, Gumustas OG S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32:174-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Crane MM, Juneja M, Allen J, Kurrasch RH, Chu ME, Quattrocchi E, Manson SC, Chang DJ. Epidemiology and Treatment of New-Onset and Established Rheumatoid Arthritis in an Insured US Population. Arthritis Care Res (Hoboken). 2015;67:1646-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Cohen S, Kay J. Biosimilars: implications for rheumatoid arthritis therapy. Curr Opin Rheumatol. 2017;29:260-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | van Overbeeke E, De Beleyr B, de Hoon J, Westhovens R, Huys I. Perception of Originator Biologics and Biosimilars: A Survey Among Belgian Rheumatoid Arthritis Patients and Rheumatologists. BioDrugs. 2017;31:447-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Boccia R, Jacobs I, Popovian R, de Lima Lopes G Jr. Can biosimilars help achieve the goals of US health care reform? Cancer Manag Res. 2017;9:197-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Mulcahy AW, Hlavka JP, Case SR. Biosimilar Cost Savings in the United States: Initial Experience and Future Potential. Rand Health Q. 2018;7:3. [PubMed] [Cited in This Article: ] |
| 7. | Araújo FC, Gonçalves J, Fonseca JE. Biosimilars in rheumatology. Pharmacol Res. 2019;149:104467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Smolen JS, Goncalves J, Quinn M, Benedetti F, Lee JY. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5:e000900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Ismailov RM, Khasanova ZD, Gascon P. Knowledge and awareness of biosimilars among oncology patients in Colorado, USA. Future Oncol. 2019;15:2577-2584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Forcino RC, Yen RW, Aboumrad M, Barr PJ, Schubbe D, Elwyn G, Durand MA. US-based cross-sectional survey of clinicians' knowledge and attitudes about shared decision-making across healthcare professions and specialties. BMJ Open. 2018;8:e022730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Légaré F, Adekpedjou R, Stacey D, Turcotte S, Kryworuchko J, Graham ID, Lyddiatt A, Politi MC, Thomson R, Elwyn G, Donner-Banzhoff N. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7:CD006732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 13. | Merchant FM, Dickert NW Jr, Howard DH. Mandatory Shared Decision Making by the Centers for Medicare & Medicaid Services for Cardiovascular Procedures and Other Tests. JAMA. 2018;320:641-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Backman WD, Levine SA, Wenger NK, Harold JG. Shared decision-making for older adults with cardiovascular disease. Clin Cardiol. 2020;43:196-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Ismailov RM, Khasanova ZD. Biosimilar Knowledge Among Oncology/Hematology Team Members in Colorado, USA: An Educational Initiative and Follow-Up Survey. BioDrugs. 2018;32:499-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Bramstedt KA, Ierna BN, Woodcroft-Brown VK. Using SurveyMonkey® to teach safe social media strategies to medical students in their clinical years. Commun Med. 2014;11:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Gimenez Verotti CC, de Miranda Torrinhas RS, Pires Corona L, Waitzberg DL. Design of quality indicators for oral nutritional therapy. Nutr Hosp. 2015;31:2692-2695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Peyrin-Biroulet L, Lönnfors S, Roblin X, Danese S, Avedano L. Patient Perspectives on Biosimilars: A Survey by the European Federation of Crohn's and Ulcerative Colitis Associations. J Crohns Colitis. 2017;11:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Peyrin-Biroulet L, Lönnfors S, Avedano L, Danese S. Changes in inflammatory bowel disease patients' perspectives on biosimilars: A follow-up survey. United European Gastroenterol J. 2019;7:1345-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Frantzen L, Cohen JD, Tropé S, Beck M, Munos A, Sittler MA, Diebolt R, Metzler I, Sordet C. Patients' information and perspectives on biosimilars in rheumatology: A French nation-wide survey. Joint Bone Spine. 2019;86:491-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Aladul MI, Fitzpatrick RW, Chapman SR. Patients' Understanding and Attitudes Towards Infliximab and Etanercept Biosimilars: Result of a UK Web-Based Survey. BioDrugs. 2017;31:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 463] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 23. | Yu CH, Ke C, Jovicic A, Hall S, Straus SE; IP-SDM Team;. Beyond pros and cons - developing a patient decision aid to cultivate dialog to build relationships: insights from a qualitative study and decision aid development. BMC Med Inform Decis Mak. 2019;19:186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32:285-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical Switching From Originators to Biosimilars: Does the Nocebo Effect Explain Treatment Failures and Adverse Events in Rheumatology and Gastroenterology? Rheumatol Ther. 2020;7:35-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Colloca L, Panaccione R, Murphy TK. The Clinical Implications of Nocebo Effects for Biosimilar Therapy. Front Pharmacol. 2019;10:1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |